Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Intact Dlucosinolates and Isothiocyanates in Cruciferous Sprouts
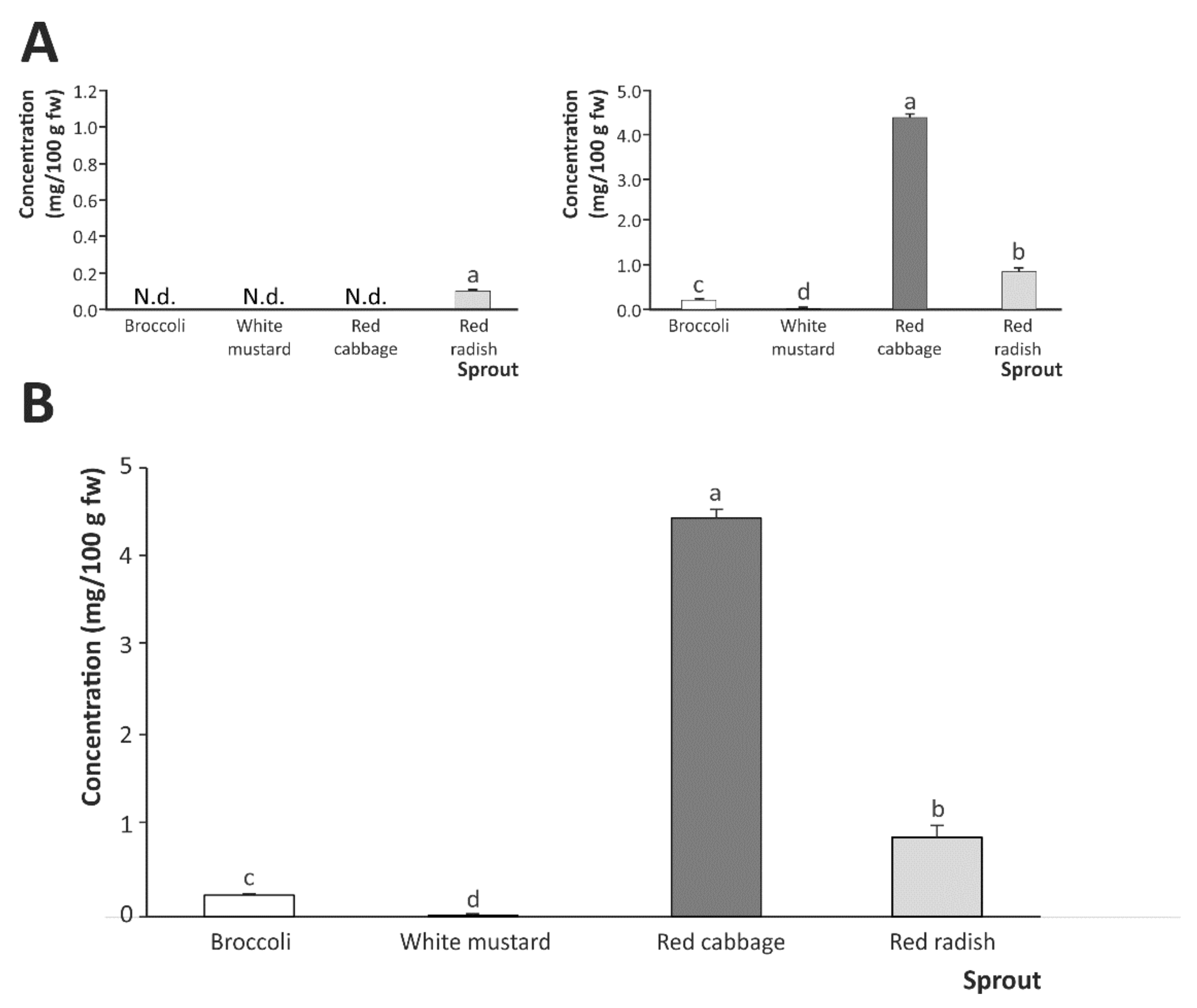
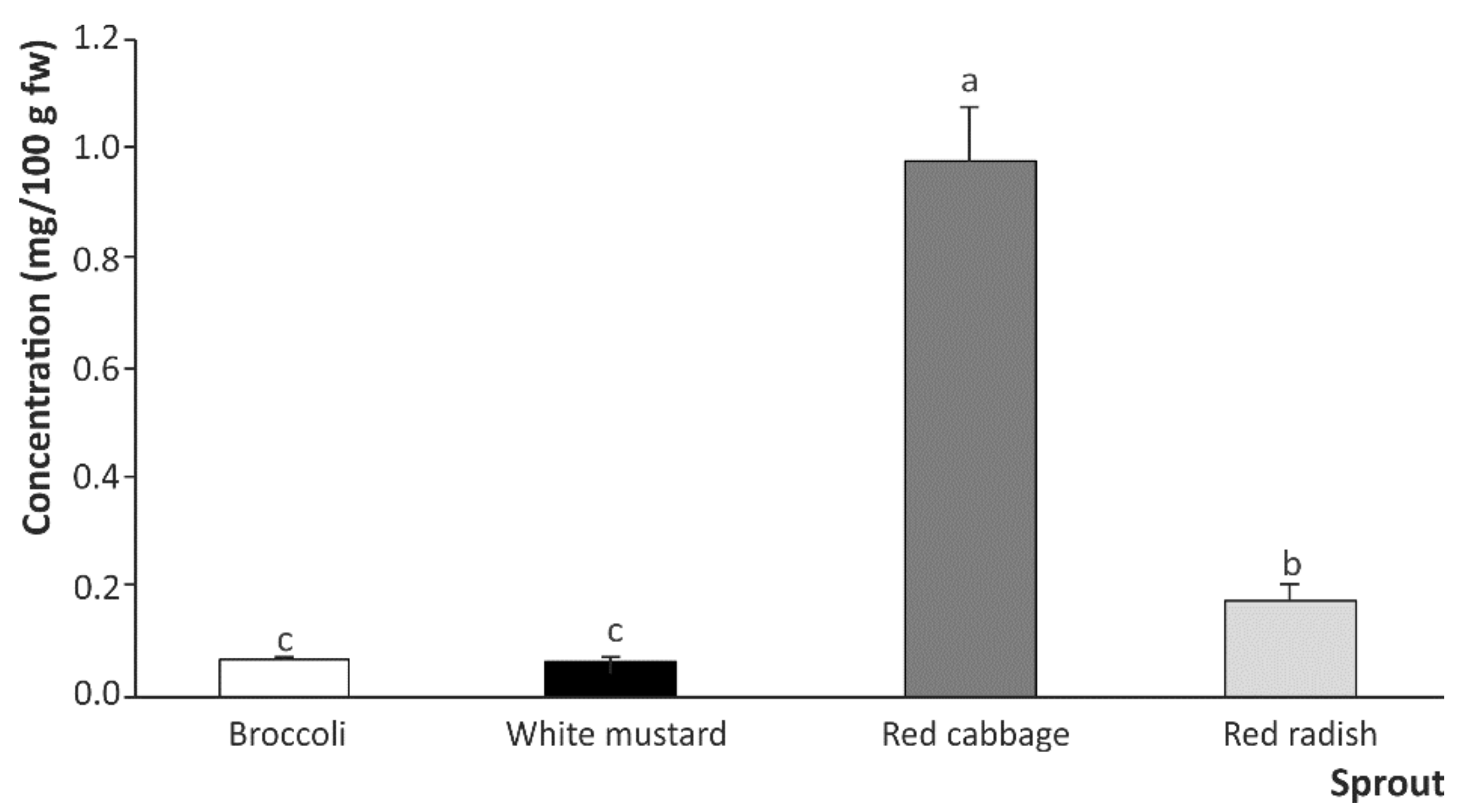
2.2. Breakdown Products from Sulfur-Based Glucosinolates after In Vitro Digestion of Cruciferous Sprouts
Influence of Gastric, Intestinal, and Gastrointestinal Digestion
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Plant Material
3.3. Processing Cruciferous Sprouts by a Simulated In Vitro Static Digestion Method
3.4. UHPLC-ESI-QqQ-MS/MS Analysis of Analytical Extracts and Digestates of Brassica Sprouts
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Domínguez-Perles, R.; Baenas, N.; García-Viguera, C. New Insights in (Poly)phenolic Compounds: From Dietary Sources to Health Evidence. Foods 2020, 9, 543. [Google Scholar]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenas, N.; Fusari, C.; Moreno, D.A.; Valero, D.; Cristina, G.-V. Biostimulation of bioactive compounds in radish sprouts (Raphanus sativus ‘Rambo’) by priming seeds and spray treatments with elicitors. Acta Hortic. 2019, 659–663. [Google Scholar] [CrossRef]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar]
- Becker, T.M.; Juvik, J.A. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Kawakishi, S.; Kaneko, T. Interaction of proteins with allyl isothiocyanate. J. Agric. Food Chem. 1987, 35, 85–88. [Google Scholar] [CrossRef]
- Murthy, N.V.K.K.; Rao, M.S.N. Interaction of allylisothiocyanate with bovine serum albumin. Int. J. Pept. Protein Res. 1986, 27, 433–439. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Hernández-Cánovas, L.; Abellán-Victorio, Á.; Moreno, D.A. The Quality and Glucosinolate Composition of Cruciferous Sprouts under Elicitor Treatments Using MeJA and LED Lights. Proceedings 2021, 70, 67. [Google Scholar] [CrossRef]
- Puangkam, K.; Muanghorm, W.; Konsue, N. Stability of Bioactive Compounds and Antioxidant Activity of Thai Cruciferous Vegetables During in Vitro Digestion. Curr. Res. Nutr. Food Sci. J. 2017, 5, 100–108. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Kozłowska, H. Effect of Light Conditions on the Contents of Glucosinolates in Germinating Seeds of White Mustard, Red Radish, White Radish, and Rapeseed. J. Agric. Food Chem. 2008, 56, 9087–9093. [Google Scholar] [CrossRef]
- Baenas, N.; Suárez Martínez, C.; Cristina, G.-V.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Medina, S.; Moreno, D.Á.; García-Viguera, C.; Ferreres, F.; Gil-Izquierdo, Á. A new ultra-rapid UHPLC/MS/MS method for assessing glucoraphanin and sulforaphane bioavailability in human urine. Food Chem. 2014, 143, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Fernández-León, A.M.; Fernández-León, M.F.; González-Gómez, D.; Ayuso, M.C.; Bernalte, M.J. Quantification and bioaccessibility of intact glucosinolates in broccoli ‘Parthenon’ and Savoy cabbage ‘Dama’. J. Food Compos. Anal. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- Hernández, M.d.C.; Medina, S.; Martínez-Ballesta, M.; Moreno, D.A. Broccoli isothiocyanate content and in vitro availability according to variety and origin. Maced. J. Chem. Chem. Eng. 2013, 32, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Kuljarachanan, T.; Fu, N.; Chiewchan, N.; Devahastin, S.; Chen, X.D. Evolution of important glucosinolates in three common Brassica vegetables during their processing into vegetable powder and in vitro gastric digestion. Food Funct. 2020, 11, 211–220. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Tipping the scales--specifier proteins in glucosinolate hydrolysis. IUBMB Life 2007, 59, 744–751. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, e0134. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; Mesa-Plata, O.; Cartea-González, M.E.; de Haro-Bailón, A. Quantification and in vitro bioaccessibility of glucosinolates and trace elements in Brassicaceae leafy vegetables. Food Chem. 2021, 339, 127860. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.-J.; Ruan, S.-Y.; Xie, Z.-H.; Dong, Y.; Yu, L. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chem. 2012, 133, 1569–1576. [Google Scholar] [CrossRef]
- Sarvan, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]


| Compound | MRM Quantitative Transition | MRM Qualitative Transition | Fragmentation (V) | Collision Energy (eV) | ESI Mode |
|---|---|---|---|---|---|
| Aliphatic glucosinolates | |||||
| GR | 436.0 > 97.0 | 436.0 > 372.0 | 90 | 25 | Negative |
| GRE | 434.0 > 97.0 | 434.0 > 259.0 | 90 | 25 | Negative |
| GE | 420.0 > 97.1 | 420.0 > 259.0 | 60 | 20 | Negative |
| GI | 422.0 > 357.7 | 422.0 > 259.0 | 100 | 26 | Negative |
| GN | 372.0 > 97.0 | 372.0 > 259.0 | 90 | 25 | Negative |
| Indolic glucosinolates | |||||
| GB | 447.2 > 97.0 | 447.2 > 259.0 | 80 | 20 | Negative |
| MeGB Y | 477.0 > 97.0 | 477.0 > 259.0 | 90 | 25 | Negative |
| OHGB Y | Negative | ||||
| NeoGB | 463.0 > 97.0 | 463.0 > 259.0 | 90 | 25 | Negative |
| Aromatic glucosinolates | |||||
| GNS | 422.0 > 97.0 | 422.0 > 259.0 | 90 | 25 | Negative |
| GT | 408.0 > 97.0 | N.d. | 90 | 25 | Negative |
| GSB | 424.4 > 97.0 | 424.10 > 259.0 | 90 | 25 | Negative |
| Isothiocyanates | |||||
| SFN | 178.0 > 114.0 | 178.0 > 95.0 | 74 | 4 | Positive |
| SFE | 176.0 > 114.0 | N.d. | 75 | 20 | Positive |
| E | 141.0 > 59.0 | 161.0 > 70.0 | 70 | 6 | Negative |
| IB | 164.0 > 105.0 | N.d. | 90 | 6 | Positive |
| Indoles | |||||
| I3C | 130.0 > 77.0 | 247.0 > 130.0 | 70 | 25 | Positive |
| Sprouts | Glucosinolates | Isothiocyanates | Indoles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic | Indolic | Aromatic | ||||||||||
| GR | GRE | GE | GB | MeBG | OHGB | NeoGB | GSB | SFN | SFE | IB | I3C | |
| Broccoli | 2.16 ± 0.13 a | N.d. | N.d. | 0.78 ± 0.39 c | 1.61 ± 0.02 b | 0.46 ± 0.08 b | 0.80 ± 0.15 c | N.d. | 0.235 ± 0.001 a | N.d. | 0.125 ± 0.002 a | 0.196 ± 0.003 b |
| White mustard | N.d. | N.d. | N.d. | 1.08 ± 0.11 bc | 1.30 ± 0.19 c | N.d. | 1.18 ± 0.05 a | 15.43 ± 0.81 a | 0.0020 ± 0.001 b | N.d. | N.d. | 0.150 ± 0.005 c |
| Red cabbage | 2.45 ± 0.14 a | N.d. | 2.04 ± 0.22 a | 1.13 ± 0.32 a | 2.29 ± 0.42 a | 1.40 ± 0.09 a | 1.02 ± 0.02 b | 0.05 ± 0.01 b | 0.231 ± 0.004 a | N.d. | 0.19 ± 0.01 b | 0.534 ± 0.012 a |
| Red radish | 0.30 ± 0.04 b | 0.97 ± 0.02 a | 2.26 ± 0.06 a | 0.16 ± 0.01 d | 1.48 ± 0.18 bc | N.d. | N.d. | N.d. | 0.025 ± 0.002 b | 0.077 ± 0.002 a | N.d. | 0.045 ± 0.002 d |
| Sprout | Glucosinolate Breakdown Products | |||
|---|---|---|---|---|
| SFN | SFE | IB | I3C | |
| Gastrointestinal digestion | ||||
| Broccoli | 0.129 ± 0.015 b | N.d. | 0.070 ± 0.001 c | 0.040 ± 0.004 a |
| White mustard | 0.013 ± 0.002 c | N.d. | N.d. | N.d. |
| Red cabbage | 0.204 ± 0.004 a | N.d. | 4.190 ± 0.080 a | 0.046 ± 0.004 a |
| Red radish | 0.014 ± 0.001 c | N.d. | 0.840 ± 0.100 b | 0.029 ± 0.001 b |
| Gastric digestion | ||||
| Broccoli | N.d. | N.d. | N.d. | 0.048 ± 0.001 b |
| White mustard | N.d. | N.d. | N.d. | N.d. |
| Red cabbage | N.d. | N.d. | N.d. | N.d. |
| Red radish | N.d. | 0.039 ± 0.001 a | N.d. | 0.063 ± 0.006 a |
| Intestinal (theoretical) digestion | ||||
| Broccoli | 0.129 ± 0.015 b | N.d. | 0.070 ± 0.001 c | N.d. |
| White mustard | 0.013 ± 0.002 c | N.d. | N.d. | N.d. |
| Red cabbage | 0.204 ± 0.012 a | N.d. | 4.190 ± 0.080 a | 0.046 ± 0.004 a |
| Red radish | 0.014 ± 0.001 c | N.d. | 0.890 ± 0.050 b | N.d. |
| Sprouts | Glucosinolate Breakdown Products | |||
|---|---|---|---|---|
| SFN | SFE | IB | I3C | |
| Broccoli | 0.026 ± 0.001 b | N.d. | 0.007 ± 0.001 c | 0.035 ± 0.001 c |
| White mustard | 0.006 ± 0.001 c | N.d. | N.d. | 0.061 ± 0.001 b |
| Red cabbage | 0.064 ± 0.001 a | N.d. | 0.840 ± 0.092 a | 0.076 ± 0.004 a |
| Red radish | 0.006 ± 0.001 c | 0.055 ± 0.014 a | 0.116 ± 0.002 b | 0.063 ± 0.006 b |
| Consituent | Concentration of SGF, PH 3 (mmol L−1) | Concentration of SIF, PH 7 (mmol L−1) |
|---|---|---|
| Potassium chloride (KCl) | 6.90 | 6.80 |
| Potassium dihydrogenphosphate (KH2PO4) | 0.90 | 0.80 |
| Sodium hydrogen carbonate (NaHCO3) | 25.00 | 85.00 |
| Sodium chloride (NaCl) | 47.20 | 38.40 |
| Magnesium chloride (MgCl2) | 0.10 | 0.33 |
| Ammonium carbonate ((NH4)CO3) | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. https://doi.org/10.3390/ijms222011046
Abellán Á, Domínguez-Perles R, García-Viguera C, Moreno DA. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. International Journal of Molecular Sciences. 2021; 22(20):11046. https://doi.org/10.3390/ijms222011046
Chicago/Turabian StyleAbellán, Ángel, Raúl Domínguez-Perles, Cristina García-Viguera, and Diego A. Moreno. 2021. "Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion" International Journal of Molecular Sciences 22, no. 20: 11046. https://doi.org/10.3390/ijms222011046
APA StyleAbellán, Á., Domínguez-Perles, R., García-Viguera, C., & Moreno, D. A. (2021). Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. International Journal of Molecular Sciences, 22(20), 11046. https://doi.org/10.3390/ijms222011046







