Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration
Abstract
:1. Introduction
2. Results
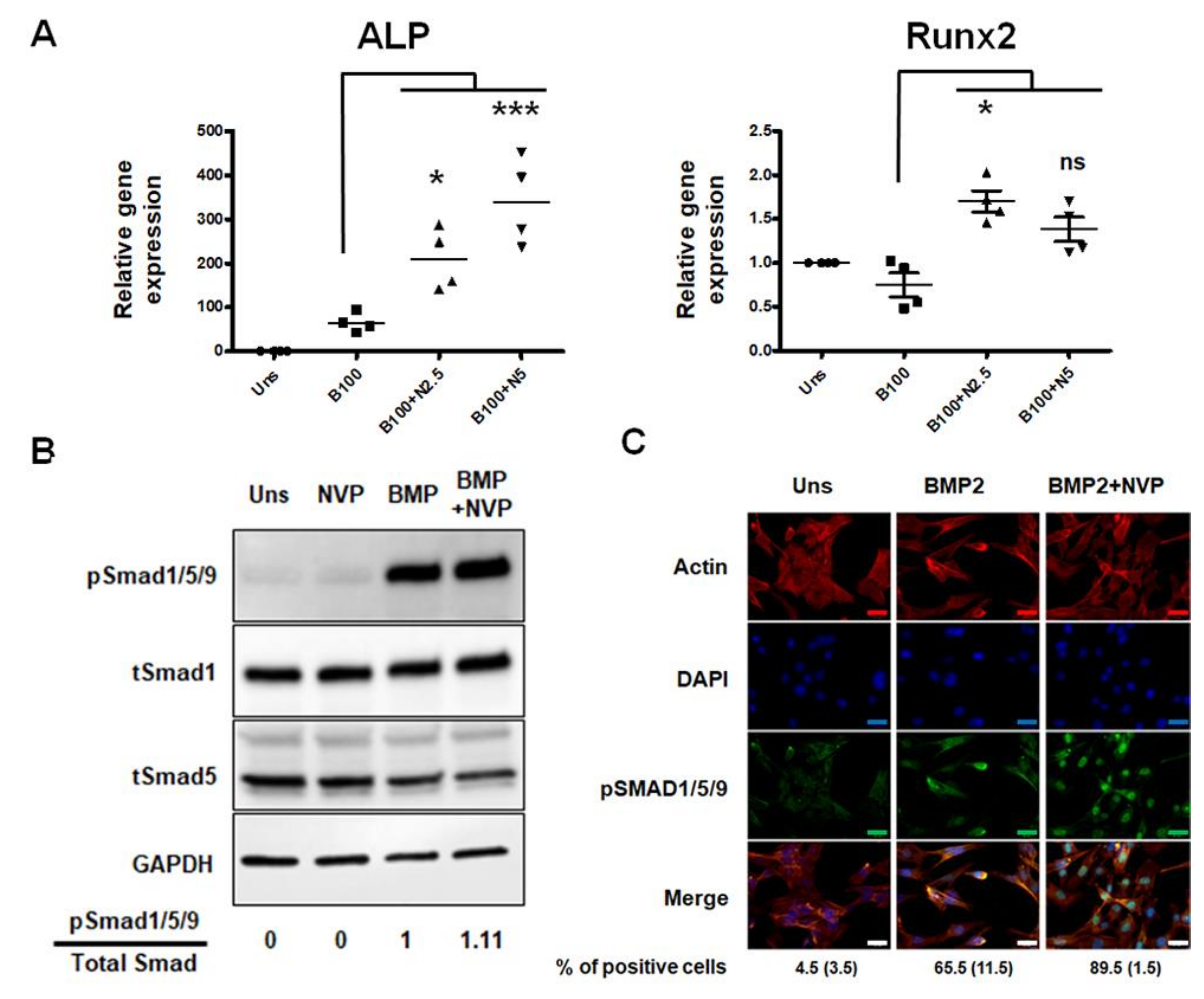
2.1. NVP Enhances BMP2-Induced Osteoblast Differentiation in C2C12 Cells
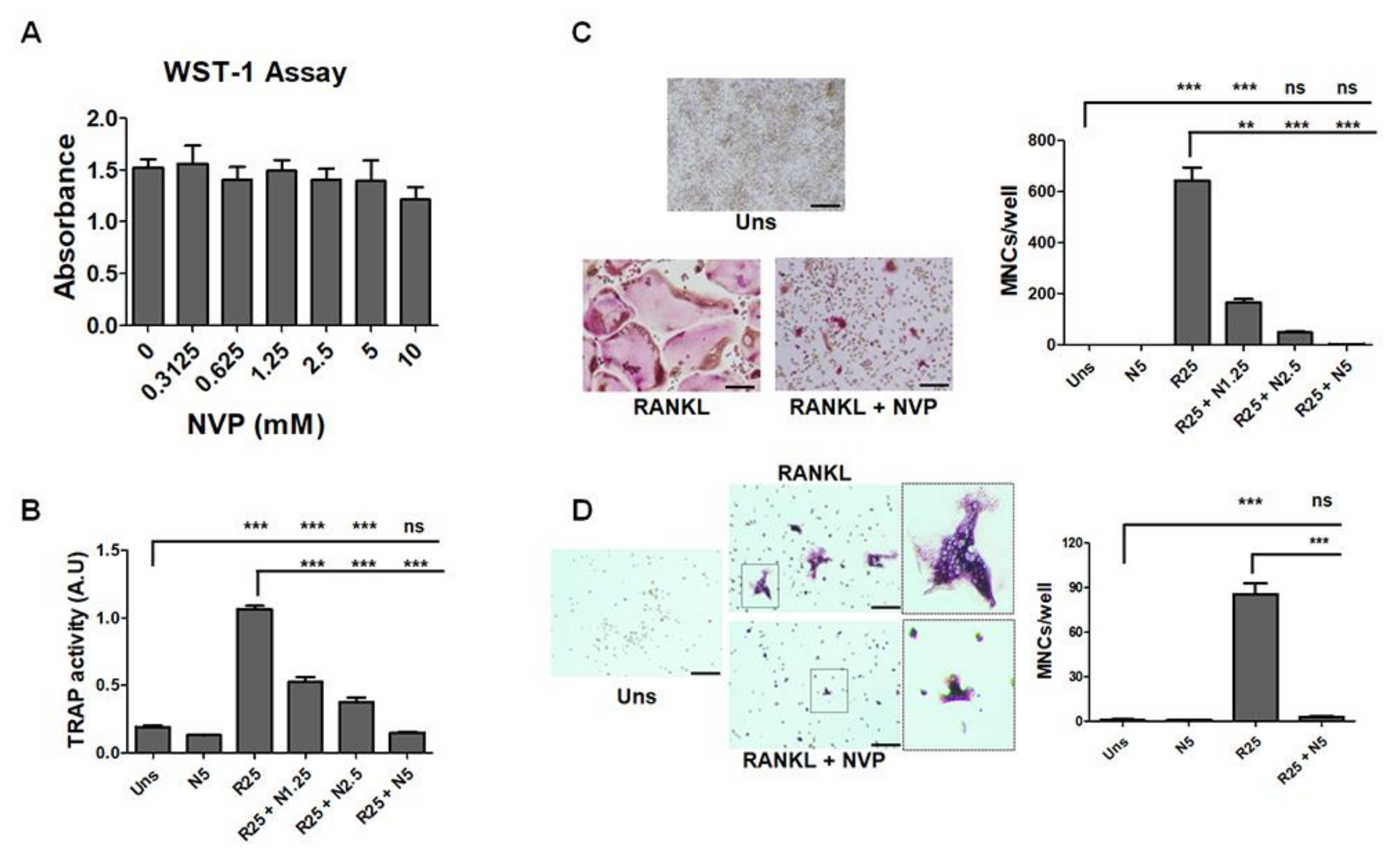
2.2. NVP Inhibits RANKL-Induced Osteoclast Differentiation
2.3. The Release of NVP from Loaded Membranes
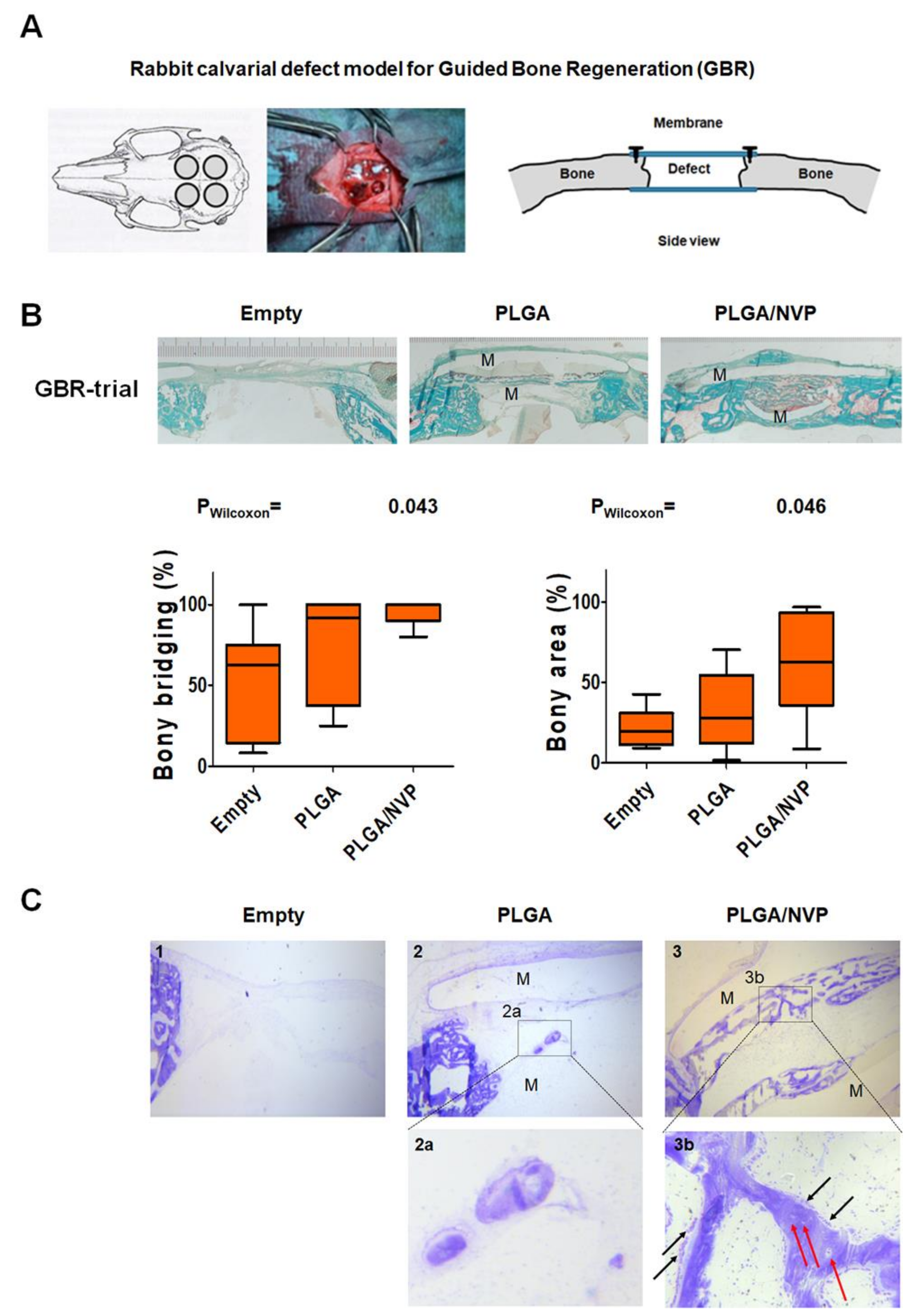
2.4. NVP Enhances Bone Regeneration In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. AlphaScreening Assay
4.3. Cell Cultures
4.4. Primary Rabbit Osteoblast
4.5. Primary Bone Marrow-Derived Monocytes (BMMs)
4.6. Cell Viability and Proliferation Assay
4.7. Assay of ALP Activity and ALP Staining
4.8. Assay of TRAP Activity and TRAP Staining
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Protein Preparation and Western Blot Analysis
4.11. Immunofluorescence Staining, pSmad1/5/9 Nuclear Translocation
4.12. NVP Loading of PLGA Membranes
4.13. In Vitro Release of NVP
4.14. Scanning Electron Microscopy for Structural Analysis
4.15. Animal Model for Bone Regeneration
4.16. Histology
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Moffitt, E.A. Blood substitutes. Can. Anaesth. Soc. J. 1975, 22, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, E. Safety evaluation of substances consumed as technical ingredients (food additives). Food Addit. Contam. 1991, 8, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Waleka, E.; Stojek, Z.; Karbarz, M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics 2021, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghayor, C.; Weber, F.E. Epigenetic Regulation of Bone Remodeling and Its Impacts in Osteoporosis. Int. J. Mol. Sci 2016, 17, 1446. [Google Scholar] [CrossRef] [Green Version]
- Gjoksi, B.; Ghayor, C.; Siegenthaler, B.; Ruangsawasdi, N.; Zenobi-Wong, M.; Weber, F.E. The epigenetically active small chemical N-methyl pyrrolidone (NMP) prevents estrogen depletion induced osteoporosis. Bone 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.H.; Weber, F.E.; Malina-Altzinger, J.; Ghayor, C. Epigenetic drugs as new therapy for tumor necrosis factor-alpha-compromised bone healing. Bone 2019, 127, 49–58. [Google Scholar] [CrossRef]
- Ghayor, C.; Correro, R.M.; Lange, K.; Karfeld-Sulzer, L.S.; Gratz, K.W.; Weber, F.E. Inhibition of osteoclast differentiation and bone resorption by N-methylpyrrolidone. J. Biol. Chem. 2011, 286, 24458–24466. [Google Scholar] [CrossRef] [Green Version]
- Boyson, S.P.; Gao, C.; Quinn, K.; Boyd, J.; Paculova, H.; Frietze, S.; Glass, K.C. Functional Roles of Bromodomain Proteins in Cancer. Cancers 2021, 13, 3606. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Chiu, L.Y.; Miller, K.M. Acetylation Reader Proteins: Linking Acetylation Signaling to Genome Maintenance and Cancer. PLoS Genet. 2016, 12, e1006272. [Google Scholar] [CrossRef] [PubMed]
- Mirguet, O.; Lamotte, Y.; Chung, C.W.; Bamborough, P.; Delannee, D.; Bouillot, A.; Gellibert, F.; Krysa, G.; Lewis, A.; Witherington, J.; et al. Naphthyridines as novel BET family bromodomain inhibitors. Chem. Med. Chem. 2014, 9, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, J.; Muller, M.; Yan, S.; Mujtaba, S.; Pan, C.; Wang, Z.; Zhou, M.M. Selective small molecules blocking HIV-1 Tat and coactivator PCAF association. J. Am. Chem. Soc. 2005, 127, 2376–2377. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2009, CD003607. [Google Scholar] [CrossRef]
- Miguel, B.S.; Ghayor, C.; Ehrbar, M.; Jung, R.E.; Zwahlen, R.A.; Hortschansky, P.; Schmoekel, H.G.; Weber, F.E. N-methyl pyrrolidone as a potent bone morphogenetic protein enhancer for bone tissue regeneration. Tissue Eng. Part A 2009, 15, 2955–2963. [Google Scholar] [CrossRef] [Green Version]
- Siegenthaler, B.; Ghayor, C.; Ruangsawasdi, N.; Weber, F.E. The Release of the Bromodomain Ligand N,N-Dimethylacetamide Adds Bioactivity to a Resorbable Guided Bone Regeneration Membrane in a Rabbit Calvarial Defect Model. Materials 2020, 13, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjoksi, B.; Ghayor, C.; Bhattacharya, I.; Zenobi-Wong, M.; Weber, F.E. The bromodomain inhibitor N-methyl pyrrolidone reduced fat accumulation in an ovariectomized rat model. Clin. Epigenet. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjoksi, B.; Ruangsawasdi, N.; Ghayor, C.; Siegenthaler, B.; Zenobi-Wong, M.; Weber, F.E. Influence of N-methyl pyrrolidone on the activity of the pulp-dentine complex and bone integrity during osteoporosis. Int. Endod. J. 2017, 50, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Pennypacker, B.; Shea, M.; Liu, Q.; Masarachia, P.; Saftig, P.; Rodan, S.; Rodan, G.; Kimmel, D. Bone density, strength, and formation in adult cathepsin K (-/-) mice. Bone 2009, 44, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Hayashi, K.; Hoshijima, M.; Ogawa, T.; Koga, S.; Miyatake, Y.; Kumegawa, M.; Kimura, T.; Takeya, T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 2002, 277, 41147–41156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Khorsand, B.; Elangovan, S.; Hong, L.; Kormann, M.S.D.; Salem, A.K. A bioactive collagen membrane that enhances bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1824–1832. [Google Scholar] [CrossRef]
- Karfeld-Sulzer, L.S.; Ghayor, C.; Siegenthaler, B.; Gjoksi, B.; Pohjonen, T.H.; Weber, F.E. Comparative study of NMP-preloaded and dip-loaded membranes for guided bone regeneration of rabbit cranial defects. J. Tissue Eng. Regen. Med. 2017, 11, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Karfeld-Sulzer, L.S.; Ghayor, C.; Siegenthaler, B.; de Wild, M.; Leroux, J.C.; Weber, F.E. N-methyl pyrrolidone/bone morphogenetic protein-2 double delivery with in situ forming implants. J. Control. Release 2015, 203, 181–188. [Google Scholar] [CrossRef]
- Ghayor, C.; Gjoksi, B.; Dong, J.; Siegenthaler, B.; Caflisch, A.; Weber, F.E. N,N Dimethylacetamide a drug excipient that acts as bromodomain ligand for osteoporosis treatment. Sci. Rep. 2017, 7, 42108. [Google Scholar] [CrossRef] [Green Version]
- Siegenthaler, B.; Ghayor, C.; Gjoksi-Cosandey, B.; Ruangsawasdi, N.; Weber, F.E. The Bromodomain Inhibitor N-Methyl pyrrolidone Prevents Osteoporosis and BMP-Triggered Sclerostin Expression in Osteocytes. Int. J. Mol. Sci. 2018, 19, 3332. [Google Scholar] [CrossRef] [Green Version]
- Shortt, J.; Hsu, A.K.; Martin, B.P.; Doggett, K.; Matthews, G.M.; Doyle, M.A.; Ellul, J.; Jockel, T.E.; Andrews, D.M.; Hogg, S.J.; et al. The drug vehicle and solvent N-methylpyrrolidone is an immunomodulator and antimyeloma compound. Cell Rep. 2014, 7, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinjha, R.K.; Witherington, J.; Lee, K. Place your BETs: The therapeutic potential of bromodomains. Trends Pharmacol. Sci. 2012, 33, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Baud’huin, M.; Rodriguez Calleja, L.; Jacques, C.; Berreur, M.; Redini, F.; Lecanda, F.; Bradner, J.E.; Heymann, D.; Ory, B. Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nat. Commun. 2014, 5, 3511. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, L.; Tang, Y.; Tu, Q.; Zheng, L.; Yu, L.; Murray, D.; Cheng, J.; Kim, S.H.; Zhou, X.; et al. BET Inhibitor JQ1 Blocks Inflammation and Bone Destruction. J. Dent. Res. 2014, 93, 657–662. [Google Scholar] [CrossRef]
- Celil, A.B.; Hollinger, J.O.; Campbell, P.G. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J. Cell Biochem. 2005, 95, 518–528. [Google Scholar] [CrossRef]
- Drissi, M.H.; Li, X.; Sheu, T.J.; Zuscik, M.J.; Schwarz, E.M.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J. Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J. Cell Biochem. 2003, 90, 1287–1298. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Hong, S.H.; Bae, S.C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 2002, 21, 7156–7163. [Google Scholar] [CrossRef] [Green Version]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Wagner, E.F.; Eferl, R. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 2005, 208, 126–140. [Google Scholar] [CrossRef]
- Wilson, S.R.; Peters, C.; Saftig, P.; Bromme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhang, B.; Chang, X. Emerging roles of circular RNAs in osteoporosis. J. Cell Mol. Med. 2021, 25, 9089–9101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, J.; Xu, G.; Ma, D. Recent advances in the epigenetics of bone metabolism. J. Bone Miner. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Ehrbar, M.; San Miguel, B.; Gratz, K.W.; Weber, F.E. cAMP enhances BMP2-signaling through PKA and MKP1-dependent mechanisms. Biochem. Biophys. Res. Commun. 2009, 381, 247–252. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemmer, V.A.; Khera, N.; Siegenthaler, B.M.; Bhattacharya, I.; Weber, F.E.; Ghayor, C. Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 11052. https://doi.org/10.3390/ijms222011052
Klemmer VA, Khera N, Siegenthaler BM, Bhattacharya I, Weber FE, Ghayor C. Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration. International Journal of Molecular Sciences. 2021; 22(20):11052. https://doi.org/10.3390/ijms222011052
Chicago/Turabian StyleKlemmer, Viviane A., Nupur Khera, Barbara M. Siegenthaler, Indranil Bhattacharya, Franz E. Weber, and Chafik Ghayor. 2021. "Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration" International Journal of Molecular Sciences 22, no. 20: 11052. https://doi.org/10.3390/ijms222011052
APA StyleKlemmer, V. A., Khera, N., Siegenthaler, B. M., Bhattacharya, I., Weber, F. E., & Ghayor, C. (2021). Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration. International Journal of Molecular Sciences, 22(20), 11052. https://doi.org/10.3390/ijms222011052






