Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite
Abstract
:1. Introduction
2. Results and Discussion
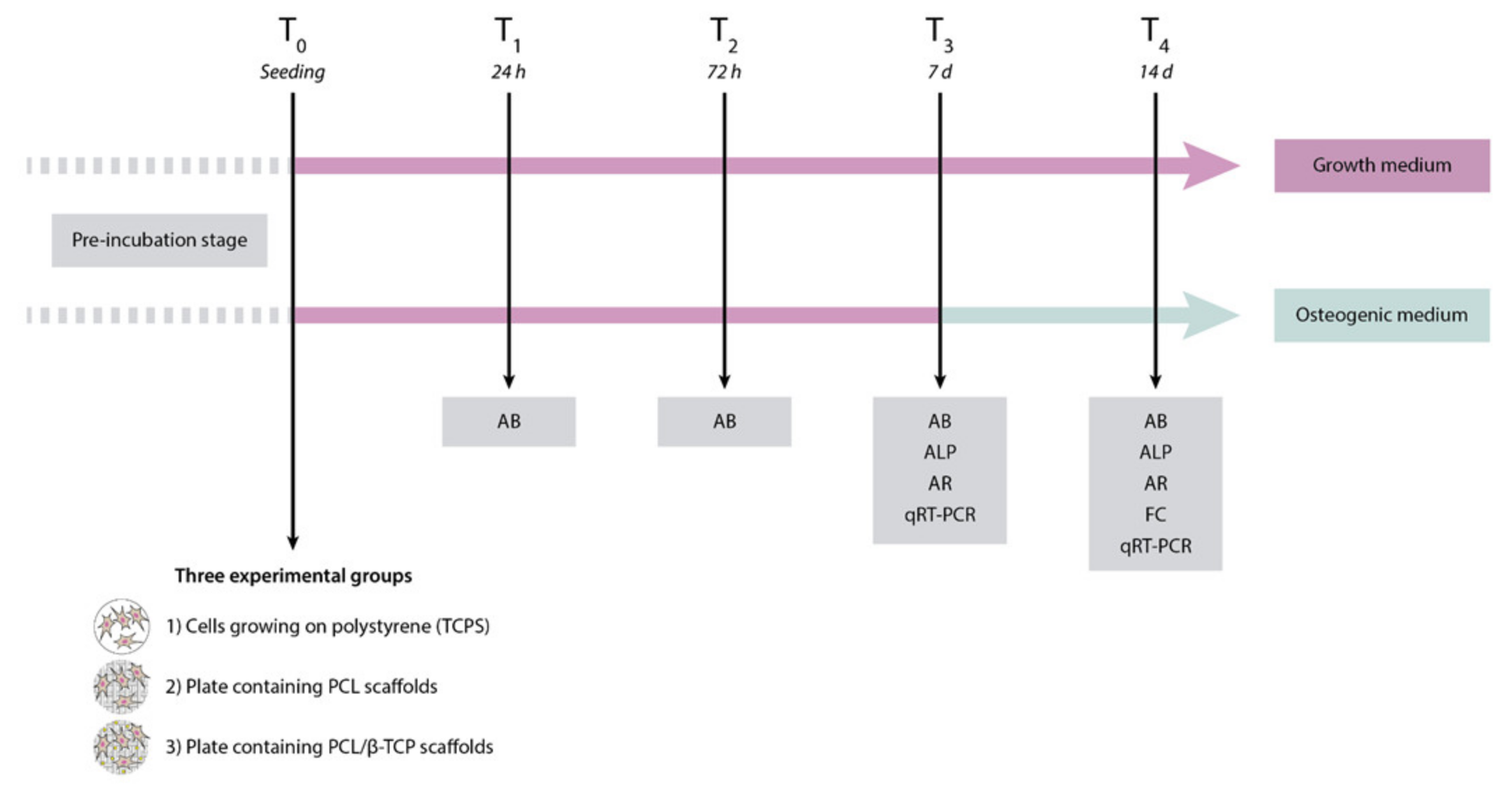
2.1. Study Design
2.2. Material Composition and Characterization
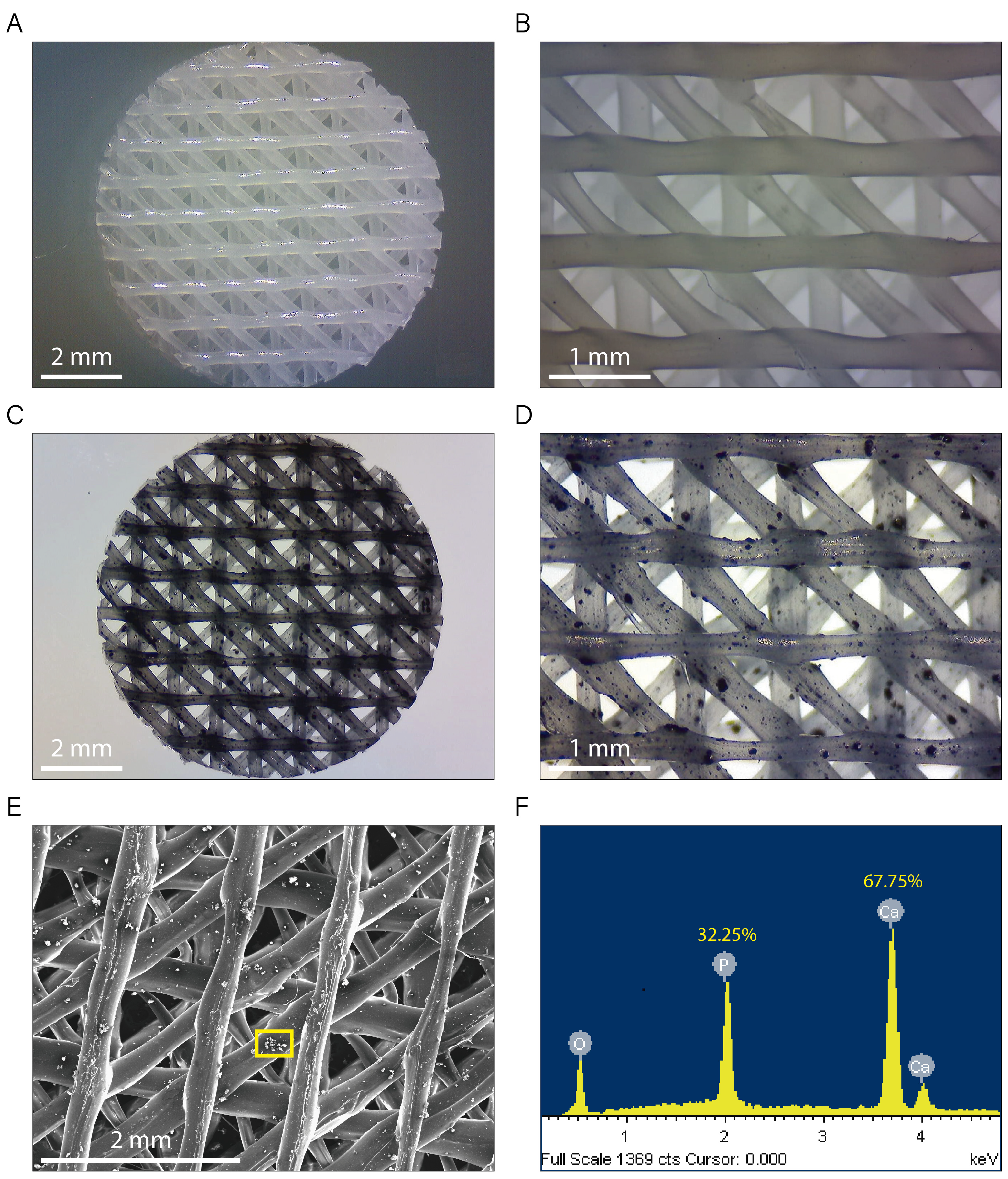
2.2.1. Morphological Characterization and Microanalysis of the Composite Filaments and the 3D-Printed Scaffolds
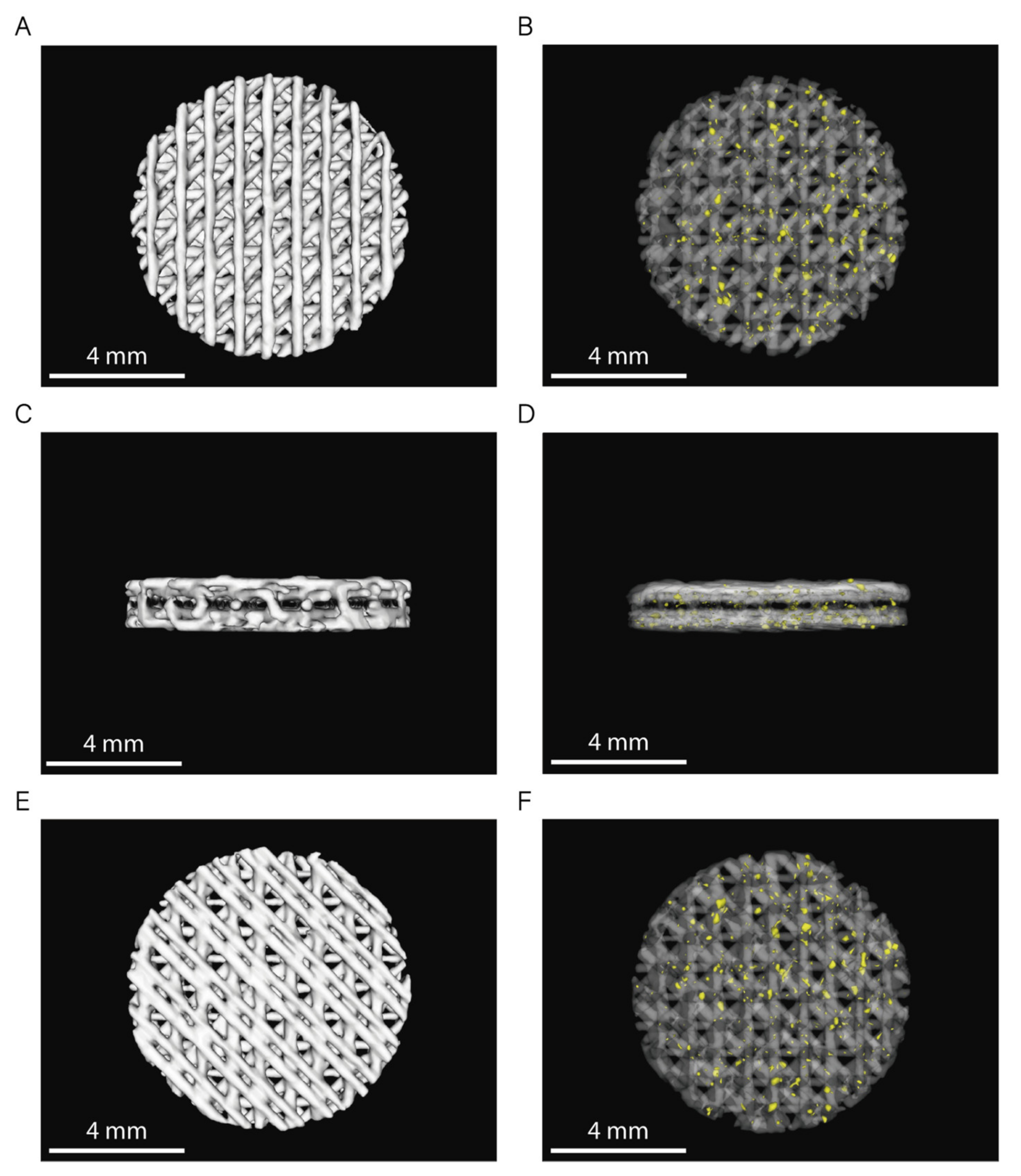
2.2.2. Pore Structure Characterization by Micro-CT
2.3. Influence of the Scaffold Composition
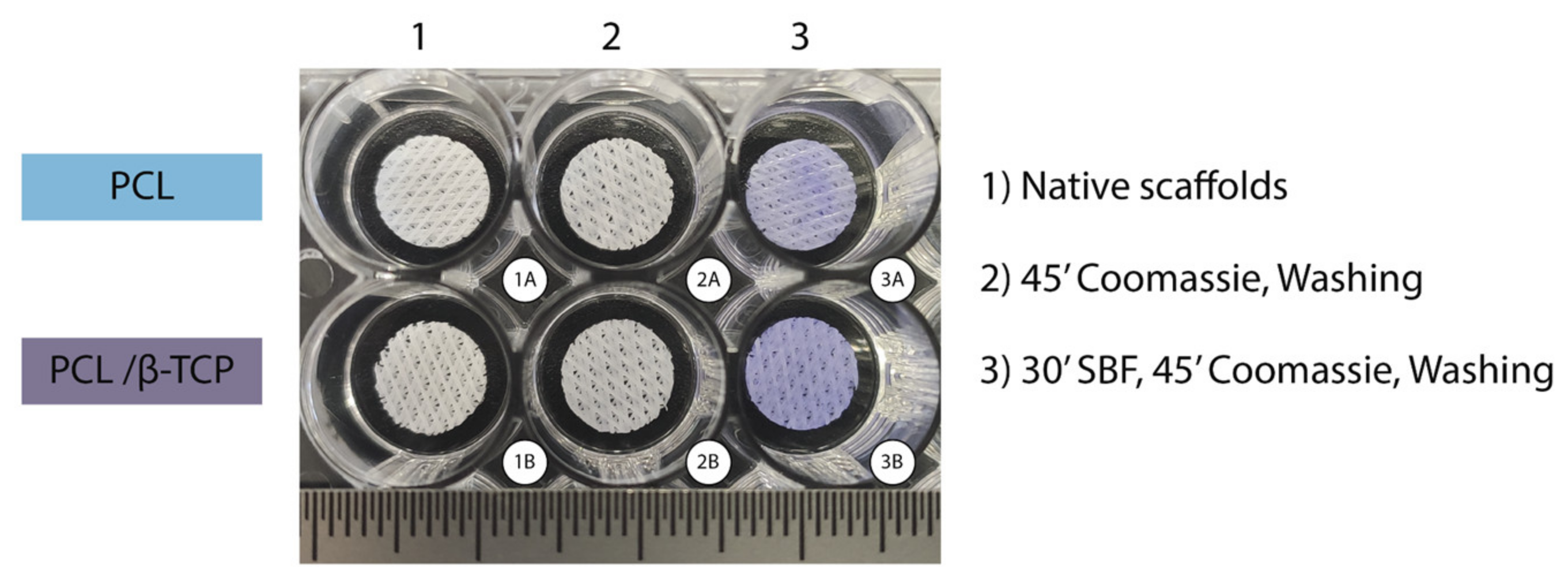
2.3.1. Protein Adhesion: Coomassie Brilliant Blue Test
2.3.2. In Vitro Degradation Kinetics
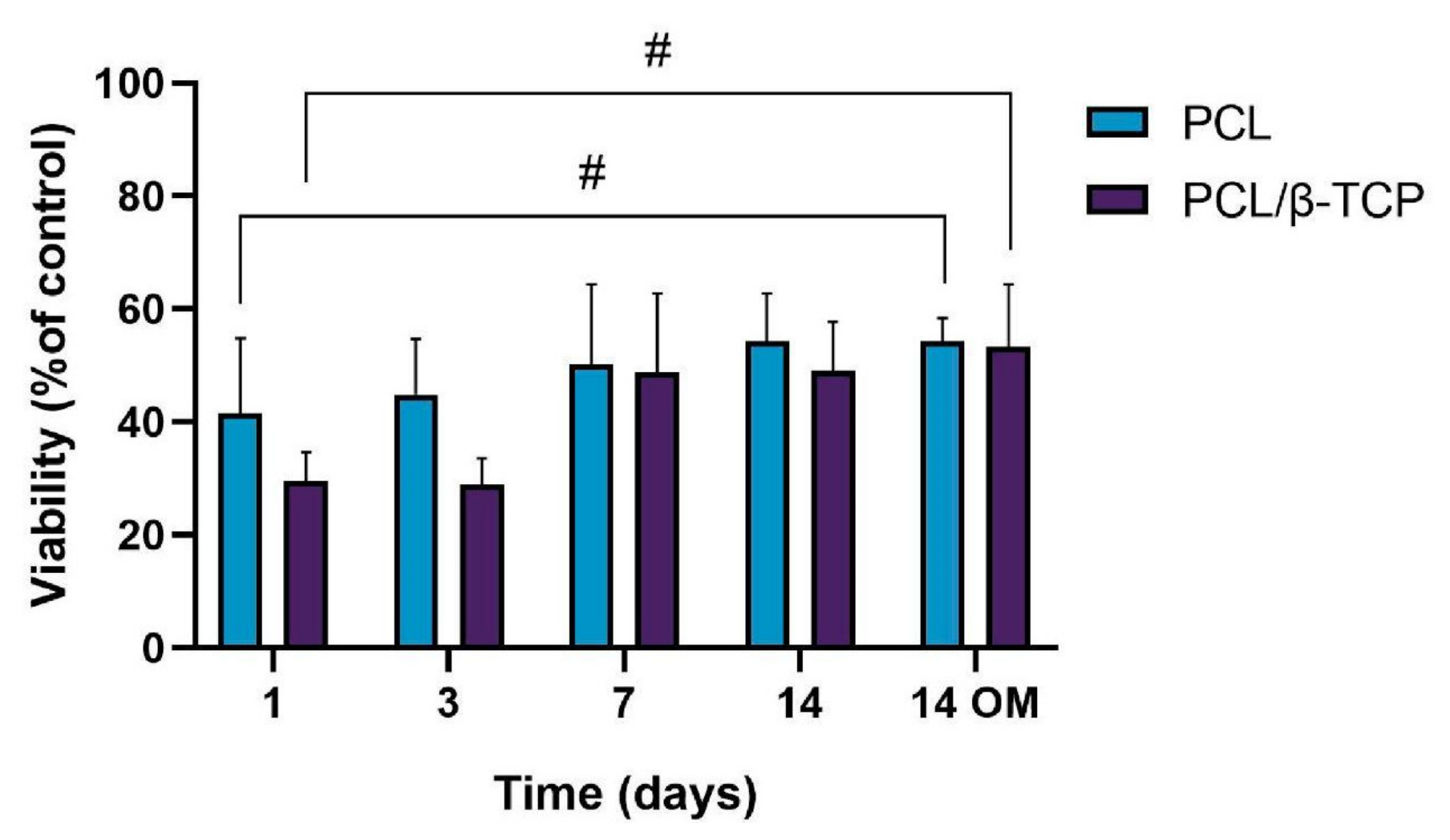
2.4. Cell Viability and Proliferation
2.4.1. ah-BM-MSC Characterization
2.4.2. Cytotoxicity Assay
2.4.3. Cellular Metabolic Activity Assay
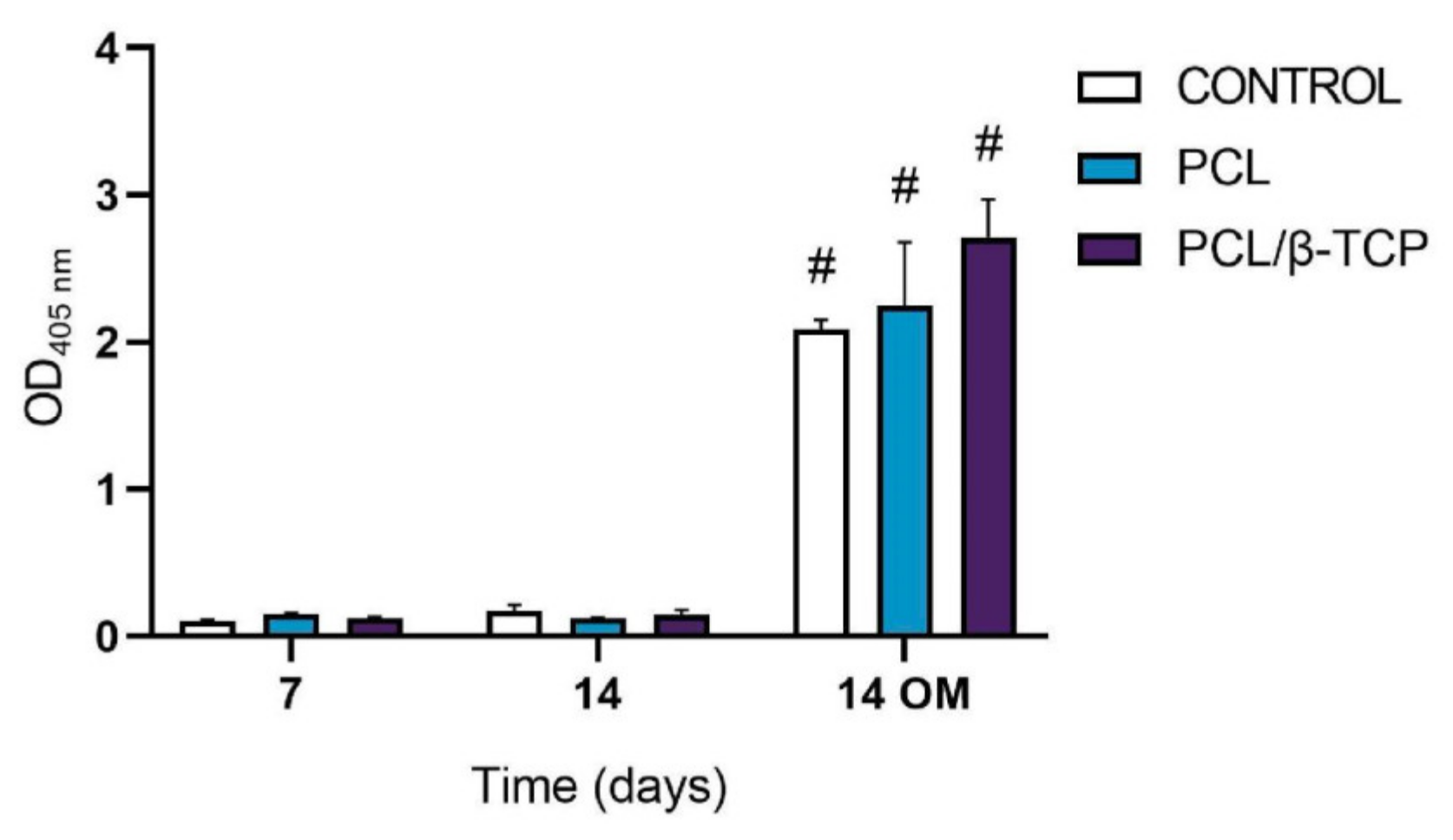
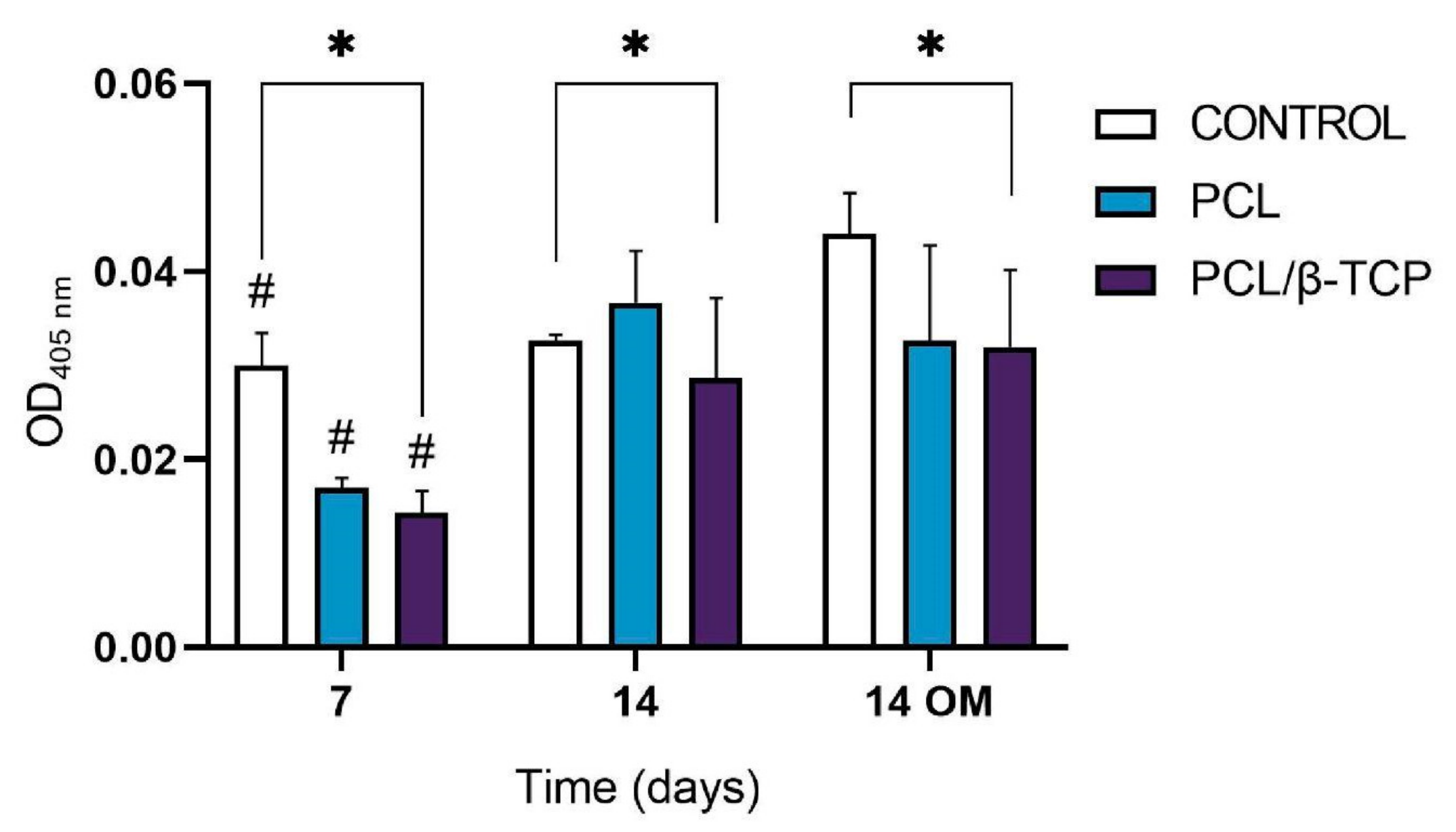
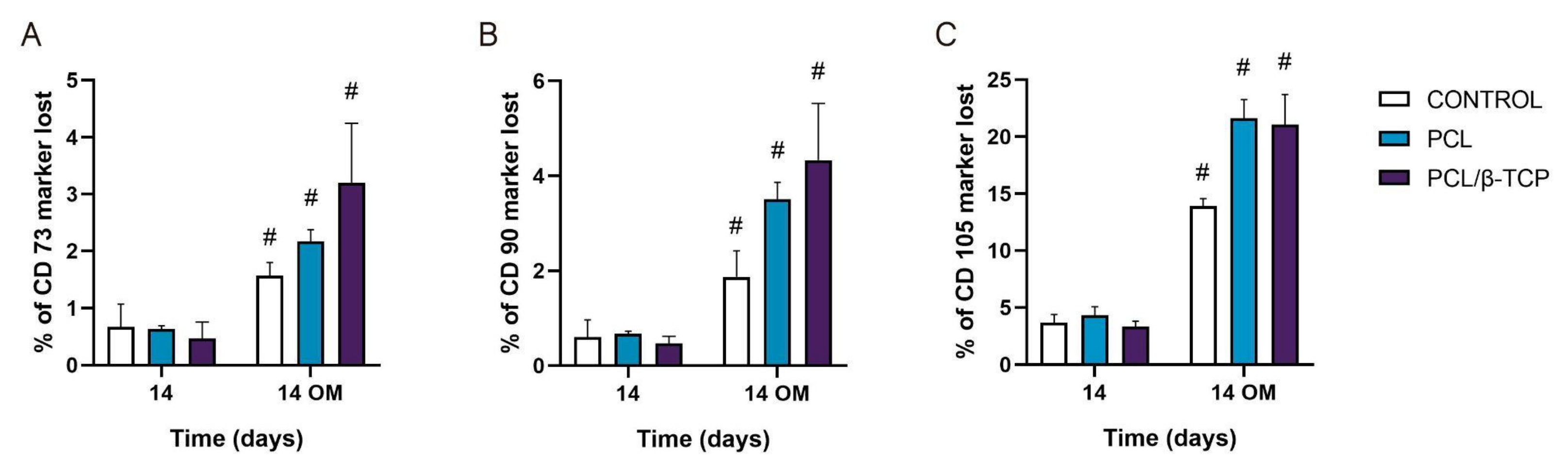
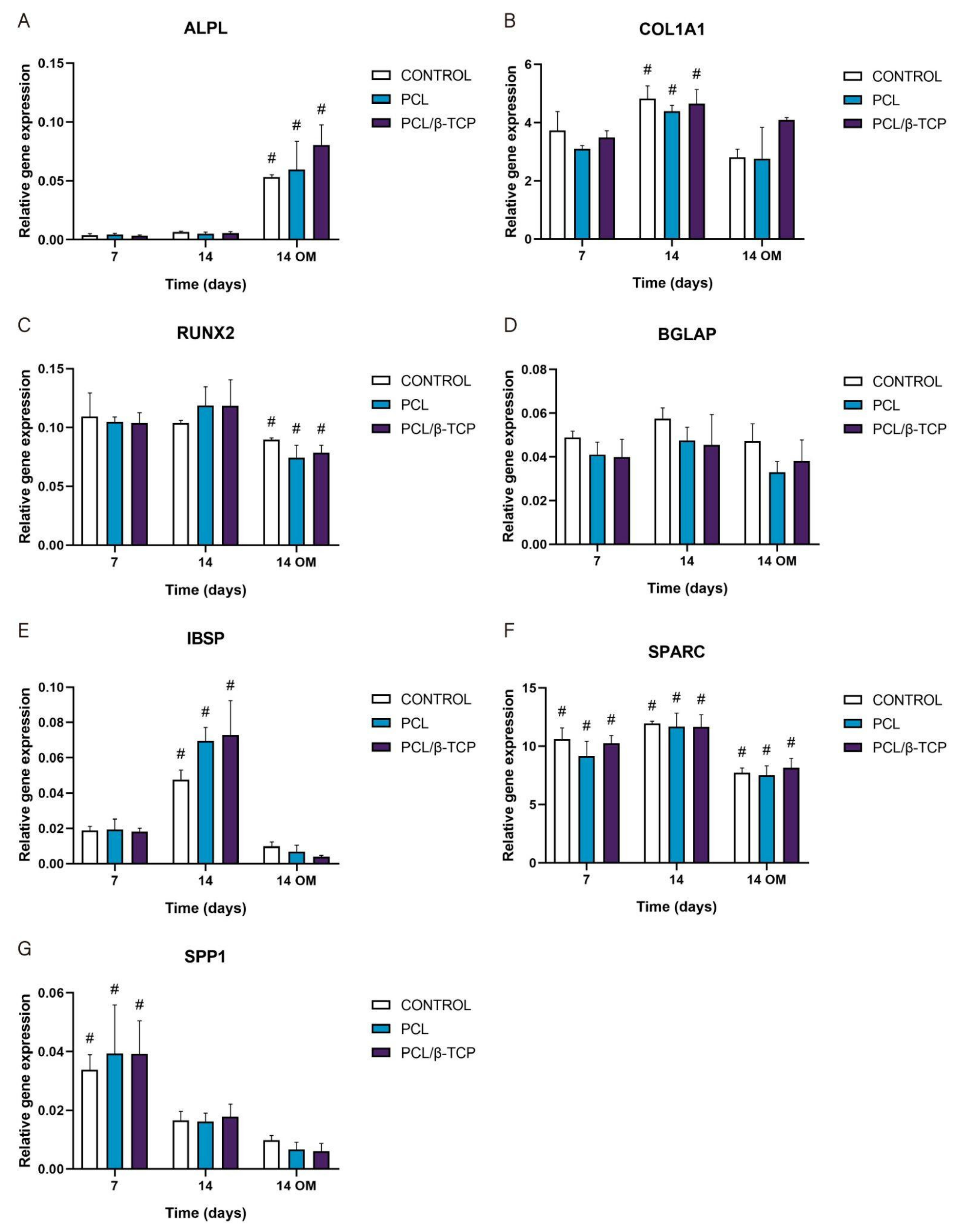
2.5. Cell Differentiation Studies
2.5.1. Alkaline Phosphatase (ALP) Activity
2.5.2. In Vitro Mineralization. Alizarin Red Solution (ARS) Staining
2.5.3. Monitoring Surface Markers on ah-BM-MSCs Seeded on the Scaffolds
2.5.4. Osteogenic Gene Expression: Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
3. Materials and Methods
3.1. Fabrication of Polymer-Based Porous 3D-Printed Scaffolds
3.1.1. Preparation of PCL Filaments Coated with β-TCP Microparticles
3.1.2. Design and Printing of 3D Scaffolds with Controlled Porosity Using the Fused Deposition Modeling (FDM) Method
3.2. Characterization of the Composite Filaments and the 3D-Printed Scaffolds
3.2.1. Morphological Characterization and Microanalysis of the Filaments and the 3D Scaffolds
Scanning Electron Microscopy and Energy-Dispersive X-ray Analysis (SEM-EDX)
Micro-Computed Tomography (µCT) Scaffold Imaging: Porosity and β-TCP Particle Distribution
3.2.2. Protein Adhesion: Coomassie Brilliant Blue Test
3.2.3. In Vitro Degradation Kinetics
3.3. Isolation, Characterization and Culture of Adult Human Bone Marrow-Derived Mesenchymal Stem Cells (ah-BM-MSCs)
3.3.1. ah-BM-MSC Characterization
3.3.2. Cell Seeding Methods
3.4. Cell Viability, Adhesion, and Proliferation Assays
3.4.1. Cytotoxicity Assay
3.4.2. Cellular Metabolic Activity Assay
3.5. Osteoblastic Differentiation Assays
3.5.1. Alkaline Phosphatase (ALP) Activity
3.5.2. In Vitro Mineralization of Alizarin Red Solution (ARS) Staining
3.5.3. Monitoring Surface Markers in the Cells Seeded on the Scaffolds: Cluster of Differentiation (CD)
3.5.4. Osteogenic Gene Expression: Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
3.6. Statistic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.T.; Gonçalves, A.I.; Babo, P.S.; Gomez-Florit, M.; Reis, R.L.; Gomes, M.E. Bioinspired materials and tissue engineering approaches applied to the regeneration of musculoskeletal tissues. In Engineering Strategies for Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2020; pp. 73–105. [Google Scholar]
- Diaz-Arca, A.; Ros-Tarraga, P.; Tome, M.J.M.; De Aza, A.H.; Meseguer-Olmo, L.; Mazon, P.; De Aza, P.N. Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone. Materials 2021, 14, 1439. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 2424–2435. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, R.G.; Schatkoski, V.M.; Montanheiro, T.L.D.A.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Rodríguez-Valencia, C.; Serra, J.; González, P. Bio-inspired Ceramics: Promising Scaffolds for Bone Tissue Engineering. Procedia Eng. 2013, 59, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Lugo, G.J.; Mazón, P.; De Aza, P.N. Phase transitions in single phase Si–Ca–P-based ceramic under thermal treatment. J. Eur. Ceram. Soc. 2015, 35, 3693–3700. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Hild, N.; Schneider, O.D.; Mohn, D.; Luechinger, N.A.; Koehler, F.M.; Hofmann, S.; Vetsch, J.R.; Thimm, B.W.; Muller, R.; Stark, W.J. Two-layer membranes of calcium phosphate/collagen/PLGA nanofibres: In vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2011, 3, 401–409. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Production and Potential of Bioactive Glass Nanofibers as a Next-Generation Biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Xie, J.; Blough, E.R.; Wang, C.H. Submicron bioactive glass tubes for bone tissue engineering. Acta Biomater. 2012, 8, 811–819. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Lee, S.; Choi, D.; Shim, J.H.; Nam, W. Efficacy of three-dimensionally printed polycaprolactone/β tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 2020, 10, 4979. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, T.H.; Ryu, J.Y.; Kim, D.K.; Oh, E.J.; Kim, H.M.; Shim, J.H.; Yun, W.S.; Huh, J.B.; Moon, S.H.; et al. Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. Int. J. Mol. Sci. 2021, 22, 5409. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Yeh, Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 52–65. [Google Scholar] [CrossRef]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/Hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, P.Q.; Hedberg, E.L.; Padron, N.T.; Spauwen, P.H.; Jansen, J.A.; Mikos, A.G. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J. Bone Joint Surg. Am. 2003, 85-A (Suppl. 3), 75–81. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Bernabeu-Esclapez, A.; Ros-Martinez, E.; Sanchez-Salcedo, S.; Padilla, S.; Martin, A.I.; Vallet-Regi, M.; Clavel-Sainz, M.; Lopez-Prats, F.; Meseguer-Ortiz, C.L. In vitro behaviour of adult mesenchymal stem cells seeded on a bioactive glass ceramic in the SiO(2)-CaO-P(2)O(5) system. Acta Biomater. 2008, 4, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Burg, K.J.L.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β-TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112197. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for Hard Tissue Engineering Applications: A Review. Int. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Urist, M.R.; Lietze, A.; Dawson, E. Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin. Orthop. Relat. Res. 1984, 187, 277–280. [Google Scholar]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Yuan, X.; Xia, Y. Coating electrospun poly(epsilon-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir 2008, 24, 14145–14150. [Google Scholar] [CrossRef]
- Liu, W.; Yeh, Y.C.; Lipner, J.; Xie, J.; Sung, H.W.; Thomopoulos, S.; Xia, Y. Enhancing the stiffness of electrospun nanofiber scaffolds with a controlled surface coating and mineralization. Langmuir 2011, 27, 9088–9093. [Google Scholar] [CrossRef]
- Bae, E.B.; Park, K.H.; Shim, J.H.; Chung, H.Y.; Choi, J.W.; Lee, J.J.; Kim, C.H.; Jeon, H.J.; Kang, S.S.; Huh, J.B. Efficacy of rhBMP-2 Loaded PCL/β-TCP/bdECM Scaffold Fabricated by 3D Printing Technology on Bone Regeneration. Biomed. Res. Int. 2018, 2018, 2876135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bachman, H.; Nicosia, J.; Dysart, M.; Barker, T.H. Utilizing Fibronectin Integrin-Binding Specificity to Control Cellular Responses. Adv. Wound Care 2015, 4, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Pirraco, R.P.; Marques, A.P.; Reis, R.L. Cell interactions in bone tissue engineering. J. Cell Mol. Med. 2010, 14, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.C.; Wullschleger, M.E.; Cipitria, A.; Lienau, J.; Cheng, T.K.; Schutz, M.A.; Duda, G.N.; Noth, U.; Eulert, J.; Hutmacher, D.W. Custom-made composite scaffolds for segmental defect repair in long bones. Int. Orthop. 2011, 35, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Park, J.-S.; Jeong, S.I.; An, S.-J.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, C.-Y. Promotion of human mesenchymal stem cell differentiation on bioresorbable polycaprolactone/biphasic calcium phosphate composite scaffolds for bone tissue engineering. Biotechnol. Bioprocess Eng. 2014, 19, 341–349. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.A.; Kang, Y.G.; Shin, J.W.; Park, Y.S.; Gu, S.R.; Wu, Y.R.; Wei, J.; Shin, J.W. PCL/β-TCP Composite Scaffolds Exhibit Positive Osteogenic Differentiation with Mechanical Stimulation. Tissue Eng. Regen. Med. 2017, 14, 349–358. [Google Scholar] [CrossRef]
- Yang, D.H.; Heo, G.M.; Park, H.J.; Oh, H.K.; Kook, M.S. Comparative Effectiveness of Surface Functionalized Poly-epsilon-Caprolactone Scaffold and β-TCP Mixed PCL Scaffold for Bone Regeneration. J. Nanosci. Nanotechnol. 2020, 20, 5349–5355. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Gao, S.; Wang, P.; Li, Y.; Guo, W.; Zhang, Y.; Wang, M.; Xiao, T.; Zhang, Z.; Zhang, X.; et al. The application of electrospinning used in meniscus tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 461–475. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111752. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Tang, M.; Weir, M.D.; Bao, C.; Xu, H.H. Gas-foaming calcium phosphate cement scaffold encapsulating human umbilical cord stem cells. Tissue Eng. Part A 2012, 18, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Yoo, J.J. Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Williams, D.F. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Fan, H.S.; Zhao, C.Y.; Lu, J.; Ikoma, T.; Tanaka, J.; Zhang, X.D. Competitive adsorption of bovine serum albumin and lysozyme on characterized calcium phosphates by polyacrylamide gel electrophoresis method. J. Mater. Sci. Mater. Med. 2007, 18, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.J.; Hsu, H.I.; Lin, C.C.; Huang, T.H.; Wu, B.C.; Kao, C.T.; Shie, M.Y. The role of integrin αv in proliferation and differentiation of human dental pulp cell response to calcium silicate cement. J. Endod. 2014, 40, 1802–1809. [Google Scholar] [CrossRef]
- Sukanya, V.S.; Mohanan, P.V. Degradation of Poly(epsilon-caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 163, 107–118. [Google Scholar]
- Huang, S.H.; Hsu, T.T.; Huang, T.H.; Lin, C.Y.; Shie, M.Y. Fabrication and characterization of polycaprolactone and tricalcium phosphate composites for tissue engineering applications. J. Dent. Sci. 2017, 12, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Rai, B.; Sju, E.; Cheong, J.J.; Teoh, S.H. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: An in vitro and in vivo study. J. Biomed. Mater. Res. A 2008, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Rai, B.; Ho, K.H.; Teoh, S.H. In vitro degradation of novel bioactive polycaprolactone—20% tricalcium phosphate composite scaffolds for bone engineering. Mater. Sci. Eng. C 2007, 27, 293–298. [Google Scholar] [CrossRef]
- Pena, J.; Corrales, T.; Izquierdo-Barba, I.; Serrano, M.C.; Portoles, M.T.; Pagani, R.; Vallet-Regi, M. Alkaline-treated poly(epsilon-caprolactone) films: Degradation in the presence or absence of fibroblasts. J. Biomed. Mater. Res. A 2006, 76, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, F.; Lin, K.; Lu, J.; Sun, J.; Chang, J.; Dai, K.; Fan, C. The effect of calcium silicate on in vitro physiochemical properties and in vivo osteogenesis, degradability and bioactivity of porous β-tricalcium phosphate bioceramics. Biomed. Mater. 2013, 8, 025008. [Google Scholar] [CrossRef]
- Khan, A.F.; Saleem, M.; Afzal, A.; Ali, A.; Khan, A.; Khan, A.R. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Maxim, M.A.; Soritau, O.; Baciut, M.; Bran, S.; Baciut, G. The role of dental stem cells in regeneration. Clujul Med. 2015, 88, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Asadi Rad, A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar]
- Kim, Y.B.; Kim, G. Functionally graded PCL/β-TCP biocomposites in a multilayered structure for bone tissue regeneration. Appl. Phys. A 2012, 108, 949–959. [Google Scholar] [CrossRef]
- Petit, C.; Tulliani, J.M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel calcium phosphate/PCL graded samples: Design and development in view of biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, Z.; Zhao, Y.; Tang, Y.; Zhou, S.; Sun, Y.; Chen, X. In Vitro and In Vivo Study of a Novel Nanoscale Demineralized Bone Matrix Coated PCL/β-TCP Scaffold for Bone Regeneration. Macromol. Biosci. 2021, 21, e2000336. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Dou, Y.; Fa, X.; Gu, Y.; Liang, L.; Wen, J.; Qin, A.; Ou, J. Fabrication and characterization of PVA/CS-PCL/gel multi-scale electrospun scaffold: Simulating extracellular matrix for enhanced cellular infiltration and proliferation. J. Biomater. Sci. Polym. Ed. 2020, 31, 729–746. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Cheng, J.; Sun, M.; Yang, P.; Zhao, F.; Zhang, J.; Duan, X.; Fu, X.; Zhang, J.; et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics 2020, 10, 5090–5106. [Google Scholar] [CrossRef]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Zernik, J.; Twarog, K.; Upholt, W.B. Regulation of alkaline phosphatase and α 2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation 1990, 44, 207–215. [Google Scholar] [CrossRef]
- Fishman, W.H.; Ghosh, N.K. Isoenzymes of Human Alkaline Phosphatase. In Advances in Clinical Chemistry Volume 10; Academic Press: Cambridge, MA, USA, 1967; pp. 255–370. [Google Scholar]
- Jansen, J.H.; van der Jagt, O.P.; Punt, B.J.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H.; Jahr, H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: An in vitro study. BMC Musculoskelet. Disord. 2010, 11, 188. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiao, X.; Lan, P.; Li, M.; Geng, L.; Liu, D.; et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016, 6, 23367. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Le, D.Q.; Kjems, J.; Bunger, C.; Lysdahl, H. Improvement of Distribution and Osteogenic Differentiation of Human Mesenchymal Stem Cells by Hyaluronic Acid and β-Tricalcium Phosphate-Coated Polymeric Scaffold In Vitro. Biores. Open Access 2015, 4, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.; Kraft, D.C.; Lysdahl, H.; Foldager, C.B.; Chen, M.; Kristiansen, A.A.; Rolfing, J.H.; Bunger, C.E. Functionalization of polycaprolactone scaffolds with hyaluronic acid and β-TCP facilitates migration and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Eng. Part A 2015, 21, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurel Pekozer, G.; Ramazanoglu, M.; Schlegel, K.A.; Kok, F.N.; Torun Kose, G. Role of STRO-1 sorting of porcine dental germ stem cells in dental stem cell-mediated bone tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W.S. Adult mesenchymal stem cells and cell surface characterization—A systematic review of the literature. Open Orthop. J. 2011, 5 (Suppl. 2), 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng-Juan, L.V.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar]
- Viti, F.; Landini, M.; Mezzelani, A.; Petecchia, L.; Milanesi, L.; Scaglione, S. Osteogenic Differentiation of MSC through Calcium Signaling Activation: Transcriptomics and Functional Analysis. PLoS ONE 2016, 11, e0148173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabadan-Ros, R.; Revilla-Nuin, B.; Mazón, P.; Aznar-Cervantes, S.; Ros-Tarraga, P.; De Aza, P.N.; Meseguer-Olmo, L. Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells. Appl. Sci. 2018, 8, 46. [Google Scholar]
- Karsenty, G.; Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Geoffroy, V.; Amling, M. Cbfa1 as a regulator of osteoblast differentiation and function. Bone 1999, 25, 107–108. [Google Scholar] [CrossRef]
- Ducy, P. CBFA1: A molecular switch in osteoblast biology. Dev. Dyn. 2000, 219, 461–471. [Google Scholar] [CrossRef]
- Ziros, P.G.; Basdra, E.K.; Papavassiliou, A.G. Runx2: Of bone and stretch. Int. J. Biochem. Cell Biol. 2008, 40, 1659–1663. [Google Scholar] [CrossRef]
- Marie, P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008, 473, 98–105. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, R.T.; Xiao, G. Regulation of the osteoblast-specific transcription factor, Runx2: Responsiveness to multiple signal transduction pathways. J. Cell Biochem. 2003, 88, 446–454. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization [ISO]. Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; ISO/FDIS Standard No. 23317; ISO: London, UK, 2014. [Google Scholar]
- Cudemos, E.; Izquier, A.; Medina-Martínez, M.S.; Gómez-López, V.M. Effects of shading and growth phase on the microbial inactivation by pulsed light. Czech J. Food Sci. 2013, 31, 189–193. [Google Scholar] [CrossRef] [Green Version]
- De Aza, P.N.; Garcia-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of Ca2SiO4-Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Aznar-Cervantes, S.; Mazon, P.; De Aza, P.N. “In vitro” behaviour of adult mesenchymal stem cells of human bone marrow origin seeded on a novel bioactive ceramics in the Ca2SiO4-Ca3(PO4)2 system. J. Mater. Sci. Mater. Med. 2012, 23, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Blumberg, J.; Pirun, M.; Volloch, V.; Vunjak-Novakovic, G.; Kaplan, D.L. Osteogenic differentiation of human bone marrow stromal cells on partially demineralized bone scaffolds in vitro. Tissue Eng. 2004, 10, 81–92. [Google Scholar] [CrossRef] [PubMed]


| Antigen | Percentage of Positive Cells |
|---|---|
| CD73 | 99.67 ± 0.06 |
| CD90 | 98.27 ± 0.25 |
| CD105 | 97.93 ± 0.06 |
| CD44 | 97.87 ± 0.23 |
| CD14/19/34/45 * | 3.10 ± 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-González, I.; Zamora-Ledezma, C.; Sanchez-Lorencio, M.I.; Tristante Barrenechea, E.; Gabaldón-Hernández, J.A.; Meseguer-Olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. https://doi.org/10.3390/ijms222011216
López-González I, Zamora-Ledezma C, Sanchez-Lorencio MI, Tristante Barrenechea E, Gabaldón-Hernández JA, Meseguer-Olmo L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. International Journal of Molecular Sciences. 2021; 22(20):11216. https://doi.org/10.3390/ijms222011216
Chicago/Turabian StyleLópez-González, Ivan, Camilo Zamora-Ledezma, María Isabel Sanchez-Lorencio, Elena Tristante Barrenechea, José Antonio Gabaldón-Hernández, and Luis Meseguer-Olmo. 2021. "Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite" International Journal of Molecular Sciences 22, no. 20: 11216. https://doi.org/10.3390/ijms222011216
APA StyleLópez-González, I., Zamora-Ledezma, C., Sanchez-Lorencio, M. I., Tristante Barrenechea, E., Gabaldón-Hernández, J. A., & Meseguer-Olmo, L. (2021). Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. International Journal of Molecular Sciences, 22(20), 11216. https://doi.org/10.3390/ijms222011216










