A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
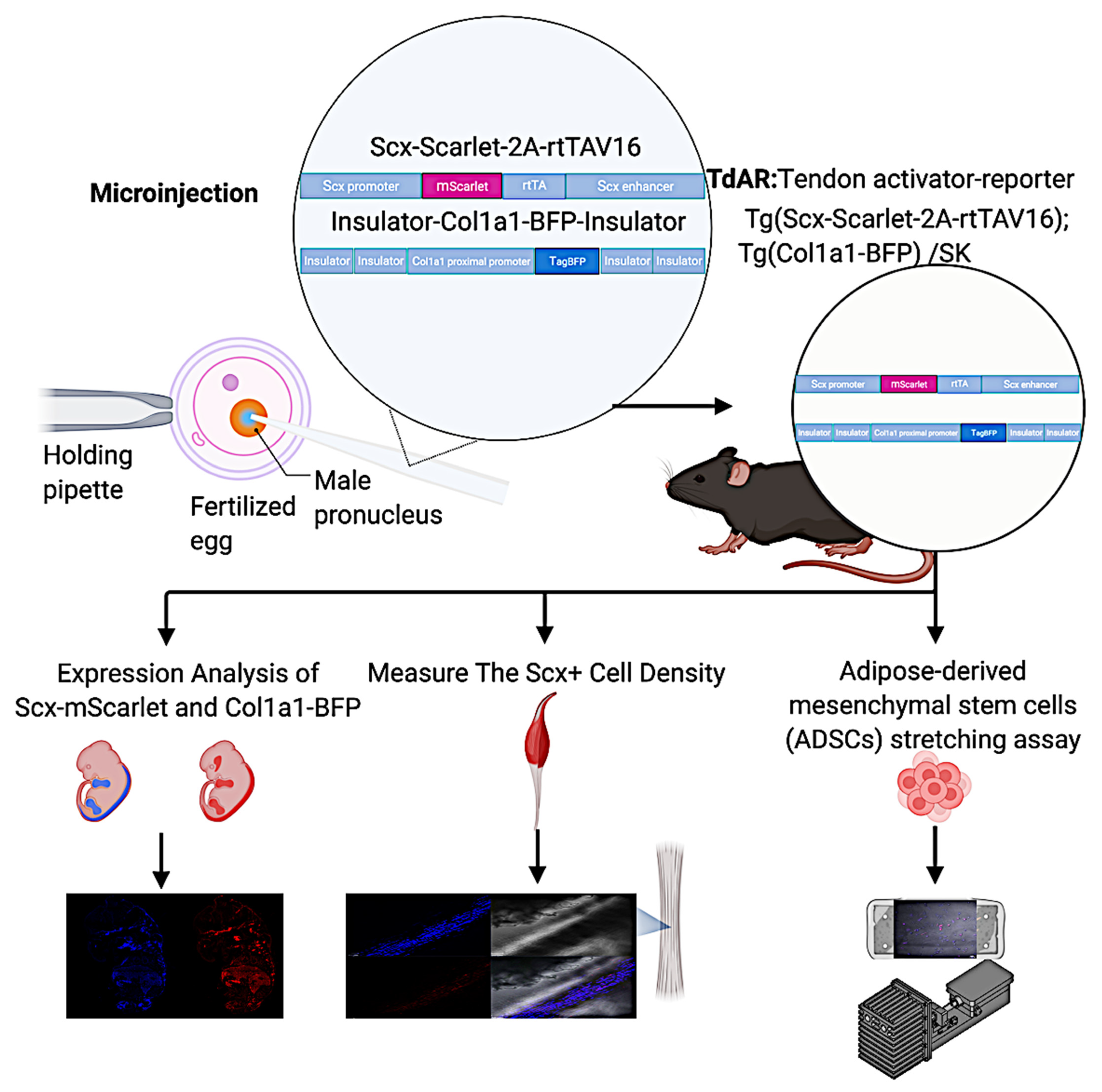
2.1. Generation of Tendon-Specific Double Transgenic Reporter Mice
2.2. Expression Profile of Scx-mScarlet and Col1a1-BFP Transgenic Mice
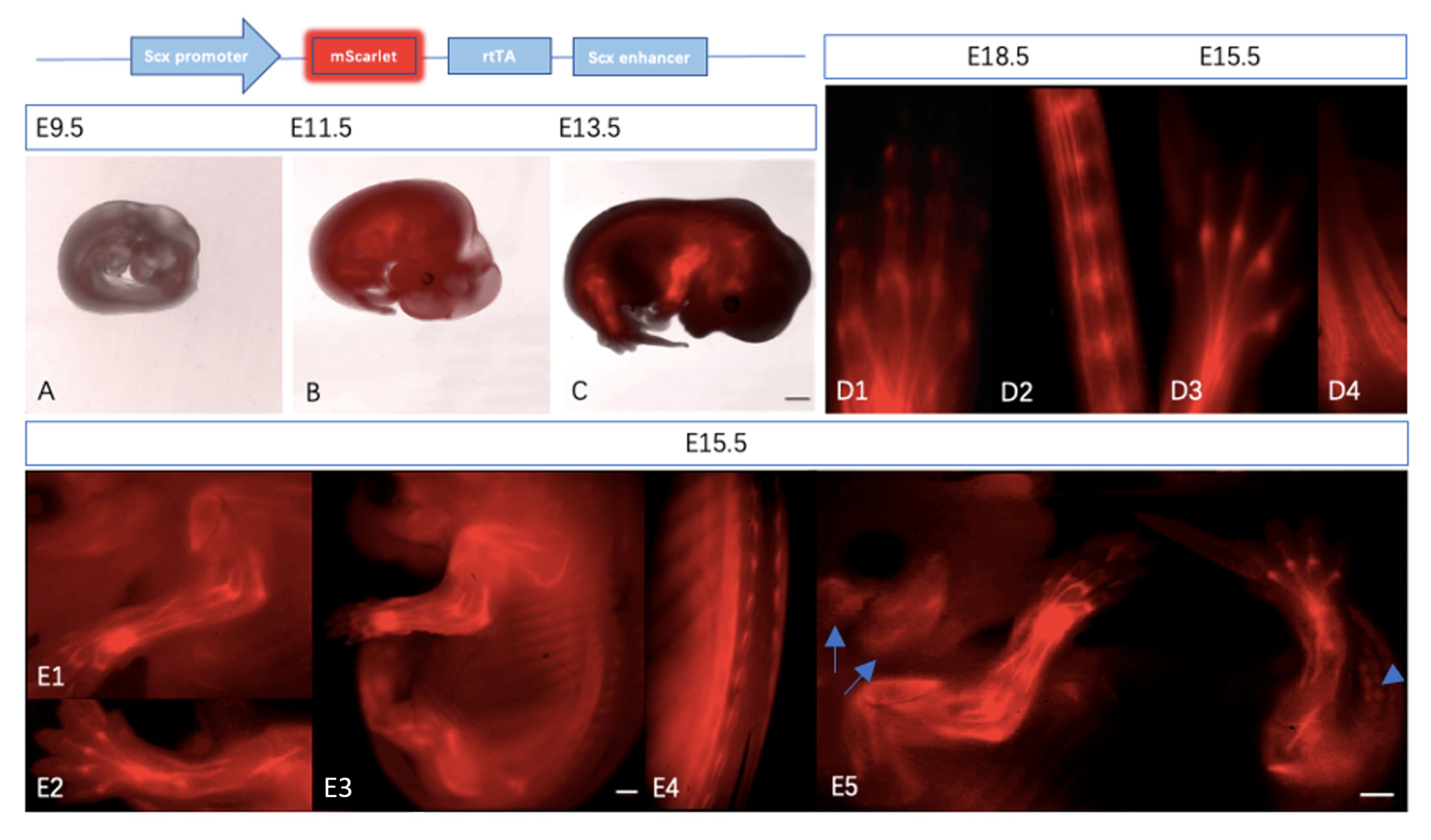
2.2.1. Expression of Scx-mScarlet Reporter Is Tendon-Specific
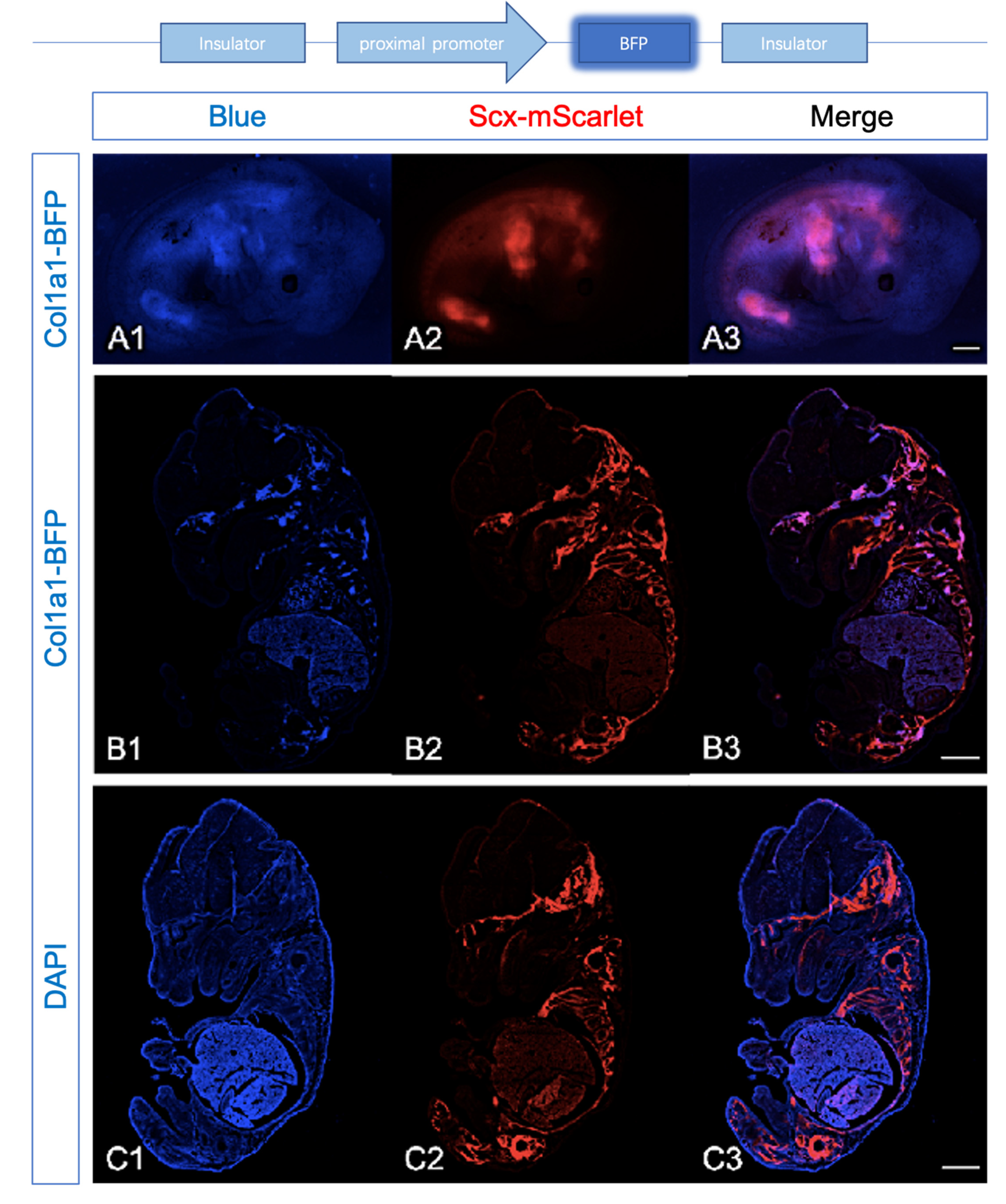
2.2.2. Expression of Col1a1-BFP and Scx-mScarlet Were Comparable
2.3. The Density of Scx+ Cells Declined in Achilles Tendon of Aged Mouse
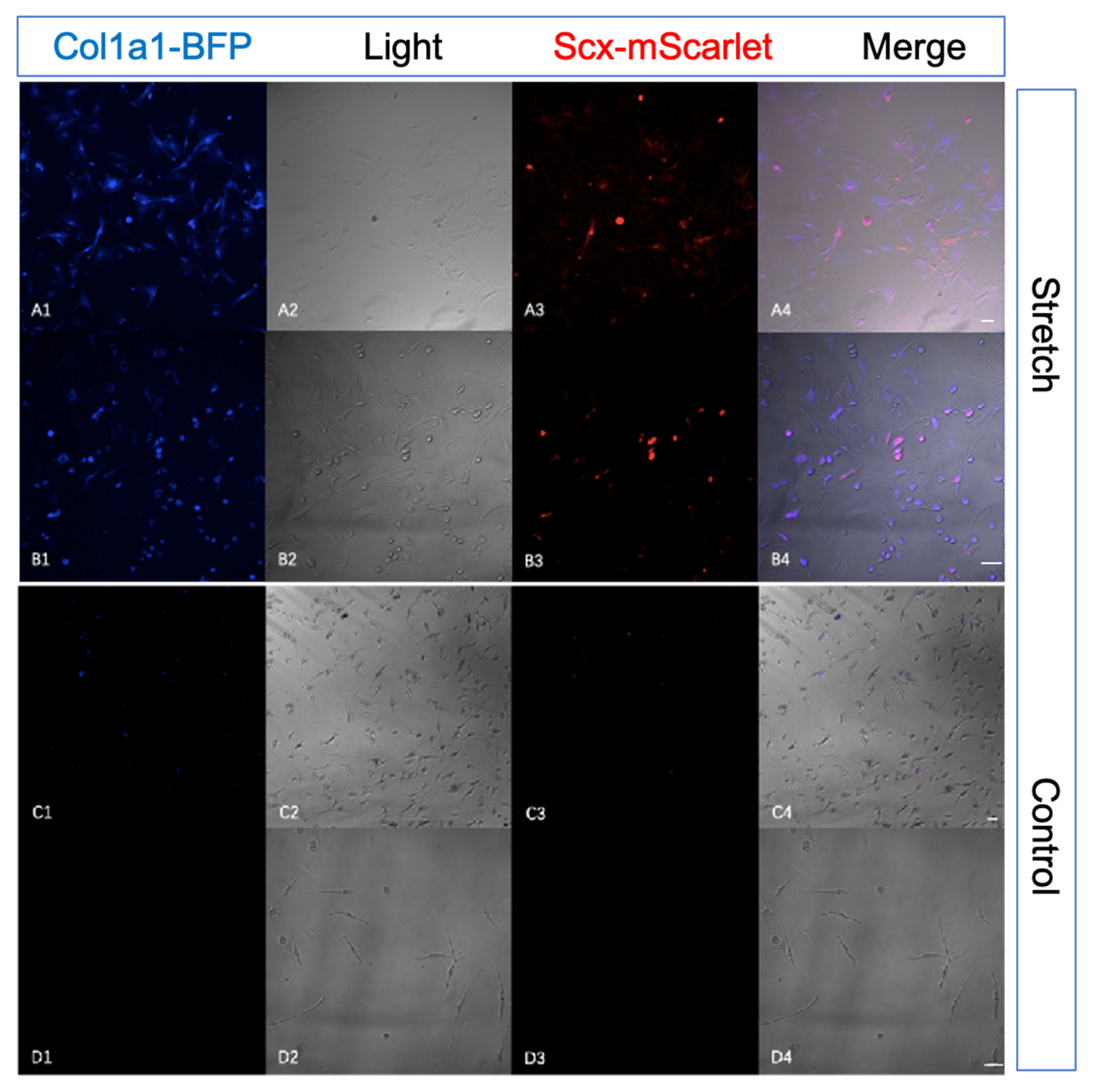
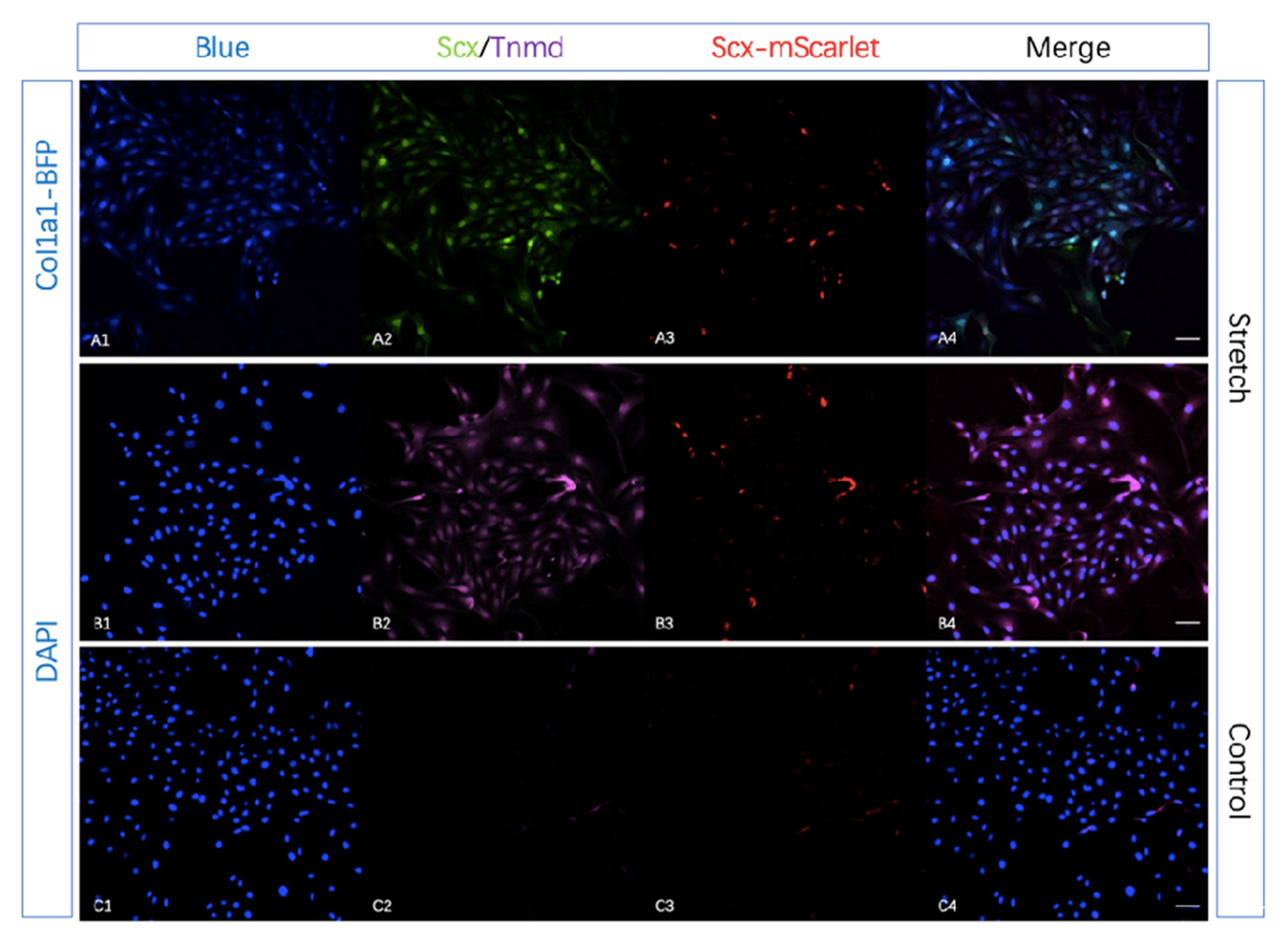
2.4. Tendon-Specific Double Reporter System Functioning Properly In Vitro
3. Discussion
4. Materials and Methods
4.1. Cloning of the Scx-mScarlet-2A-rtTAV16 and Col1a1-BFP Transgenic Constructs
4.2. Mechanical Stimulation In Vivo
4.3. Immunohistochemistry and Immunocytochemical Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Screen, H.R.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Kalson, N.S.; Lu, Y.; Taylor, S.H.; Starborg, T.; Holmes, D.F.; Kadler, K.E. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife 2015, 4, e05958. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.V.; Perrine, M.; Brainard, N.; Vogel, K.G. Scleraxis (Scx) directs lacZ expression in tendon of transgenic mice. Mech. Dev. 2003, 120, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Brent, A.E.; Braun, T.; Tabin, C.J. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 2005, 132, 515–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, K.; Chien, C.; Bell, R.; Laudier, D.; Tufa, S.F.; Keene, D.R.; Andarawis-Puri, N.; Huang, A.H. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci. Rep. 2017, 7, 45238. [Google Scholar] [CrossRef] [Green Version]
- Pryce, B.A.; Brent, A.E.; Murchison, N.D.; Tabin, C.J.; Schweitzer, R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 2007, 236, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Liska, D.J.; Reed, M.J.; Sage, E.H.; Bornstein, P. Cell-specific expression of alpha 1(I) collagen-hGH minigenes in transgenic mice. J. Cell Biol. 1994, 125, 695–704. [Google Scholar] [CrossRef]
- Terraz, C.; Brideau, G.; Ronco, P.; Rossert, J. A combination of cis-acting elements is required to activate the pro-alpha 1(I) collagen promoter in tendon fibroblasts of transgenic mice. J. Biol. Chem. 2002, 277, 19019–19026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.H.; Whiteley, M.; Felsenfeld, G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 1993, 74, 505–514. [Google Scholar] [CrossRef]
- Brent, A.E.; Schweitzer, R.; Tabin, C.J. A somitic compartment of tendon progenitors. Cell 2003, 113, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Takimoto, A.; Hiraki, Y.; Shukunami, C. Generation and characterization of ScxCre transgenic mice. Genesis 2013, 51, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, T.; Koga, Y.; Nakamura, A.; Fujishita, A.; Kohara, H.; Moriuchi, E.; Yoshimi, K.; Tsai, C.Y.; Yoshida, N. Mechanism of motor coordination of masseter and temporalis muscles for increased masticatory efficiency in mice. J. Oral Rehabil. 2017, 44, 363–374. [Google Scholar] [CrossRef]
- Delaurier, A.; Burton, N.; Bennett, M.; Baldock, R.; Davidson, D.; Mohun, T.J.; Logan, M.P. The Mouse Limb Anatomy Atlas: An interactive 3D tool for studying embryonic limb patterning. BMC Dev. Biol. 2008, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossert, J.; Eberspaecher, H.; de Crombrugghe, B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 1995, 129, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossert, J.A.; Chen, S.S.; Eberspaecher, H.; Smith, C.N.; de Crombrugghe, B. Identification of a minimal sequence of the mouse pro-alpha 1(I) collagen promoter that confers high-level osteoblast expression in transgenic mice and that binds a protein selectively present in osteoblasts. Proc. Natl. Acad. Sci. USA 1996, 93, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Sakabe, T.; Sunaga, A.; Sakai, K.; Rivera, A.L.; Keene, D.R.; Sasaki, T.; Stavnezer, E.; Iannotti, J.; Schweitzer, R.; et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol. 2011, 21, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Harvey, T.; Flamenco, S.; Fan, C.M. A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 2019, 21, 1490–1503. [Google Scholar] [CrossRef]
- Park, A.; Hogan, M.V.; Kesturu, G.S.; James, R.; Balian, G.; Chhabra, A.B. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng. Part A 2010, 16, 2941–2951. [Google Scholar] [CrossRef] [Green Version]
- Kapacee, Z.; Yeung, C.Y.; Lu, Y.; Crabtree, D.; Holmes, D.F.; Kadler, K.E. Synthesis of embryonic tendon-like tissue by human marrow stromal/mesenchymal stem cells requires a three-dimensional environment and transforming growth factor β3. Matrix Biol. 2010, 29, 668–677. [Google Scholar] [CrossRef]
- Cardwell, R.D.; Kluge, J.A.; Thayer, P.S.; Guelcher, S.A.; Dahlgren, L.A.; Kaplan, D.L.; Goldstein, A.S. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J. Biomech. Eng. 2015, 137, 0710101–0710108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Zhou, Y.; Cheng, T.; Lu, X.; Yu, K.; Zhou, Y.; Hong, J.; Chen, Y. Hypoxia enhances tenocyte differentiation of adipose-derived mesenchymal stem cells by inducing hypoxia-inducible factor-1α in a co-culture system. Cell Prolif. 2016, 49, 173–184. [Google Scholar] [CrossRef]
- de Aro, A.A.; Carneiro, G.D.; Teodoro, L.F.R.; da Veiga, F.C.; Ferrucci, D.L.; Simões, G.F.; Simões, P.W.; Alvares, L.E.; de Oliveira, A.L.R.; Vicente, C.P.; et al. Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5. Cells 2018, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Sartori, J.; Köhring, S.; Bruns, S.; Moosmann, J.; Hammel, J.U. Gaining Insight into the Deformation of Achilles Tendon Entheses in Mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Best, K.T.; Korcari, A.; Mora, K.E.; Nichols, A.E.; Muscat, S.N.; Knapp, E.; Buckley, M.R.; Loiselle, A.E. Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. eLife 2021, 10, e62203. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zhou, X.; Skutella, T. A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 11189. https://doi.org/10.3390/ijms222011189
Chen R, Zhou X, Skutella T. A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(20):11189. https://doi.org/10.3390/ijms222011189
Chicago/Turabian StyleChen, Rui, Xunlei Zhou, and Thomas Skutella. 2021. "A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 20: 11189. https://doi.org/10.3390/ijms222011189





