Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model
Abstract
:1. Introduction
2. Results
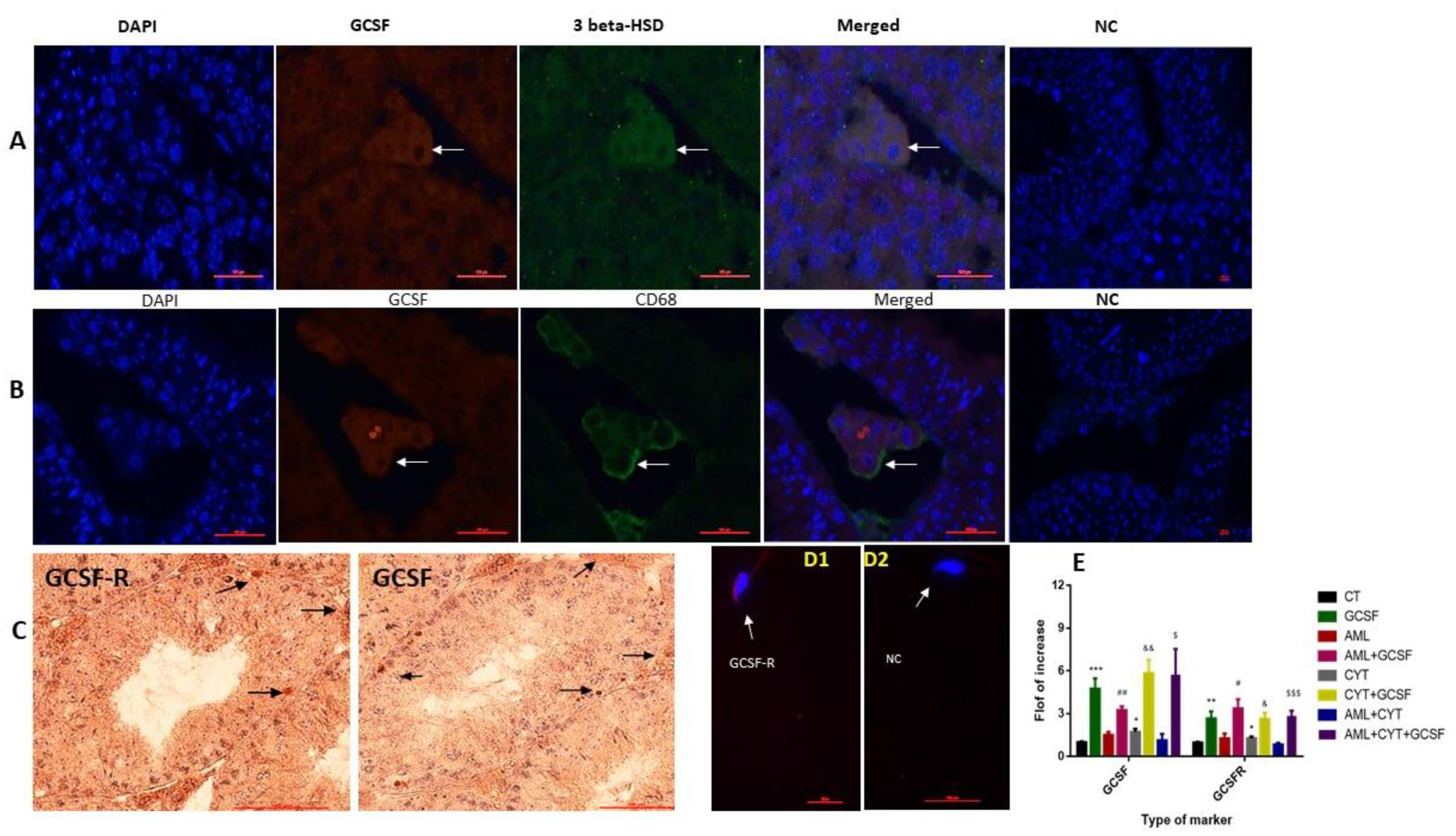
2.1. Localization of GCSF and GCSF-R in Testicular Cells and Spermatozoa, and the Effect of AML and CYT on Their Expression in the Testis
2.2. Effect of GCSF on the Survival, Testicular Weight and Seminiferous Tubules Histology and Parameters of AML- and CYT-Treated Mice
2.2.1. Mouse Survival
2.2.2. Testis Weight
2.2.3. Seminiferous Tubule Histology (Diameters and Cell Layer)
2.3. Effect of GCSF on Sperm Parameters, Fertility Capacity and Number of Offspring in AML- and CYT-Treated Groups
2.4. Effect of GCSF on Apoptosis of Testicular Cells in AML- and CYT-Treated Groups
2.5. Effect of GCSF on the Pre-Meiotic, Meiotic and Post-Meiotic Cells and Their Expression Levels in Testes of AML- and CYT-Treated Groups
2.5.1. Pre-Meiotic Markers
2.5.2. Meiotic Marker
2.5.3. Post-Meiotic Marker
2.6. Effect of GCSF on the Expression Levels of Testicular Growth Factors and Pro-Inflammatory Cytokines of AML- and CYT-Treated Mice
2.7. Effect of GCSF on the Expression Levels of Interstitial Pro-Inflammatory and Anti-Inflammatory Cytokines in AML- and CYT-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. C1498/Cell Line and Cytarabine Preparation and Injection
4.3. Mouse Survival
4.4. Testis Weight and Other Evaluations
4.5. Evaluation of Sperm Parameters
4.6. Immunohistochemical (IHC) Staining of Testicular Tissue was Performed according to Our Previous Study [54]
4.7. Double Immunofluorescence Staining
4.8. Evaluation of Apoptosis by TUNEL Assay
4.9. Isolation of Interstitial Cells
4.10. RNA Extraction and Real-Time Quantitative PCR
4.11. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, R.M.; Sutcliffe, S.B.; Malpas, J.S. Male gonadal dysfunction in Hodgkin’s disease. A prospective study. J. Am. Med. Assoc. 1981, 245, 1323–1328. [Google Scholar] [CrossRef]
- Rueffer, U.; Breuer, K.; Josting, A.; Lathan, B.; Sieber, M.; Manzke, O.; Grotenhermen, F.J.; Tesch, H.; Bredenfeld, H.; Koch, P.; et al. Male gonadal dysfunction in patients with Hodgkin’s disease prior to treatment. Ann. Oncol. 2011, 2, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Hallak, J.; Kolettis, P.N.; Sekhon, V.S.; Thomas, A.J.; Agarwal, A. Sperm cryopreservation in patients with testicular cancer. Urology 1999, 54, 894–899. [Google Scholar] [CrossRef]
- Berthelsen, J.G.; Skakkebaek, N.E. Gonadal function in men with testis cancer. Fertil. Steril. 1983, 39, 68–75. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S. Disruption of Spermatogenesis by the Cancer Disease Process. JNCI Monogr. 2005, 34, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Irani, J.; Knee, G.; Efymow, B.; Blasco, L.; Patrizio, P. Sperm cryopreservation for male patients with cancer: An epidemiological analysis at the University of Pennsylvania. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 7–11. [Google Scholar] [CrossRef]
- Auger, J.; Sermondade, N.; Eustache, F. Semen quality of 4480 young cancer and systemic disease patients: Baseline data and clinical considerations. Basic Clin. 2016, 26, 3. [Google Scholar]
- Hotaling, J.M.; Lopushnyan, N.A.; Davenport, M.; Christensen, H.; Pagel, E.R.; Muller, C.H.; Walsh, T.J. Raw and test-thaw semen parameters after cryopreservation among men with newly diagnosed cancer. Fertil. Steril. 2013, 99, 464–469. [Google Scholar] [CrossRef]
- Ku, J.Y.; Park, N.C.; Jeon, T.G.; Park, H.J. Semen Analysis in Cancer Patients Referred for Sperm Cryopreservation before Chemotherapy over a 15-Year Period in Korea. World J. Men’s Health 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Hallak, J.; Kolettis, P.N.; Sekhon, V.S.; Thomas, A.J.; Agarwal, A. Cryopreservation of sperm from patients with leukemia: Is it worth the effort? Cancer 1999, 85, 1973–1978. [Google Scholar] [CrossRef]
- Johnson, M.D.; Cooper, A.R.; Jungheim, E.S.; Lanzendorf, S.E.; Odem, R.R.; Ratts, V.S. Sperm banking for fertility preservation: A 20-year experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 177–182. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Alcazer, V.; Huguet, F.; Cony-Makhoul, P.; Heiblig, M.; Fort, M.P.; Morisset, S.; Guerci-Bresler, A.; Soula, V.; Sobh, M.; et al. CML patients show sperm alterations at diagnosis that are not improved with imatinib treatment. Leuk. Res. 2016, 48, 80–83. [Google Scholar] [CrossRef]
- Wigny, K.M.; van Dorp, W.; van der Kooi, A.L.; de Rijke, Y.B.; de Vries, A.C.; Smit, M.; Pluijm, S.M.; van den Akker, E.L.; Pieters, R.; Laven, J.S.; et al. Gonadal function in boys with newly diagnosed cancer before the start of treatment. Hum. Reprod. 2016, 31, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- van Casteren, N.J.; Boellaard, W.P.; Romijn, J.C.; Dohle, G.R. Gonadal dysfunction in male cancer patients before cytotoxic treatment. Int. J. 2010, 33, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meirow, D.; Schenker, J.G. Cancer and male infertility. Hum. Reprod. 1995, 10, 2017–2022. [Google Scholar] [CrossRef]
- Michailov, Y.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Leukemia and male infertility: Past, present, and future. Leuk. Lymphoma 2019, 60, 1126–1135. [Google Scholar] [CrossRef]
- Michailov, Y.; Lunenfeld, E.; Kapilushnik, J.; Friedler, S.; Meese, E.; Huleihel, M. Acute Myeloid Leukemia Affects Mouse Sperm Parameters, Spontaneous Acrosome Reaction, and Fertility Capacity. Int. J. Mol. Sci. 2019, 20, 219. [Google Scholar] [CrossRef] [Green Version]
- Van Etten, R.A. Aberrant cytokine signaling in leukemia. Oncogene 2007, 26, 6738–6749. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Lee, Y.M.; Tsai, W.H.; Jiang, M.L.; Ho, C.H.; Ho, C.K.; Wangn, S.Y. Circulating levels of thrombopoietic and inflammatory cytokines in patients with acute myeloblastic leukemia and myelodysplastic syndrome. Oncology 2002, 63, 64–69. [Google Scholar] [CrossRef]
- Dokter, W.H.; Tuyt, L.; Sierdsema, S.J.; Esselink, M.T.; Vellenga, E. The spontaneous expression of interleukin-1 beta and interleukin-6 is associated with spontaneous expression of AP-1 and NF-kappa B transcription factor in acute myeloblastic leukemia cells. Leukemia 1995, 9, 425–432. [Google Scholar]
- Griffin, J.D.; Rambaldi, A.; Vellenga, E.; Young, D.C.; Ostapovicz, D.; Cannistra, S.A. Secretion of interleukin-1 by acute myeloblastic leukemia cells in vitro induces endothelial cells to secrete colony stimulating factors. Blood 1987, 70, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp, K.U.; Esselink, M.T.; Kruijer, W.; Vellenga, E. Differential effects of interleukin-3 and interleukin-1 on the proliferation and interleukin- 6 protein secretion of acute myeloid leukemic cells; the involvement of ERK; p38 and STAT5. Eur. Cytokine Netw. 1999, 10, 479–490. [Google Scholar] [PubMed]
- Loveland, K.L.; Klein, B.; Puesch, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Abuelhija, M.; Lunenfeld, E. In vitro culture of testicular germ cells: Regulatory factors and limitations. Growth Factors 2007, 25, 236–252. [Google Scholar] [CrossRef]
- Plant, T.M.; Marshall, G.R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001, 22, 764–786. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, G.F.; Luetjens, C.M.; Simoni, M.; Nieschlag, E. Physiology of Testicular Function. In Andrology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 11–59. [Google Scholar] [CrossRef]
- Pesce, M.; Farrace, M.G.; Piacentini, M.; Dolci, S.; De Felici, M. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis). Development 1993, 118, 1089–1094. [Google Scholar] [CrossRef]
- Mauduit, C.; Hamamah, S.; Benahmed, M. Stem cell factor/c-kit system in spermatogenesis. Hum. Reprod. Update 1999, 5, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, Y.; Yomogida, K.; Ohta, H.; Tohda, A.; Nishimune, Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 2002, 113, 29–39. [Google Scholar] [CrossRef]
- Hofmann, M.C.; Braydich-Stolle, L.; Dym, M. Isolation of male germ-line stem cells; influence of GDNF. Dev. Biol. 2005, 279, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huleihel, M.; Fadlon, E.; Abuelhija, A.; Piltcher Haber, E.; Lunenfeld, E. Glial cell line-derived neurotrophic factor (GDNF) induced migration of spermatogonial cells in vitro via MEK and NF-kB pathways. Differentiation 2013, 86, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sawaied, A.; Arazi, E.; AbuElhija, A.; Lunenfeld, E.; Huleihel, M. The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis. In Vitro. Int. J. Mol. Sci. 2021, 22, 2325. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Griffin, J.D. Granulocyte colony-stimulating factor and its receptor. Blood 1991, 78, 2791–2808. [Google Scholar] [CrossRef] [Green Version]
- Geissler, K.; Koller, E.; Hubmann, E.; Niederwieser, D.; Hinterberger, W.; Geissler, D.; Kyrle, P.; Knöbl, P.; Pabinger, I.; Thalhammer, R.; et al. Granulocyte colony-stimulating factor as an adjunct to induction chemotherapy for adult acute lymphoblastic leukemia–a randomized phase-III study. Blood 1997, 90, 590–596. [Google Scholar] [CrossRef]
- Muhonen, T.; Jantunen, I.; Pertovaara, H.; Voutilainen, L.; Maiche, A.; Blomqvist, C.; Pyrhönen, S.; Kellokumpu-Lehtinen, P. Prophylactic filgrastim (G-CSF) during mitomycin-C; mitoxantrone, and methotrexate (MMM) treatment for metastatic breast cancer. A randomized study. Am. J. Clin. Oncol. 1996, 19, 232–234. [Google Scholar] [CrossRef]
- Eftekhar, M.; Naghshineh, E.; Khani, P. Role of granulocyte colony-stimulating factor in human reproduction. J. Res. Med. Sci. 2018, 23, 7. [Google Scholar]
- Kotzur, T.; Benavides-Garcia, R.; Mecklenburg, J.; Sanchez, J.R.; Reilly, M.; Hermann, B.P. Granulocyte Colony-Stimulating Factor (G-CSF) Promotes Spermatogenic Regeneration from Surviving Spermatogonia after High-Dose Alkylating Chemotherapy. Reprod. Biol. Endocrinol. 2017, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Benavides-Garcia, R.; Joachim, R.; Pina, N.A.; Mutoji, K.N.; Reilly, M.A.; Hermann, B.P. Granulocyte colony-stimulating factor prevents loss of spermatogenesis after sterilizing busulfan chemotherapy. Fertil. Steril. 2015, 103, 270–280. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Jeon, B.; Jang, W.; Moon, C.; Kim, S. Protection of spermatogenesis against gammaray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia 2010, 43, 87–93. [Google Scholar] [CrossRef]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: A new regulatory model. J. Reprod. Immunol. 2013, 97, 147–158. [Google Scholar] [CrossRef]
- Bhushan, S.; Meinhardt, A.C.J. The macrophages in testis function. J. Reprod. Immunol. 2017, 119, 107–112. [Google Scholar] [CrossRef]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [PubMed]
- Bhushan, S.; Tchatalbachev, S.; Lu, Y.; Fröhlich, S.; Fijak, M.; Vijayan, V.; Meinhardt, A. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol. 2015, 19, 5455–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, D.C.; Crawford, J.; Klippel, Z.; Reiner, M.; Osslund, T.; Fan, E.; Morrow, P.K.; Allcott, K.; Lyman, G.H. A systematic literature review of the efficacy, effectiveness, and safety of filgrastim. Supportive Care Cancer 2018, 26, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Harada, M.; Qin, Y.; Takano, H.; Minamino, T.; Zou, Y.; Toko, H.; Ohtsuka, M.; Matsuura, K.; Sano, M.; Nishi, J.; et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat. Med. 2005, 11, 305–311. [Google Scholar] [CrossRef]
- Boneberg, E.M.; Hartung, T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm. Res. 2002, 51, 119–128. [Google Scholar] [CrossRef]
- Saito, M.; Kiyokawa, N.; Taguchi, T.; Suzuki, K.; Sekino, T.; Mimori, K.; Suzuki, T.; Nakajima, H.; Katagiri, Y.U.; Fujimura, J.; et al. Granulocyte colony-stimulating factor directly affects human monocytes and modulates cytokine secretion. Exp. Hematol. 2002, 30, 1115–1123. [Google Scholar] [CrossRef]
- Lin, J.M.; Li, B.; Rimmer, E.; VanRoey, M.; Jooss, K. Enhancement of the anti-tumor efficacy of a GM-CSF–secreting tumor cell immunotherapy in preclinical models by cytosine arabinoside. Exp. Hematol. 2008, 36, 319–328. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of Spermatogenesis In Vitro in Three-Dimensional Culture from Spermatogonial Cells of Busulfan-Treated Immature Mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Spermatozoa and Semen-Cervical Mucus Interaction, 3rd ed.; Cambridge University Press: Cambridge, UK, 1992; pp. 13–18. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailov, Y.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model. Int. J. Mol. Sci. 2021, 22, 11157. https://doi.org/10.3390/ijms222011157
Michailov Y, AbuMadighem A, Lunenfeld E, Kapelushnik J, Huleihel M. Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model. International Journal of Molecular Sciences. 2021; 22(20):11157. https://doi.org/10.3390/ijms222011157
Chicago/Turabian StyleMichailov, Yulia, Ali AbuMadighem, Eitan Lunenfeld, Joseph Kapelushnik, and Mahmoud Huleihel. 2021. "Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model" International Journal of Molecular Sciences 22, no. 20: 11157. https://doi.org/10.3390/ijms222011157
APA StyleMichailov, Y., AbuMadighem, A., Lunenfeld, E., Kapelushnik, J., & Huleihel, M. (2021). Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model. International Journal of Molecular Sciences, 22(20), 11157. https://doi.org/10.3390/ijms222011157






