Identification of Salicylates in Willow Bark (Salix Cortex) for Targeting Peripheral Inflammation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity-Guided Fractionation of Salix Bark Extract
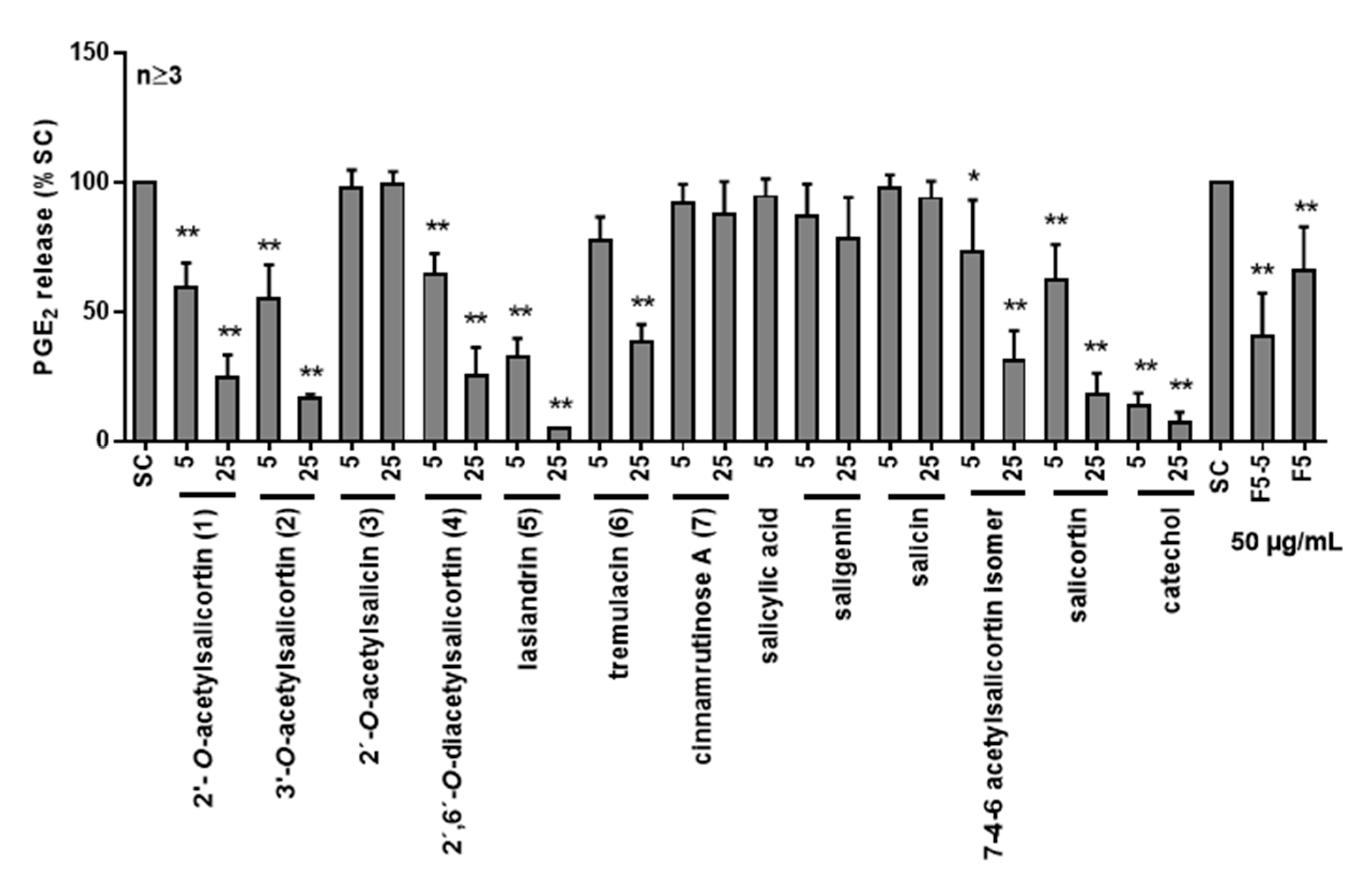
2.2. Anti-Inflammatory Activity of Salix Compounds
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Sequential Solvent Extraction of Willow Bark
4.3. Fractionation of the Methanol Fraction by Means of Solid-Phase Extraction
4.4. Isolation and Identification of (Non)Bioactive Salix Compounds
4.5. Determination of the Absolute Configuration
4.6. Sugar Determination
4.7. Acetalization Reaction
4.8. High-Performance Liquid Chromatography
4.9. Ultra-Performance Liquid Chromatography Time-of-Flight Mass Spectrometry
4.10. Quadrupole LC-MS/MS Spectrometer
4.11. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.12. Isolation and Exposure of Human Peripheral Blood Mononuclear Cells
4.13. Preparation of Extracts, Fractions, and Compounds for Bioactivity Assays
4.14. Quantification of PGE2 Release by Enzyme-Linked Immunosorbent Assay Assay
4.15. Determination of COX-1 and COX-2 Enzyme Activity Inhibition
4.16. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- EMA. Assessment Report on Salix [Various Species Including S. purpurea, S. daphnoides Vill., S. fragilis L.], Cortex; European Medicines Agency: London, UK, 2017.
- Förster, N.; Ulrichs, C.; Zander, M.; Katzel, R.; Mewis, I. Influence of the season on the salicylate and phenolic glycoside contents in the bark of Salix daphnoides, Salix pentandra, and Salix purpurea. J. Appl. Bot. Food Qual. Angew. Bot. 2008, 82, 99–102. [Google Scholar]
- Donaldson, J.R.; Stevens, M.T.; Barnhill, H.R.; Lindroth, R.L. Age-related shifts in leaf chemistry of clonal aspen (Populus tremuloides). J. Chem. Ecol. 2006, 32, 1415–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruuhola, T.M.; Julkunen-Tiitto, M.-R.K. Salicylates of intact Salix myrsinifolia plantlets do not undergo rapid metabolic turnover. Plant Physiol. 2000, 122, 895–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fötsch, G.; Pfeifer, S. Die Biotransformation der Phenolglycoside Leiocarposid und Salicin―Beispiele für Besonderheiten von Absorption und Metabolismus glycosidischer Verbindungen. Pharmazie 1989, 44, 710–712. [Google Scholar] [PubMed]
- Krantz, M.J.; Berger, J.S.; Hiatt, W.R. An Aspirin a Day: Are We Barking Up the Wrong Willow Tree? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Bonaterra, G.A.; Heinrich, E.U.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine 2010, 17, 1106–1113. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Okpanyi, S.N.; Kelber, O.; Weiser, D. Mechanisms Involved in the Anti-inflammatory Effect of a Standardized Willow Bark Extract. Arzneimittelforschung 2005, 55, 677–687. [Google Scholar] [CrossRef]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef]
- Knuth, S.; Abd el salam, R.; Khayyal, M.; Schweda, F.; Heilmann, J.; Kees, M.; Mair, G.; Kees, F.; Jürgenliemk, G. Catechol is a bioactive metabolite of Willow bark. Planta Med. 2013, 79, SL49. [Google Scholar] [CrossRef]
- Knuth, S.; Schübel, H.; Hellemann, M.; Jürgenliemk, G. Catechol, a bioactive degradation product of salicortin, reduces TNF-α induced ICAM-1 expression in human endothelial cells. Planta Med. 2011, 77, 1024–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Kinneer, K. Chemoprotection by phenolic antioxidants. Inhibition of tumor necrosis factor alpha induction in macrophages. J. Biol. Chem. 2002, 277, 2477–2484. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.T.; Ryu, G.M.; Kwon, B.M.; Lee, W.H.; Suk, K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. Eur. J. Pharmacol. 2008, 588, 106–113. [Google Scholar] [CrossRef]
- Förster, N.; Ulrichs, C.; Zander, M.; Kätzel, R.; Mewis, I. Factors influencing the variability of antioxidative phenolic glycosides in Salix species. J. Agric. Food Chem. 2010, 58, 8205–8210. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Sugier, D.; Dziki, D.; Sugier, P. Bioaccessibility In Vitro of Nutraceuticals from Bark of Selected Salix Species. Sci. World J. 2014, 2014, 782763. [Google Scholar] [CrossRef] [Green Version]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef] [Green Version]
- Ruuhola, T.; Julkunen-Tiitto, R. Trade-Off Between Synthesis of Salicylates and Growth of Micropropagated Salix pentandra. J. Chem. Ecol. 2003, 29, 1565–1588. [Google Scholar] [CrossRef] [PubMed]
- Ruuhola, T.; Julkunen-Tiitto, R.; Vainiotalo, P. In vitro degradation of willow salicylates. J. Chem. Ecol. 2003, 29, 1083–1097. [Google Scholar] [CrossRef]
- Shao, Y.; Lahloub, M.; Meier, B.; Sticher, O. Isolation of phenolic compounds from the bark of Salix pentandra. Planta Med. 1989, 55, 617–618. [Google Scholar] [CrossRef]
- Herz, C.; Márton, M.-R.; Tran, H.T.T.; Gründemann, C.; Schell, J.; Lamy, E. Benzyl isothiocyanate but not benzyl nitrile from Brassicales plants dually blocks the COX and LOX pathway in primary human immune cells. J. Funct. Foods 2016, 23, 135–143. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Márton, M.-R.; Herz, C.; Maul, R.; Baldermann, S.; Schreiner, M.; Lamy, E. Nasturtium (Indian cress, Tropaeolum majus nanum) dually blocks the COX and LOX pathway in primary human immune cells. Phytomedicine 2016, 23, 611–620. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflammation 2020, 17, 30. [Google Scholar] [CrossRef]
- Kawabata, A. Prostaglandin E2 and Pain—An Update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef] [Green Version]
- Vane, J.R.; Botting, R.M. Mechanism of action of aspirin-like drugs. Semin. Arthritis Rheum 1997, 26 (Suppl. 1), 2–10. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brune, K.; Patrignani, P. Patrignani, New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, N.P.K.; Herz, C.; Gomes, J.V.D.; Förster, N.; Antoniadou, K.; Mittermeier-Kleßinger, V.K.; Mewis, I.; Dawid, C.; Ulrichs, C.; Lamy, E. Comparative Anti-Inflammatory Effects of Salix Cortex Extracts and Acetylsalicylic Acid in SARS-CoV-2 Peptide and LPS-Activated Human In Vitro Systems. Int. J. Mol. Sci. 2021, 22, 6766. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Shao, Y.; Julkunen-Tiitto, R.; Bettschart, A.; Sticher, O. A chemotaxonomic survey of phenolic compounds in Swiss willow species. Proc. R. Soc. Edinburgh. Sect. B. Boil. Sci. 1992, 98, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, P.B.; Merken, H.M.; Clausen, T.P.; Wu, J. Phenolic glycosides from Salix lasiandra. J. Nat. Prod. 1992, 55, 970–973. [Google Scholar] [CrossRef]
- Kim, C.; Subedi, L.; Park, K.; Kim, S.; Choi, S.; Kim, K.H.; Lee, K. Salicin derivatives from Salix glandulosa and their biological activities. Fitoterapia 2015, 106, 147–152. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, C.; Dawid, C.; Peters, V.; Hofmann, T. Saponins from European Licorice Roots (Glycyrrhiza glabra ). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Jossang, A.; Jossang, P.; Bodo, B. Cinnamrutinoses A and B, glycosides of Populus tremula. Phytochemistry 1994, 35, 547–549. [Google Scholar] [CrossRef]
- Noleto-Dias, C.; Wu, Y.; Bellisai, A.; Macalpine, W.; Beale, M.H.; Ward, J.L. Phenylalkanoid Glycosides (Non-Salicinoids) from Wood Chips of Salix triandra × dasyclados Hybrid Willow. Molecules 2019, 24, 1152. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Rena, K.; Yang, X.-W. New salicin derivatives from the leaves ofPopulus euphratica. J. Asian Nat. Prod. Res. 2015, 17, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Feistel, F.; Paetz, C.; Lorenz, S.; Schneider, B. The absolute configuration of salicortin, HCH-salicortin and tremulacin from Populus trichocarpa× deltoides Beaupré. Molecules 2015, 20, 5566–5573. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Babst, B.A.; Harding, S.A.; Tsai, C.-J. Biosynthesis of Phenolic Glycosides from Phenylpropanoid and Benzenoid Precursors in Populus. J. Chem. Ecol. 2010, 36, 286–297. [Google Scholar] [CrossRef]
- ESCOP, Salicis cortex—Willow Bark. ESCOP Monographs. The Scientific Foundation for Herbal Medicinal Products; European Scientific Cooperative on Phytotherapy (ESCOP): Exeter, UK, 2017; p. 10. [Google Scholar]
- Knuth, S. Pharmakologische und pharmakokinetische Untersuchungen zu Salicylalkoholderivaten aus Salicis Cortex. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2013. [Google Scholar]
- Freischmidt, A.; Jürgenliemk, G.; Kraus, B.; Okpanyi, S.N.; Müller, J.; Kelber, O.; Weiser, D.; Heilmann, J. Contribution of flavonoids and catechol to the reduction of ICAM-1 expression in endothelial cells by a standardised Willow bark extract. Phytomedicine 2012, 19, 245–252. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien. Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef]
- Förster, N.; Antoniadou, K.; Zander, M.; Baur, S.; Mittermeier-Kleßinger, V.K.; Dawid, C.; Ulrichs, C.; Mewis, I. Chemoprofiling as Breeding Tool for Pharmaceutical Use of Salix. Front. Plant Sci. 2021, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Kreissl, J.K.; Daschner, A.; Hofmann, T. Accurate Determination of Reference Materials and Natural Isolates by Means of Quantitative 1H NMR Spectroscopy. J. Agric. Food Chem. 2014, 62, 2506–2515. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniadou, K.; Herz, C.; Le, N.P.K.; Mittermeier-Kleßinger, V.K.; Förster, N.; Zander, M.; Ulrichs, C.; Mewis, I.; Hofmann, T.; Dawid, C.; et al. Identification of Salicylates in Willow Bark (Salix Cortex) for Targeting Peripheral Inflammation. Int. J. Mol. Sci. 2021, 22, 11138. https://doi.org/10.3390/ijms222011138
Antoniadou K, Herz C, Le NPK, Mittermeier-Kleßinger VK, Förster N, Zander M, Ulrichs C, Mewis I, Hofmann T, Dawid C, et al. Identification of Salicylates in Willow Bark (Salix Cortex) for Targeting Peripheral Inflammation. International Journal of Molecular Sciences. 2021; 22(20):11138. https://doi.org/10.3390/ijms222011138
Chicago/Turabian StyleAntoniadou, Kyriaki, Corinna Herz, Nguyen Phan Khoi Le, Verena Karolin Mittermeier-Kleßinger, Nadja Förster, Matthias Zander, Christian Ulrichs, Inga Mewis, Thomas Hofmann, Corinna Dawid, and et al. 2021. "Identification of Salicylates in Willow Bark (Salix Cortex) for Targeting Peripheral Inflammation" International Journal of Molecular Sciences 22, no. 20: 11138. https://doi.org/10.3390/ijms222011138
APA StyleAntoniadou, K., Herz, C., Le, N. P. K., Mittermeier-Kleßinger, V. K., Förster, N., Zander, M., Ulrichs, C., Mewis, I., Hofmann, T., Dawid, C., & Lamy, E. (2021). Identification of Salicylates in Willow Bark (Salix Cortex) for Targeting Peripheral Inflammation. International Journal of Molecular Sciences, 22(20), 11138. https://doi.org/10.3390/ijms222011138






