Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation
Abstract
:1. Introduction
2. Results
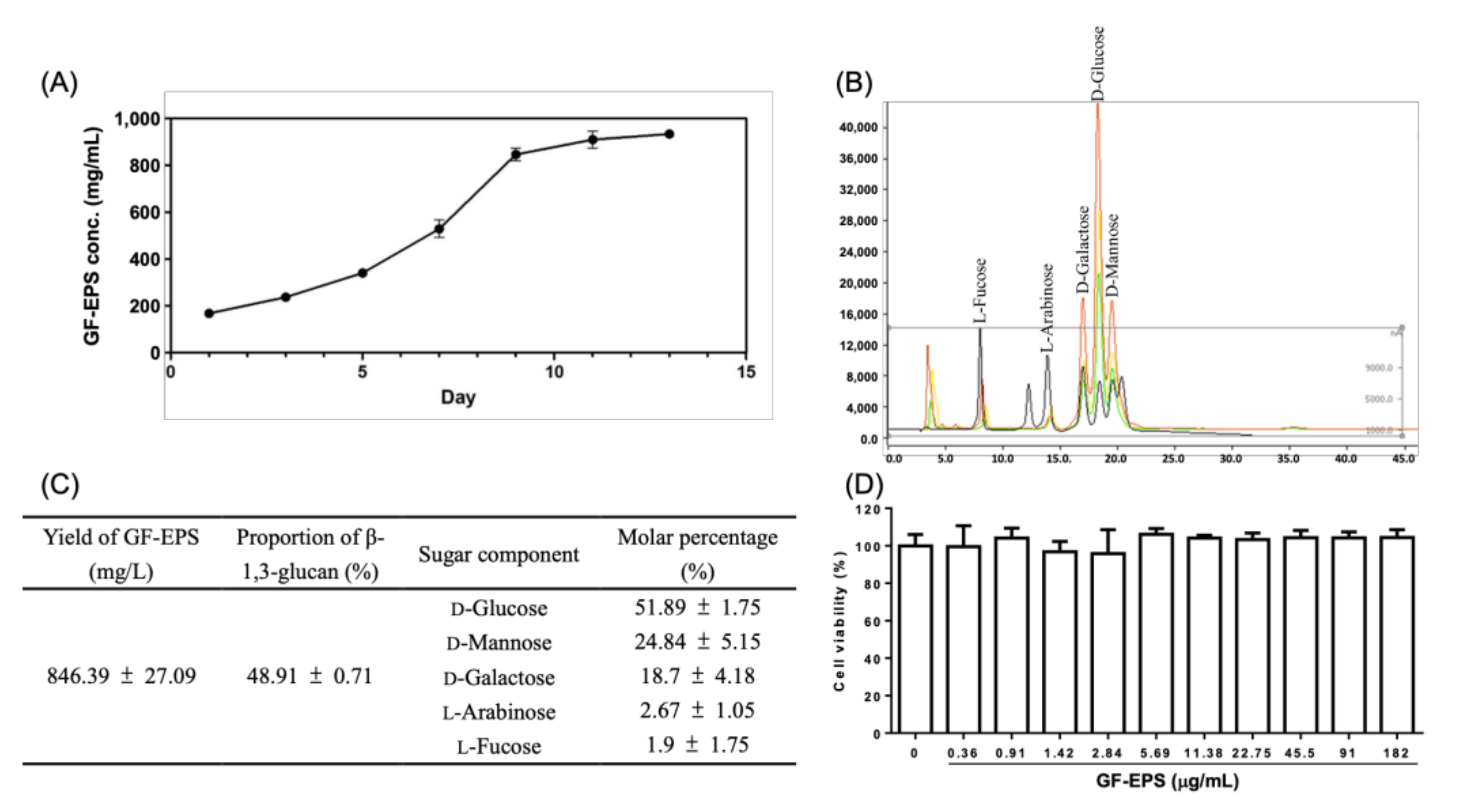
2.1. GF-EPS Preparation and Characterization
2.2. Cell Viability of LLC1 Cells Treated with GF-EPS
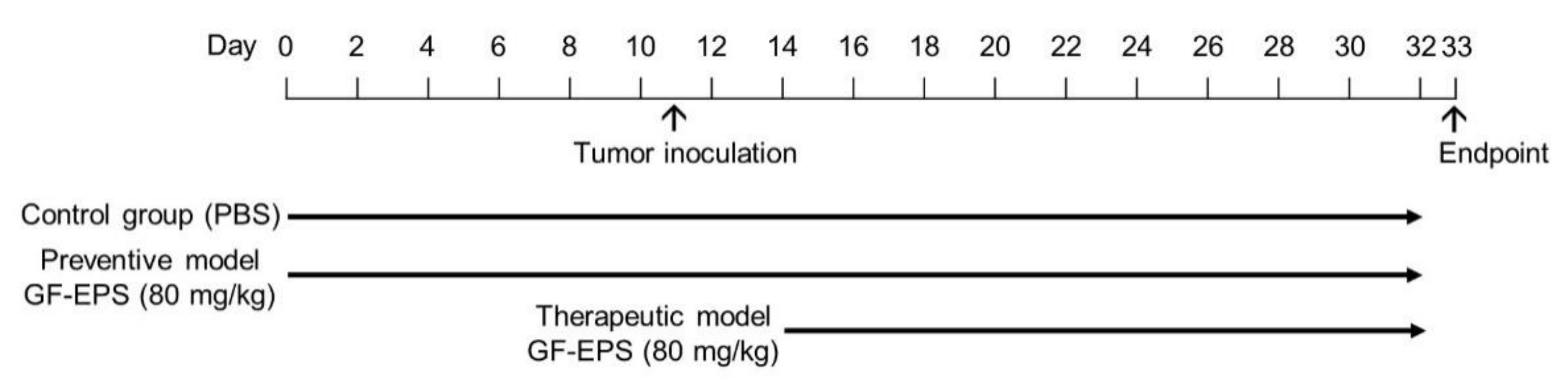
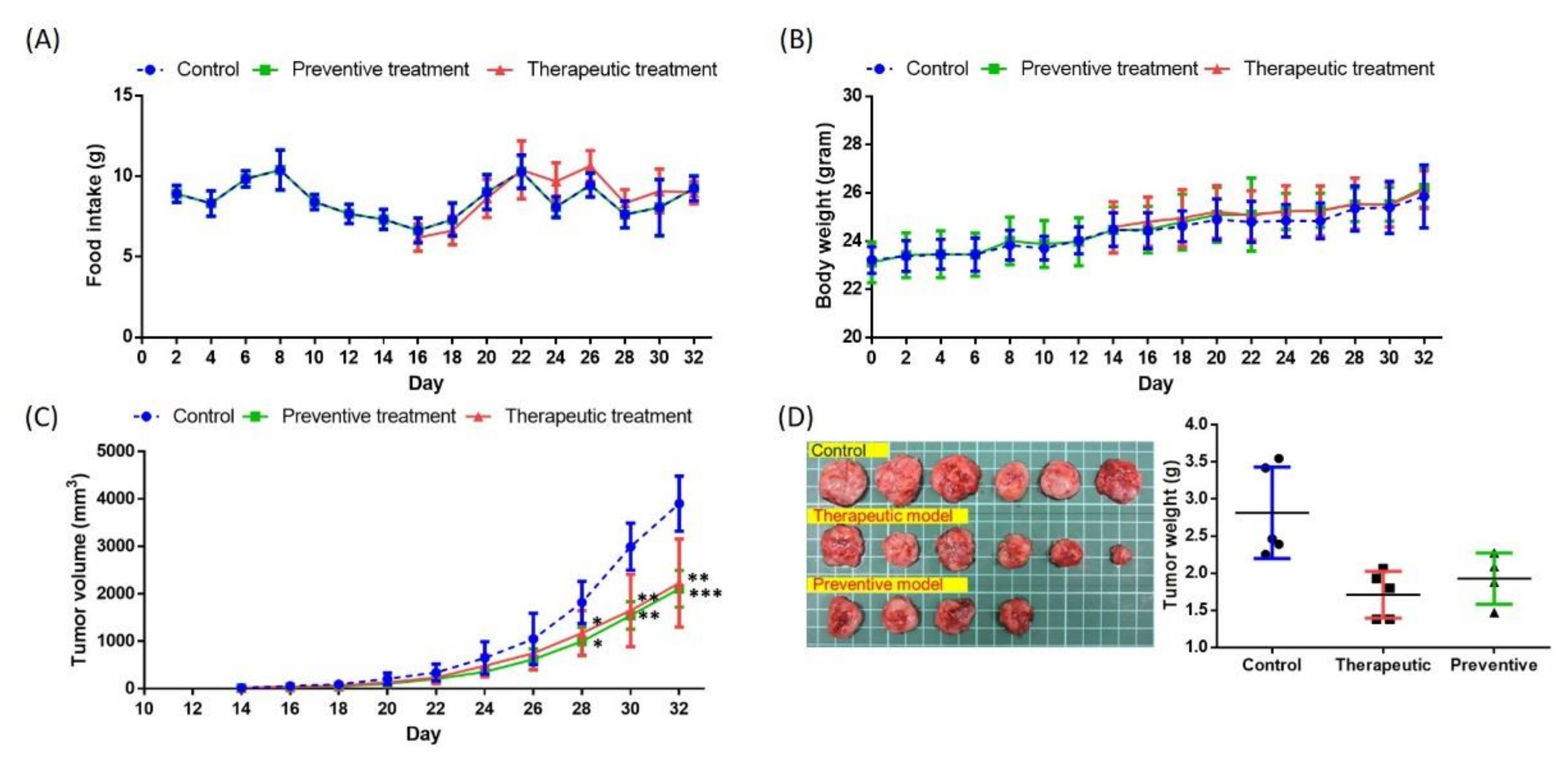
2.3. Effects of GF-EPS on Tumor-Bearing Mice
2.4. Cell Population in the Spleen
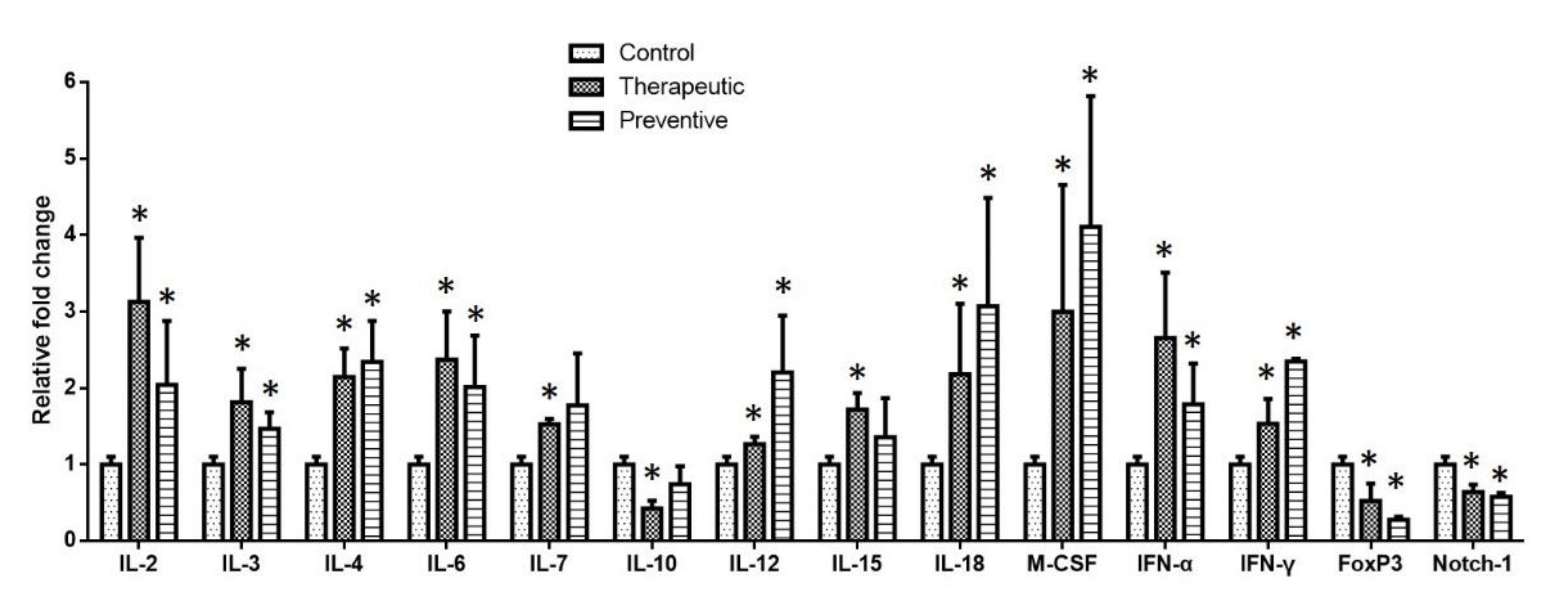
2.5. Cytokine Gene Expression in the Spleen
3. Discussion
4. Materials and Methods
4.1. GF-EPS Preparation
4.2. GF-EPS Characterization
4.3. Cytotoxicity of GF-EPS on Mouse LLC1
4.4. Effects of GF-EPS on Tumor-Bearing Mice
4.5. Cell Population Analysis by Flow Cytometry
4.6. Quantitative RT-PCR
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, K.D.; Cheng, K.C. From nutraceutical to clinical trial: Frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef]
- Hsu, K.D.; Chen, H.J.; Wang, C.S.; Lum, C.C.; Wu, S.P.; Lin, S.P.; Cheng, K.C. Extract of Ganoderma formosanum mycelium as a highly potent tyrosinase inhibitor. Sci. Rep. 2016, 6, 32854. [Google Scholar] [CrossRef]
- Hsu, K.D.; Chan, Y.H.; Chen, H.J.; Lin, S.P.; Cheng, K.C. Tyrosinase-based TLC Autography for anti-melanogenic drug screening. Sci. Rep. 2018, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Lin, C.C.; Chen, M.F.; Ujiie, T.; Takada, A. Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neo-japonicum. J. Ethnopharmacol. 1995, 47, 33–41. [Google Scholar] [CrossRef]
- Wang, C.L.; Pi, C.C.; Kuo, C.W.; Zhuang, Y.J.; Khoo, K.H.; Liu, W.H.; Chen, C.J. Polysaccharides purified from the submerged culture of Ganoderma formosanum stimulate macrophage activation and protect mice against Listeria monocytogenes infection. Biotechnol. Lett. 2011, 33, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Lu, C.Y.; Pi, C.C.; Zhuang, Y.J.; Chu, C.L.; Liu, W.H.; Chen, C.J. Extracellular polysaccharides produced by Ganoderma formosanum stimulate macrophage activation via multiple pattern-recognition receptors. BMC Complement. Altern. Med. 2012, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Pi, C.C.; Wang, H.Y.; Lu, C.Y.; Lu, F.L.; Chen, C.J. Ganoderma formosanum polysaccharides attenuate Th2 inflammation and airway hyperresponsiveness in a murine model of allergic asthma. Springerplus 2014, 3, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.F.; Liu, H.B.; Zhang, Q.W.; Li, Z.P.; Wong, T.L.; Fung, H.Y.; Zhang, J.X.; Bai, S.P.; Lu, A.P.; Han, Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 6172. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.N.; Liao, Y.M.; Kuo, L.N.; Tsai, H.J.; Chang, W.C.; Yen, Y. Cancers in Taiwan: Practical insight from epidemiology, treatments, biomarkers, and cost. J. Formos. Med. Assoc. 2020, 119, 1731–1741. [Google Scholar] [CrossRef]
- Hsu, K.D.; Wu, S.P.; Lin, S.P.; Lum, C.C.; Cheng, K.C. Enhanced active extracellular polysaccharide production from Ganoderma formosanum using computational modeling. J. Food Drug Anal. 2017, 25, 804–811. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Liu, Y.W.; Gao, J.L.; Guan, J.; Qian, Z.M.; Feng, K.; Li, S.P. Evaluation of antiproliferative activities and action mechanisms of extracts from two species of Ganoderma on tumor cell lines. J. Agric. Food Chem. 2009, 57, 3087–3093. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A mechanistic review on medicinal mushrooms-derived bioactive compounds: Potential mycotherapy candidates for alleviating neurological disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.V.; Gavaric, N.S.; Bozin, B.N. Ganoderma: Insights into anticancer effects. Eur. J. Cancer Prev. 2016, 25, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Z. Immunomodulating effect of Ganoderma (Lingzhi) and possible mechanism. Adv. Exp. Med. Biol. 2019, 1182, 1–37. [Google Scholar] [CrossRef]
- Lai, C.Y.; Hung, J.T.; Lin, H.H.; Yu, A.L.; Chen, S.H.; Tsai, Y.C.; Shao, L.E.; Yang, W.B.; Yu, J. Immunomodulatory and adjuvant activities of a polysaccharide extract of Ganoderma lucidum in vivo and in vitro. Vaccine 2010, 28, 4945–4954. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.L.; Lu, C.Y.; Hsueh, Y.C.; Liu, W.H.; Chen, C.J. Activation of antitumor immune responses by Ganoderma formosanum polysaccharides in tumor-bearing mice. Appl. Microbiol. Biotechnol. 2014, 98, 9389–9398. [Google Scholar] [CrossRef]
- Hsu, W.H.; Qiu, W.L.; Tsao, S.M.; Tseng, A.J.; Lu, M.K.; Hua, W.J.; Cheng, H.C.; Hsu, H.Y.; Lin, T.Y. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer. Int. J. Biol. Macromol. 2020, 165, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12: A cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998, 70, 83–243. [Google Scholar] [CrossRef]
- Irinoda, K.; Masihi, K.N.; Chihara, G.; Kaneko, Y.; Katori, T. Stimulation of microbicidal host defence mechanisms against aerosol influenza virus infection by lentinan. Int. J. Immunopharmacol. 1992, 14, 971–977. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Ding, Z.; Zhao, Y.; Yang, Y.; Sun, Q.; Yang, X.; Ge, W.; Xu, X.; Cheng, R.; et al. Soluble beta-glucan salecan improves vaginal infection of Candida albicans in mice. Int. J. Biol. Macromol. 2020, 148, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Masterson, C.H.; Murphy, E.J.; Gonzalez, H.; Major, I.; McCarthy, S.D.; O’Toole, D.; Laffey, J.G.; Rowan, N.J. Purified beta-glucans from the Shiitake mushroom ameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020, 71, 405–412. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chang, T.T.; Chen, T. Ganoderma formosanum sp.nov. on Formosan sweet gum in Taiwan. Trans. Br. Mycol. Soc. 1984, 82, 731–733. [Google Scholar] [CrossRef]

| Genes | Primer Sequence |
|---|---|
| IL-2 | F: 5′-GCCCCAAGGGCTCAAAAATG-3′ R: 5′-GCGCTTACTTTGTGCTGTCC-3′ |
| IL-3 | F: 5′-GCCAGGGGTCTTCATTCGAG-3′ R: 5′-TTCCACGGTTCCACGGTTAG-3′ |
| IL-4 | F: 5′-GATCCCCGGGCAGAGC-3′ R: 5′-TGTCGCATCCGTGGATATGG-3′ |
| IL-6 | F: 5′-GCCTTCTTGGGACTGATGCT-3′ R: 5′-GACAGGTCTGTTGGGAGTGG-3′ |
| IL-7 | F: 5′-GCTGCAGTCCCAGTCATCA-3′ R: 5′-TGTGACAGGCAGCAGAACAA-3′ |
| IL-10 | F: 5′-GCTCTTGCACTACCAAAGCC-3′ R: 5′-CTGCTGATCCTCATGCCAGT-3′ |
| IL-12 | F: 5′-CGCCCTCCTCACACAGATAG-3′ R: 5′-ATGCAGCCTCGGGTATTCTG-3′ |
| IL-15 | F: 5′-GGGATCCTGCTGTGTTTGGA-3′ R: 5′-AGCAAGGACCATGAAGAGGC-3′ |
| IL-18 | F: 5′-ATGCTTTCTGGACTCCTGCC-3′ R: 5′-ATTGTTCCTGGGCCAAGAGG-3′ |
| M-CSF | F: 5′-TCAAAGGGTGGGACAGCATC-3′ R: 5′-GTCTCCCTCCTTCCTGGCTA-3′ |
| IFN-α | F: 5′-GTTGGAAAGTTAGAGGAGGGCA-3′ R: 5′-TGCTCCTTCCCCTCTAGGTC-3′ |
| IFN-γ | F: 5′-ACTGTGATTGCGGGGTTGTA-3′ R: 5′-ACATTCGAGTGCTGTCTGGC-3′ |
| Foxp3 | F: 5′-ACTGACCAAGGCTTCATCTGTG-3′ R: 5′-GGAACTCTGGGAATGTGCTGT-3′ |
| Notch1 | F: 5′-CCGGTGAGACCTGCCTGAAT-3′ R: 5′-GCACTTGTACTCCGTCAGCG-3′ |
| GAPDH | F: 5′-TCAACAGCAACTCCCACTCTTCCA-3′ R: 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, H.-C.; Liu, Y.-W.; Lum, C.-C.; Hsu, K.-D.; Lin, S.-P.; Hsieh, C.-W.; Lin, H.-W.; Lu, T.-Y.; Cheng, K.-C. Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation. Int. J. Mol. Sci. 2021, 22, 11251. https://doi.org/10.3390/ijms222011251
Kuo H-C, Liu Y-W, Lum C-C, Hsu K-D, Lin S-P, Hsieh C-W, Lin H-W, Lu T-Y, Cheng K-C. Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation. International Journal of Molecular Sciences. 2021; 22(20):11251. https://doi.org/10.3390/ijms222011251
Chicago/Turabian StyleKuo, Hsing-Chun, Yen-Wenn Liu, Chi-Chin Lum, Kai-Di Hsu, Shin-Ping Lin, Chang-Wei Hsieh, Hui-Wen Lin, Tze-Ying Lu, and Kuan-Chen Cheng. 2021. "Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation" International Journal of Molecular Sciences 22, no. 20: 11251. https://doi.org/10.3390/ijms222011251






