Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species
Abstract
:1. Introduction
2. Results
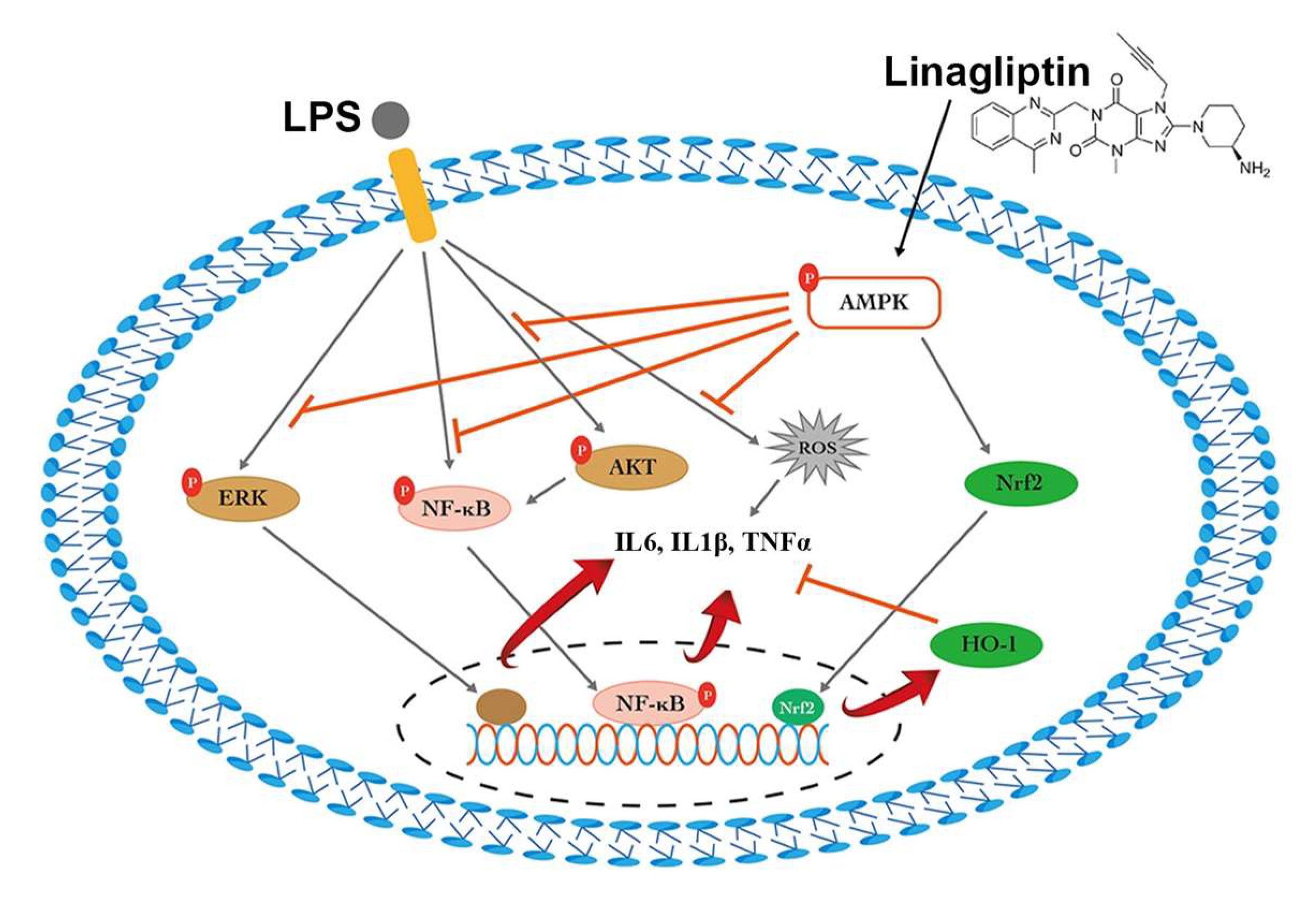
2.1. Linagliptin Restored the AMPK Pathway to Attenuate Endotoxin-Induced Overproduction of TNF-⍺, IL-1β, and ROS
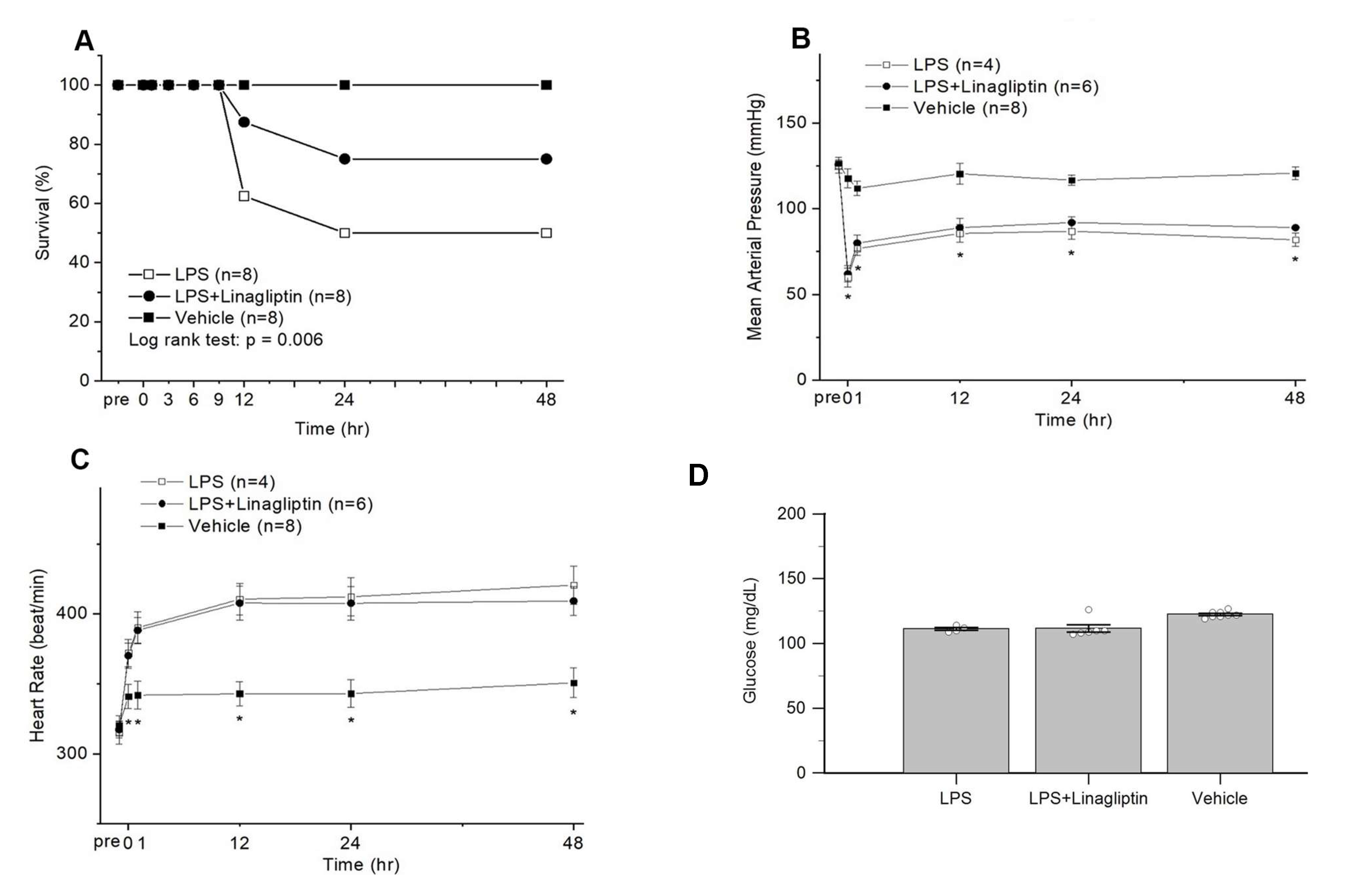
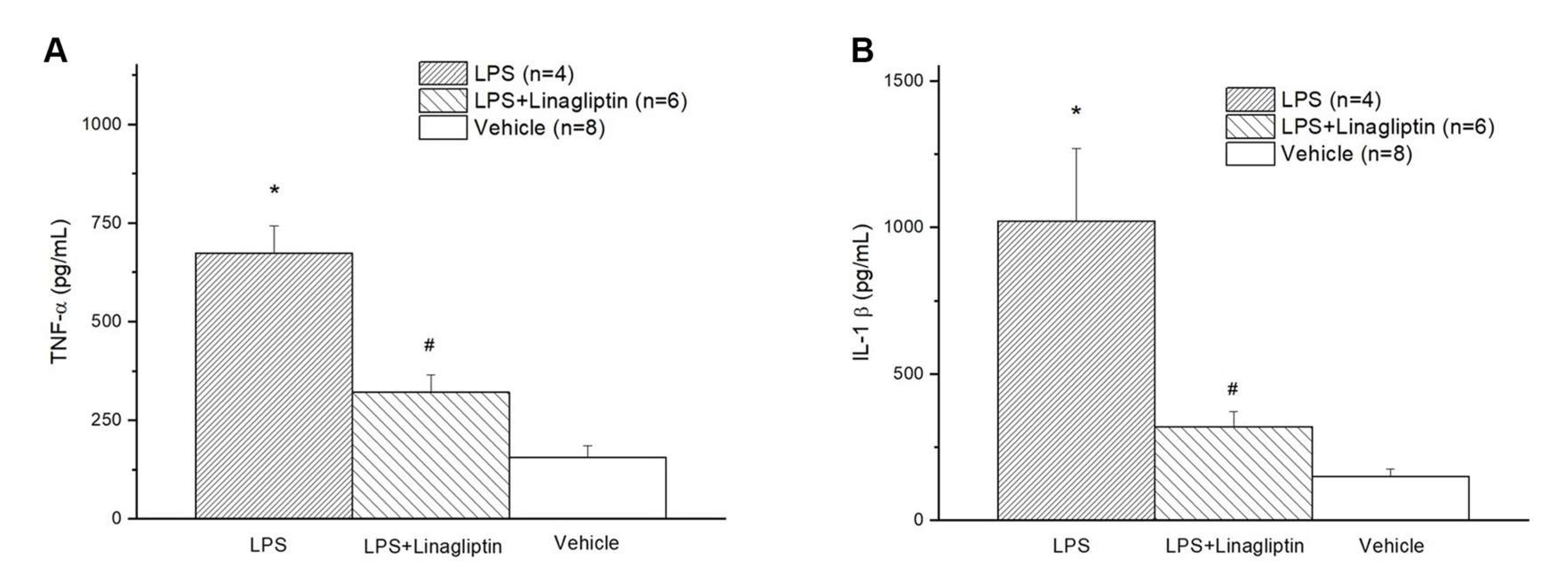
2.2. Linagliptin Attenuated Endotoxic-Shock-Induced multiorgan Damages, Inflammatory Biomarkers, and Survival in a Rat Model
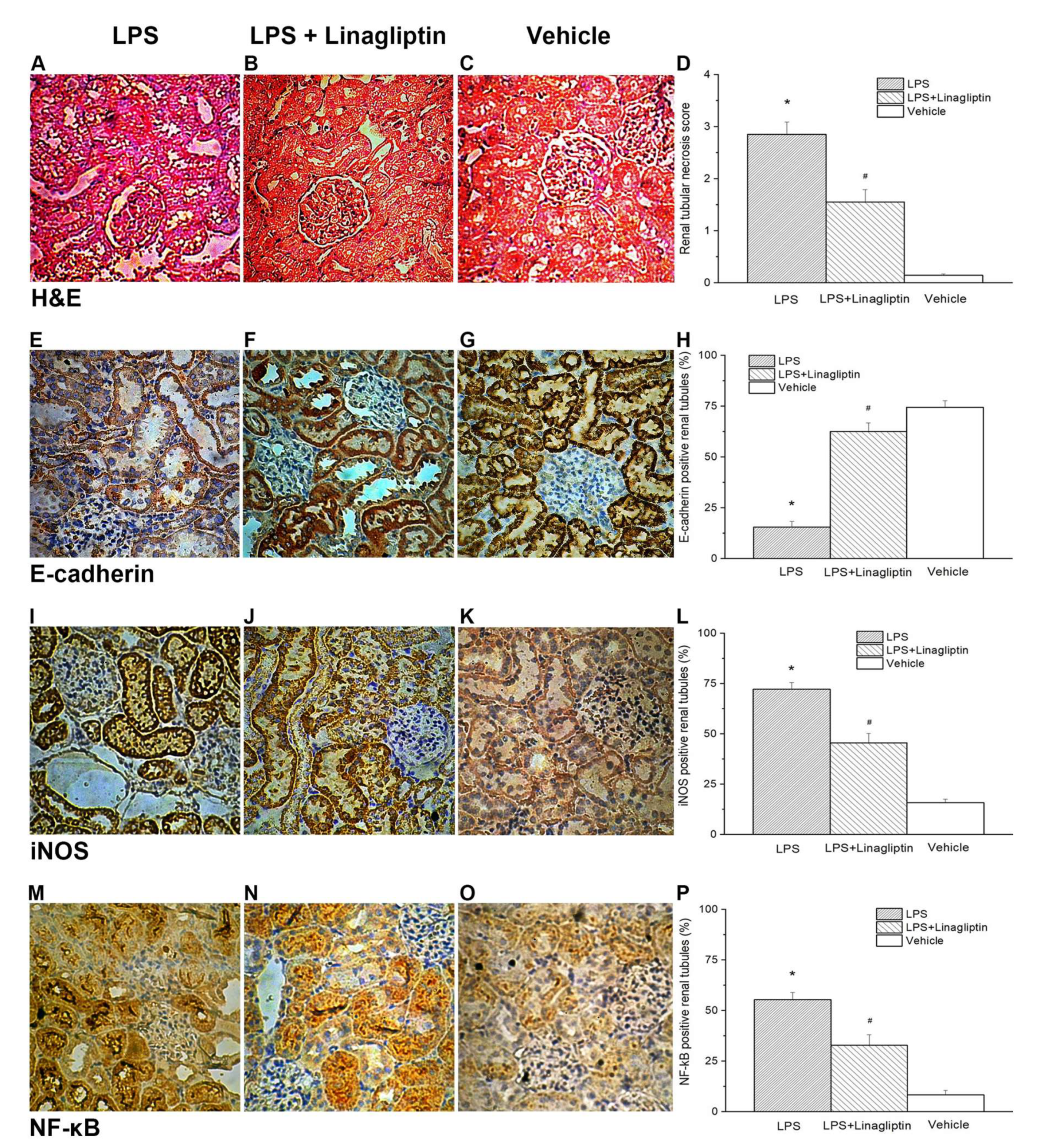
2.3. Linagliptin Attenuated Endotoxic-Shock-Induced Renal Tubular Damages
3. Discussion
4. Materials and Methods
4.1. Cell Model
4.2. Reactive Oxygen Species (ROS) Evaluation
4.3. Quantitative Real-Time Polymerase Chain Reaction and Western Blot Analysis
4.4. Animals
4.5. Blood Vessels Catheterization
4.6. Lipopolysaccharide-Induced Endotoxic Shock Rat Model
4.7. Experimental Design
4.8. Serum Cytokines and Biochemical Assessment
4.9. Histopathological and Immunohistochemical Examinations
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Murugan, R.; Karajala-Subramanyam, V.; Lee, M.; Yende, S.; Kong, L.; Carter, M.; Angus, D.C.; Kellum, J.A.; on behalf of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010, 77, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2015, 10, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Sood, M.M.; Shafer, L.A.; Ho, J.; Reslerova, M.; Martinka, G.; Keenan, S.; Dial, S.; Wood, G.; Rigatto, C.; Kumar, A.; et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J. Crit. Care 2014, 29, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006, 69, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Swaminathan, S.; Bachman, L.A.; Croatt, A.J.; Nath, K.A.; Griffin, M.D. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney. Int. 2007, 71, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pindjakova, J.; Hanley, S.A.; Duffy, M.M.; Sutton, C.E.; Weidhofer, G.A.; Miller, M.N.; Nath, K.A.; Mills, K.H.; Ceredig, R.; Griffin, M.D. Interleukin-1 accounts for intrarenal Th17 cell activation during ureteral obstruction. Kidney Int. 2012, 81, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, P.J.; Rees, A.J.; Griffin, M.D.; Hughes, J.; Kurts, C.; Duffield, J. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 2012, 23, 194–203. [Google Scholar] [CrossRef]
- Anders, H.J. Toll-like receptors and danger signaling in kidney injury. J. Am. Soc. Nephrol. 2010, 21, 1270–1274. [Google Scholar] [CrossRef] [Green Version]
- Kurts, C.; Panzer, U.; Anders, H.J.; Rees, A.J. The immune system and kidney disease: Basic concepts and clinical implications. Nat. Rev. Immunol. 2013, 13, 738–753. [Google Scholar] [CrossRef]
- Rosin, D.L.; Okusa, M.D. Dangers within: DAMP responses to damage and cell death in kidney disease. J. Am. Soc. Nephrol. 2011, 22, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Matheeussen, V.; Jungraithmayr, W.; De Meester, I. Dipeptidyl peptidase 4 as a therapeutic target in ischemia/reperfusion injury. Pharm. Ther. 2012, 136, 267–282. [Google Scholar] [CrossRef]
- Beckers, P.A.J.; Gielis, J.F.; Van Schil, P.E.; Adriaensen, D. Lung ischemia reperfusion injury: The therapeutic role of dipeptidyl peptidase 4 inhibition. Ann. Transl. Med. 2017, 5, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungraithmayr, W.; De Meester, I.; Matheeussen, V.; Inci, I.; Augustyns, K.; Scharpe, S.; Weder, W.; Korom, S. Inhibition of CD26/DPP IV attenuates ischemia/reperfusion injury in orthotopic mouse lung transplants: The pivotal role of vasoactive intestinal peptide. Peptides 2010, 31, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Kroller-Schon, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsalia, V.; Ortsater, H.; Olverling, A.; Darlof, E.; Wolbert, P.; Nystrom, T.; Klein, T.; Sjoholm, A.; Patrone, C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes 2013, 62, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Deacon, C.F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zoubi, S.; Chen, J.; Murphy, C.; Martin, L.; Chiazza, F.; Collotta, D.; Yaqoob, M.M.; Collino, M.; Thiemermann, C. Linagliptin attenuates the cardiac dysfunction associated with experimental sepsis in mice with pre-existing type 2 diabetes by inhibiting NF-kappaB. Front. Immunol. 2018, 9, 2996. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.B.; Choi, Y.K.; Woo, H.I.; Jung, Y.A.; Lee, S.; Lee, S.; Park, M.; Lee, I.K.; Jung, G.S.; Park, K.G. Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome. Diabetes. Metab. J. 2019, 43, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, L.; Dong, Y.; Fei, Y.; Wen, J.; Li, X.; Guan, J.; Liu, F.; Zhou, T.; Li, Z.; et al. Sitagliptin ameliorates renal tubular injury in diabetic kidney disease via STAT3-dependent mitochondrial homeostasis through SDF-1alpha/CXCR4 pathway. FASEB. J. 2020, 34, 7500–7519. [Google Scholar] [CrossRef] [Green Version]
- Benetti, A.; Martins, F.L.; Sene, L.B.; Shimizu, M.H.M.; Seguro, A.C.; Luchi, W.M.; Girardi, A.C.C. Urinary DPP4 correlates with renal dysfunction, and DPP4 inhibition protects against the reduction in megalin and podocin expression in experimental CKD. Am. J. Physiol. Ren. Physiol. 2021, 320, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Rao, X.; Rajagopalan, S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis 2013, 226, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin drugs in diabetic kidney disease: Biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 2021, 17, 227–244. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Yang, F.L.; Lee, R.P.; Peng, T.C.; Harn, H.J.; Chen, H.I. N-acetylcysteine ameliorates lipopolysaccharide-induced organ damage in conscious rats. J. Biomed. Sci. 2004, 11, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Dipeptidyl peptidase-4 inhibitor, linagliptin, ameliorates endothelial dysfunction and atherogenesis in normoglycemic apolipoprotein-E deficient mice. Vasc. Pharm. 2016, 79, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.P.; Lee, C.J.; Subeq, Y.M.; Lee, R.P.; Hsu, B.G. Acute alcohol intoxication exacerbates rhabdomyolysis-induced acute renal failure in rats. Int. J. Med. Sci. 2017, 14, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Hsu, B.G.; Lee, R.P.; Yang, F.L.; Harn, H.J.; Chen, H.I. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006, 79, 2010–2016. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-J.; Hsieh, Y.-J.; Lu, C.-W.; Lee, C.-J.; Hsu, B.-G. Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 11190. https://doi.org/10.3390/ijms222011190
Wu T-J, Hsieh Y-J, Lu C-W, Lee C-J, Hsu B-G. Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. International Journal of Molecular Sciences. 2021; 22(20):11190. https://doi.org/10.3390/ijms222011190
Chicago/Turabian StyleWu, Tsung-Jui, Yi-Jen Hsieh, Chia-Wen Lu, Chung-Jen Lee, and Bang-Gee Hsu. 2021. "Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species" International Journal of Molecular Sciences 22, no. 20: 11190. https://doi.org/10.3390/ijms222011190
APA StyleWu, T.-J., Hsieh, Y.-J., Lu, C.-W., Lee, C.-J., & Hsu, B.-G. (2021). Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. International Journal of Molecular Sciences, 22(20), 11190. https://doi.org/10.3390/ijms222011190








