Biphasic Roles of Clock Genes and Bone Morphogenetic Proteins in Gonadotropin Expression by Mouse Gonadotrope Cells
Abstract
:1. Introduction
2. Results
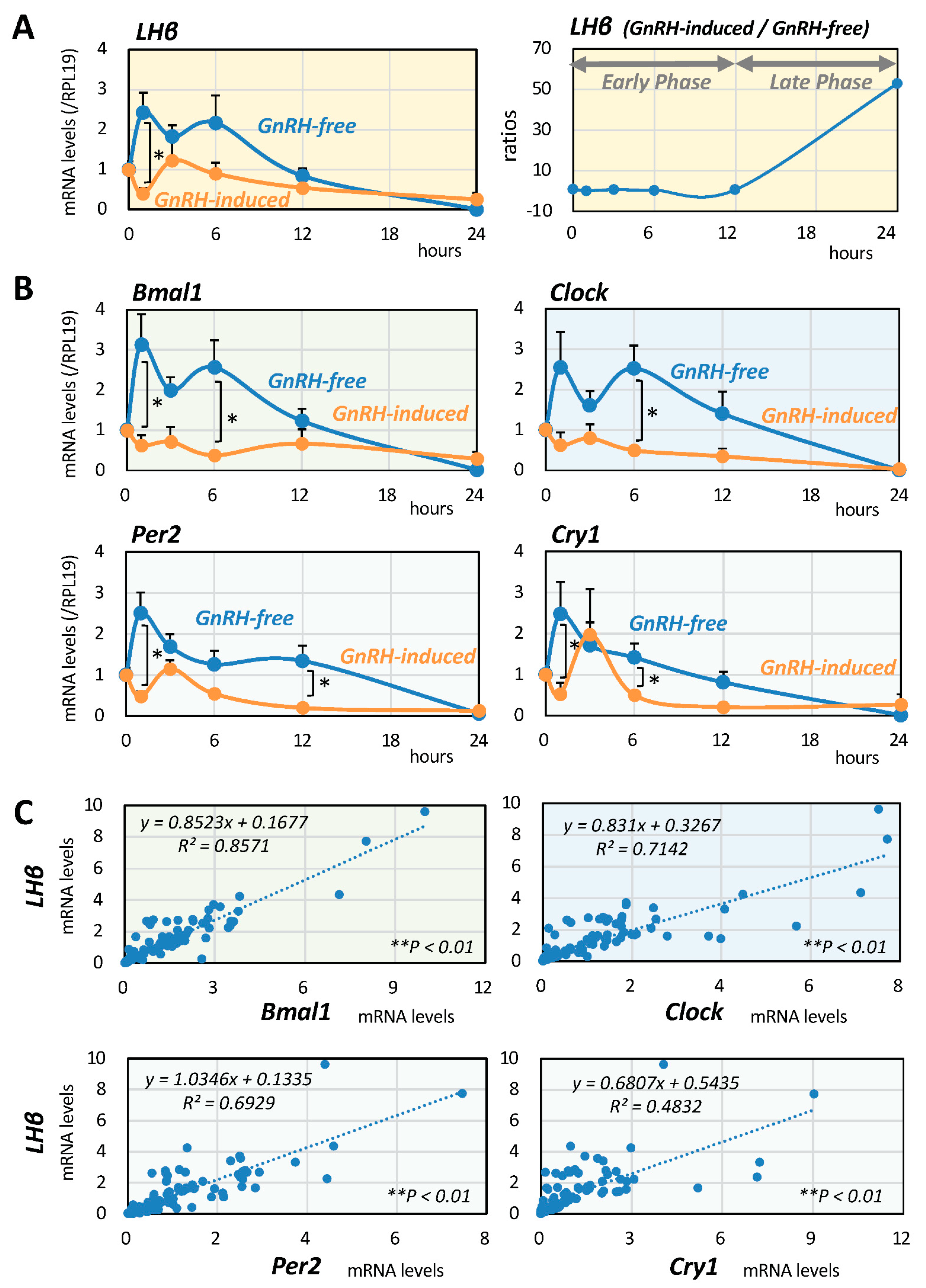
2.1. Serial Changes of LH and Clock Gene Expression Induced by GnRH
2.2. Interrelationships between LH and Clock Gene Expression
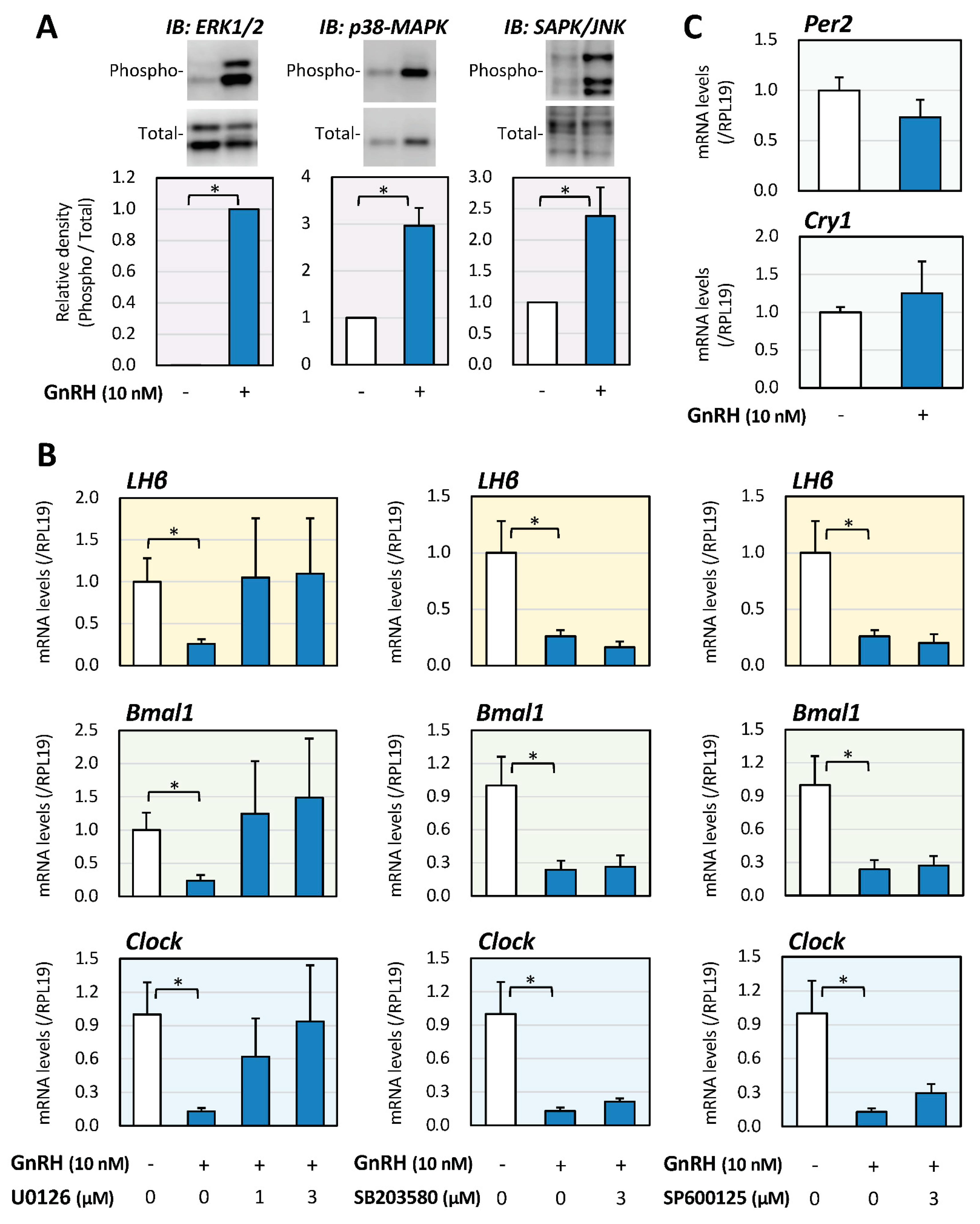
2.3. Involvement of MAPKs in GnRH-Induced LH and Clock Gene Expression
2.4. Inhibitory Effects of Clock Gene Expression and Its Involvement of BMPs
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Culture
4.2. Quantitative Real-Time PCR Analysis
4.3. Western Immunoblotting Analysis
4.4. Transient Transfection for siRNA Experiments
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMP | Bone morphogenetic protein |
| BMPRII | BMP type-II receptor |
| Cry | Cryptochrome |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| MAPK | Mitogen-activated protein kinase |
| LH | Luteinizing hormone |
| Per | Period |
References
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian rhythms in isolated brain regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian system, sleep and endocrinology. Mol. Cell. Endocrinol. 2012, 349, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonneaux, V.; Bahougne, T. A Multi-Oscillatory Circadian System Times Female Reproduction. Front. Endocrinol. (Lausanne) 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunderer, F.; Kuhne, S.; Jilg, A.; Ackermann, K.; Sebesteny, T.; Maronde, E.; Stehle, J.H. Clock gene expression in the human pituitary gland. Endocrinology 2013, 154, 2046–2057. [Google Scholar] [CrossRef] [Green Version]
- Becquet, D.; Boyer, B.; Rasolonjanahary, R.; Brue, T.; Guillen, S.; Moreno, M.; Franc, J.L.; Francois-Bellan, A.M. Evidence for an internal and functional circadian clock in rat pituitary cells. Mol. Cell. Endocrinol. 2014, 382, 888–898. [Google Scholar] [CrossRef]
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef] [Green Version]
- Mugami, S.; Dobkin-Bekman, M.; Rahamim-Ben Navi, L.; Naor, Z. Differential roles of PKC isoforms (PKCs) in GnRH stimulation of MAPK phosphorylation in gonadotrope derived cells. Mol. Cell. Endocrinol. 2018, 463, 97–105. [Google Scholar] [CrossRef]
- Naor, Z. Signaling by G-protein-coupled receptor (GPCR): Studies on the GnRH receptor. Front. Neuroendocrinol. 2009, 30, 10–29. [Google Scholar] [CrossRef]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef]
- Otsuka, F. Multiple endocrine regulation by bone morphogenetic protein system. Endocr. J. 2010, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, F.; Tsukamoto, N.; Miyoshi, T.; Iwasaki, Y.; Makino, H. BMP action in the pituitary: Its possible role in modulating somatostatin sensitivity in pituitary tumor cells. Mol. Cell. Endocrinol. 2012, 349, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, D.; Paez-Pereda, M.; Theodoropoulou, M.; Gerez, J.; Nagashima, A.C.; Chervin, A.; Berner, S.; Labeur, M.; Refojo, D.; Renner, U.; et al. Bone morphogenetic protein-4 control of pituitary pathophysiology. Front. Horm Res. 2006, 35, 22–31. [Google Scholar]
- Labeur, M.; Paez-Pereda, M.; Haedo, M.; Arzt, E.; Stalla, G.K. Pituitary tumors: Cell type-specific roles for BMP-4. Mol. Cell. Endocrinol. 2010, 326, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Paez-Pereda, M.; Giacomini, D.; Refojo, D.; Nagashima, A.C.; Hopfner, U.; Grubler, Y.; Chervin, A.; Goldberg, V.; Goya, R.; Hentges, S.T.; et al. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc. Natl. Acad. Sci. USA 2003, 100, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto-Yamauchi, N.; Terasaka, T.; Iwasaki, Y.; Otsuka, F. Interaction of pituitary hormones and expression of clock genes modulated by bone morphogenetic protein-4 and melatonin. Biochem. Biophys. Res. Commun. 2015, 459, 172–177. [Google Scholar] [CrossRef]
- Nudi, M.; Ouimette, J.F.; Drouin, J. Bone morphogenic protein (Smad)-mediated repression of proopiomelanocortin transcription by interference with Pitx/Tpit activity. Mol. Endocrinol. 2005, 19, 1329–1342. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, D.; Paez-Pereda, M.; Theodoropoulou, M.; Labeur, M.; Refojo, D.; Gerez, J.; Chervin, A.; Berner, S.; Losa, M.; Buchfelder, M.; et al. Bone morphogenetic protein-4 inhibits corticotroph tumor cells: Involvement in the retinoic acid inhibitory action. Endocrinology 2006, 147, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, N.; Otsuka, F.; Miyoshi, T.; Yamanaka, R.; Inagaki, K.; Yamashita, M.; Otani, H.; Takeda, M.; Suzuki, J.; Ogura, T.; et al. Effects of bone morphogenetic protein (BMP) on adrenocorticotropin production by pituitary corticotrope cells: Involvement of up-regulation of BMP receptor signaling by somatostatin analogs. Endocrinology 2010, 151, 1129–1141. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, N.; Otsuka, F.; Miyoshi, T.; Inagaki, K.; Nakamura, E.; Terasaka, T.; Takeda, M.; Ogura, T.; Iwasaki, Y.; Makino, H. Functional interaction of bone morphogenetic protein and growth hormone releasing peptide in adrenocorticotropin regulation by corticotrope cells. Mol. Cell. Endocrinol. 2011, 344, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Shimasaki, S. A novel function of bone morphogenetic protein-15 in the pituitary: Selective synthesis and secretion of FSH by gonadotropes. Endocrinology 2002, 143, 4938–4941. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Otsuka, F.; Suzuki, J.; Kishida, M.; Ogura, T.; Tamiya, T.; Makino, H. Involvement of activin/BMP system in development of human pituitary gonadotropinomas and nonfunctioning adenomas. Biochem. Biophys. Res. Commun. 2003, 306, 812–818. [Google Scholar] [CrossRef]
- Takeda, M.; Otsuka, F.; Otani, H.; Inagaki, K.; Miyoshi, T.; Suzuki, J.; Mimura, Y.; Ogura, T.; Makino, H. Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LβT2 cells. J. Endocrinol. 2007, 194, 87–99. [Google Scholar] [CrossRef]
- Takeda, M.; Otsuka, F.; Takahashi, H.; Inagaki, K.; Miyoshi, T.; Tsukamoto, N.; Makino, H.; Lawson, M.A. Interaction between gonadotropin-releasing hormone and bone morphogenetic protein-6 and -7 signaling in LbetaT2 gonadotrope cells. Mol. Cell. Endocrinol. 2012, 348, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.A.; Santos, S.J.; Kreidel, M.K.; Diaz, A.L.; Rey, R.; Lawson, M.A. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2. Mol. Endocrinol. 2004, 18, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Toma, K.; Otsuka, F.; Oguni, K.; Terasaka, T.; Komatsubara, M.; Tsukamoto-Yamauchi, N.; Inagaki, K.; Makino, H. BMP-6 modulates somatostatin effects on luteinizing hormone production by gonadrotrope cells. Peptides 2016, 76, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.H.; Olson, S.L.; Turek, F.W.; Levine, J.E.; Horton, T.H.; Takahashi, J.S. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 2004, 14, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.; Zhu, L.; Blum, I.D.; Mai, O.; Leliavski, A.; Fahrenkrug, J.; Oster, H.; Boehm, U.; Storch, K.F. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology 2013, 154, 2924–2935. [Google Scholar] [CrossRef] [Green Version]
- Kakar, S.S.; Winters, S.J.; Zacharias, W.; Miller, D.M.; Flynn, S. Identification of distinct gene expression profiles associated with treatment of LbetaT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene 2003, 308, 67–77. [Google Scholar] [CrossRef]
- Olcese, J.; Sikes, H.E.; Resuehr, D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): Involvement of protein kinase C and MAP kinase signaling. Chronobiol. Int. 2006, 23, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Oh-hashi, K.; Naruse, Y.; Tanaka, M. Intracellular calcium mobilization induces period genes via MAP kinase pathways in NIH3T3 cells. FEBS Lett. 2002, 516, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Resuehr, H.E.; Resuehr, D.; Olcese, J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol. Cell. Endocrinol. 2009, 311, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Scully, K.M.; Rosenfeld, M.G. Pituitary development: Regulatory codes in mammalian organogenesis. Science 2002, 295, 2231–2235. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-J.; Wu, J.C.; Su, P.; Zhirnov, O.; Miller, W.L. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology 2001, 142, 2275–2283. [Google Scholar] [CrossRef]
- Nicol, L.; Faure, M.O.; McNeilly, J.R.; Fontaine, J.; Taragnat, C.; McNeilly, A.S. Bone morphogenetic protein-4 interacts with activin and GnRH to modulate gonadotrophin secretion in LbetaT2 gonadotrophs. J. Endocrinol. 2008, 196, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Soejima, Y.; Iwata, N.; Nakano, Y.; Yamamoto, K.; Suyama, A.; Nada, T.; Ogawa, H.; Otsuka, F. Involvement of clock gene expression, bone morphogenetic protein and activin in adrenocortical steroidogenesis by human H295R cells. Endocr. J. 2021, 68, 243–250. [Google Scholar] [CrossRef]
- Fujita, S.; Hasegawa, T.; Nishiyama, Y.; Fujisawa, S.; Nakano, Y.; Nada, T.; Iwata, N.; Kamada, Y.; Masuyama, H.; Otsuka, F. Interaction between orexin A and bone morphogenetic protein system on progesterone biosynthesis by rat granulosa cells. J. Steroid Biochem. Mol. Biol. 2018, 181, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Nagao, S.; Iwata, N.; Soejima, Y.; Takiguchi, T.; Aokage, T.; Kozato, Y.; Nakano, Y.; Nada, T.; Hasegawa, T.; Otsuka, F. Interaction of ovarian steroidogenesis and clock gene expression modulated by bone morphogenetic protein-7 in human granulosa cells. Endocr. J. 2019, 66, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Suyama, A.; Iwata, N.; Soejima, Y.; Nakano, Y.; Yamamoto, K.; Nada, T.; Otsuka, F. Roles of NR5A1 and NR5A2 in the regulation of steroidogenesis by Clock gene and bone morphogenetic proteins by human granulosa cells. Endocr. J. 2021, EJ21-0223. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soejima, Y.; Iwata, N.; Nakano, Y.; Yamamoto, K.; Suyama, A.; Nada, T.; Otsuka, F. Biphasic Roles of Clock Genes and Bone Morphogenetic Proteins in Gonadotropin Expression by Mouse Gonadotrope Cells. Int. J. Mol. Sci. 2021, 22, 11186. https://doi.org/10.3390/ijms222011186
Soejima Y, Iwata N, Nakano Y, Yamamoto K, Suyama A, Nada T, Otsuka F. Biphasic Roles of Clock Genes and Bone Morphogenetic Proteins in Gonadotropin Expression by Mouse Gonadotrope Cells. International Journal of Molecular Sciences. 2021; 22(20):11186. https://doi.org/10.3390/ijms222011186
Chicago/Turabian StyleSoejima, Yoshiaki, Nahoko Iwata, Yasuhiro Nakano, Koichiro Yamamoto, Atsuhito Suyama, Takahiro Nada, and Fumio Otsuka. 2021. "Biphasic Roles of Clock Genes and Bone Morphogenetic Proteins in Gonadotropin Expression by Mouse Gonadotrope Cells" International Journal of Molecular Sciences 22, no. 20: 11186. https://doi.org/10.3390/ijms222011186
APA StyleSoejima, Y., Iwata, N., Nakano, Y., Yamamoto, K., Suyama, A., Nada, T., & Otsuka, F. (2021). Biphasic Roles of Clock Genes and Bone Morphogenetic Proteins in Gonadotropin Expression by Mouse Gonadotrope Cells. International Journal of Molecular Sciences, 22(20), 11186. https://doi.org/10.3390/ijms222011186





