Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis of Morus macroura
2.1.1. Total Phenolic Contents of Morus macroura
2.1.2. Total Flavonoid Contents of Morus macroura
2.1.3. Free Radical Scavenging Activity
2.2. Characterization of UV-Mediated Green Synthesized AgNPs, ZnONPs, and Bimetallic Ag–ZnONPs
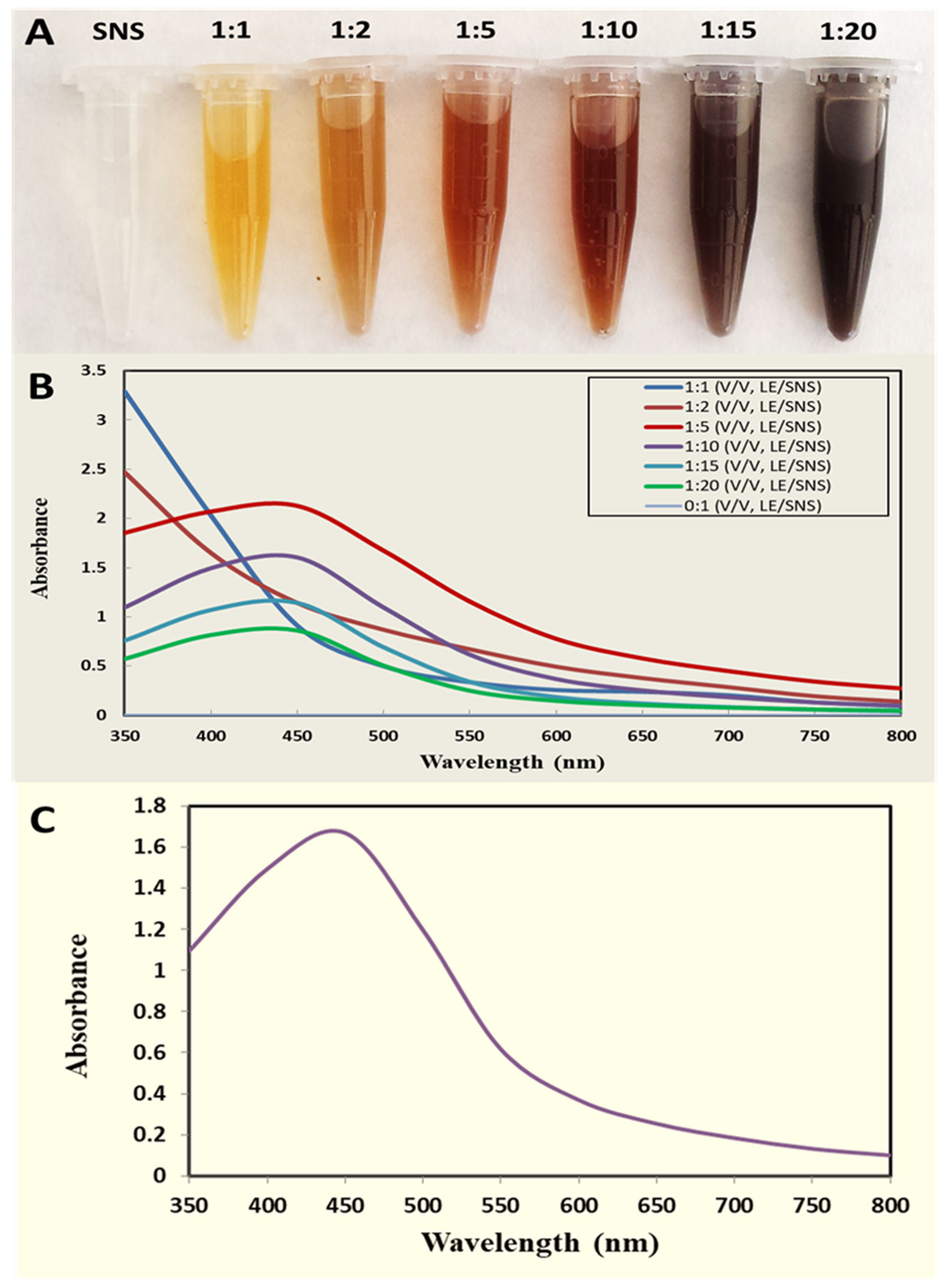
2.2.1. UV–Visible Spectroscopy
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.3. Scanning Electron Microscope Analysis
2.2.4. Energy Dispersive X-ray Analysis
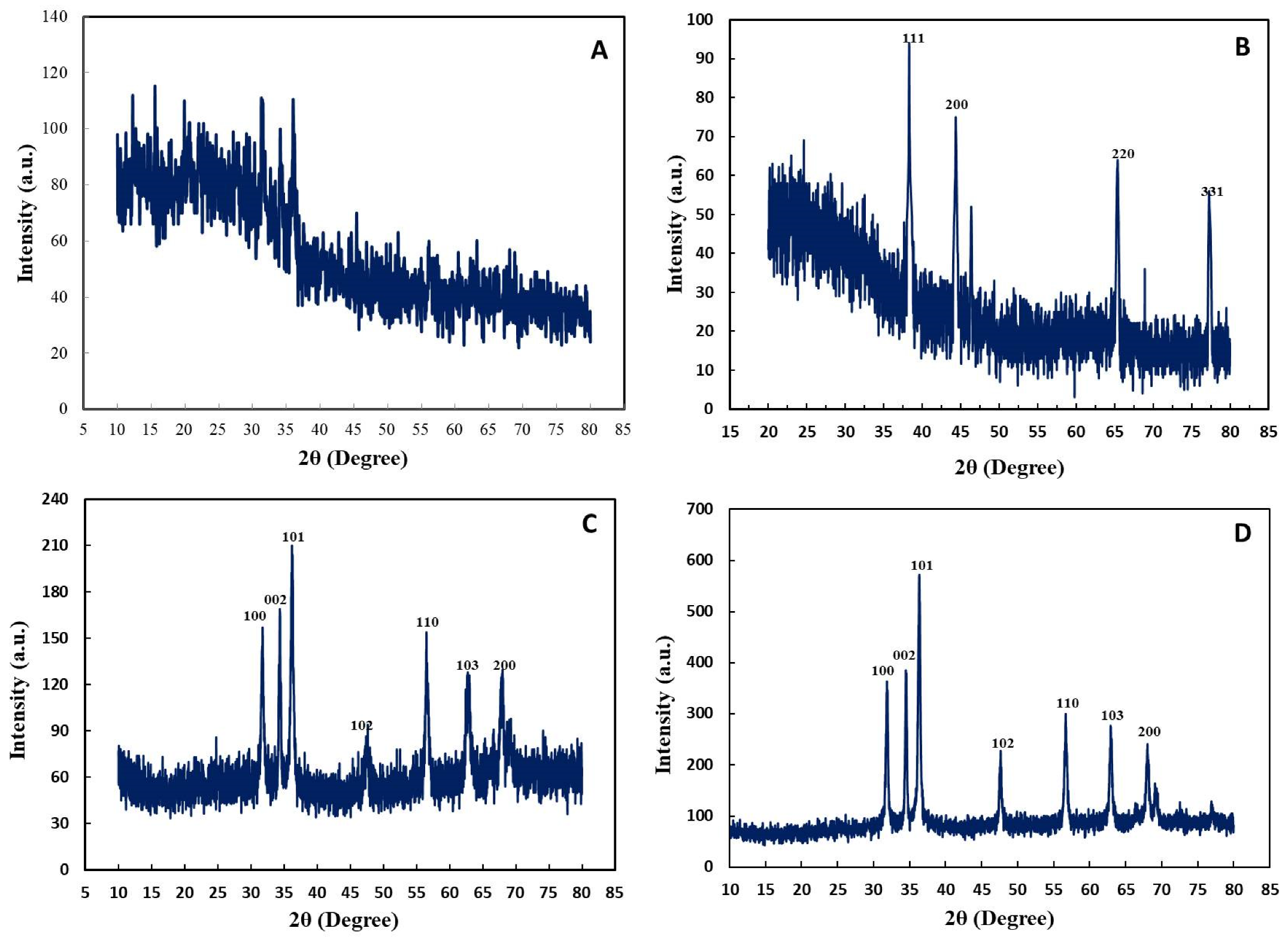
2.2.5. X-ray Diffraction Analysis
2.3. Biocompatibility Studies of Green Synthesized AgNPs, ZnONPs, and Bimetallic Ag–ZnONPs
2.4. Anti-Diabetic and Anti-Glycation Activities of Green Synthesized AgNPs, ZnONPs, and Bimetallic Ag–ZnONPs
2.5. Anti-Cancerous Activities of Green Synthesized AgNPs, ZnONPs, and Bimetallic Ag–ZnONPs
2.5.1. Cell Viability Assay by MTT
2.5.2. Measurement of Intracellular ROS/RNS Production
2.5.3. Measurement of Mitochondrial Membrane Potential
2.5.4. Caspase-3 Gene Expression and Caspase-3/7 Activity
3. Materials and Methods
3.1. Preparation of Aqueous Leaf Extract of Morus macroura
3.2. Phytochemical Analysis of Morus macroura
3.2.1. Total Phenolic Contents
3.2.2. Total Flavonoid Contents
3.2.3. Free Radical Scavenging Activity (FRSA)
3.3. UV-Mediated Green Synthesis of Nanoparticles
3.3.1. AgNPs
3.3.2. ZnONPs
3.3.3. Bimetallic Ag–ZnONPs
3.4. Characterization of UV-Mediated Green Synthesized NPs
3.4.1. UV–Visible Spectroscopy
3.4.2. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR-FT-IR)
3.4.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analyses
3.4.4. X-ray Diffraction Analysis
3.5. Biocompatibility Studies
3.5.1. Brine Shrimp Lethality Assay
3.5.2. Biocompatibility with Human Red Blood Cells (hRBCs)
3.6. Anti-Diabetic Activities of Green Synthesized NPs
3.6.1. α-Glucosidase Inhibition
3.6.2. α-Amylase Inhibition
3.7. Vesperlysine and Pentosidine-like AGEs Activity
3.8. Anti-Cancerous Activity of Green Synthesized NPs
3.8.1. Cell Viability Assay by MTT
3.8.2. Measurement of Intracellular Reactive Oxygen and Nitrogen Species (ROS/RNS)
3.8.3. Measurement of Mitochondrial Membrane Potential (MMP)
3.8.4. Caspase-3 Gene Expression and Caspase-3/7 Activity
3.9. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Handayani, D.; Putri, D.; Oktaviani, M. Antimicrobial activity of endophytic fungi from Andalas (Morus macroura Miq.) plant. J. Phys. Conf. Ser. 2021, 1940, 012050. [Google Scholar] [CrossRef]
- Farrag, E.; Kassem, M.; Bayoumi, D.; Shaker, S.; Afifi, M. Phytochemical study, phenolic profile and antigastric ulcer activity of Morus macroura Miq. fruits extract. J. Appl. Pharm. Sci. 2017, 7, 152–160. [Google Scholar]
- Jha, A.K.; Prasad, K.; Kumar, V.; Prasad, K. Biosynthesis of silver nanoparticles using Eclipta leaf. Biotechnol. Prog. 2009, 25, 1476–1479. [Google Scholar] [CrossRef]
- Mittelman, A.M.; Fortner, J.D.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J. Photochem. Photobiol. B Biol. 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef]
- Mukha, I.; Vityuk, N.; Grodzyuk, G.; Shcherbakov, S.; Lyberopoulou, A.; Efstathopoulos, E.; Gazouli, M. Anticancer Effect of Ag, Au, and Ag/Au Bimetallic Nanoparticles Prepared in the Presence of Tryptophan. J. Nanosci. Nanotechnol. 2017, 17, 8987–8994. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Dalal, S.; Kataria, S.K. A review on ameliorative green nanotechnological approaches in diabetes management. Biomed. Pharmacother. 2020, 127, 110198. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 420204. [Google Scholar] [CrossRef] [Green Version]
- Kalpana, V.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Gupta, V.; Yadav, T.P.; Gupta, V.; Singh, S.P. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater. Chem. Phys. 2018, 203, 40–48. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Zak, A.K.; Zamiri, R.; Hakimi, M. Fabrication and characterization of gelatin stabilized silver nanoparticles under UV-light. Int. J. Mol. Sci. 2011, 12, 6346–6356. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Negro, C.; Aprile, A.; De Bellis, L.; Miceli, A. Nutraceutical Properties of Mulberries Grown in Southern Italy (Apulia). Antioxidants 2019, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Kanwal, S.; Shabir, G.; Alkharfy, K.; Gilani, A.-H. Antioxidant and Antimicrobial Attributes of Different Solvent Extracts from Leaves of Four Species of Mulberry. Int. J. Pharmacol. 2015, 11, 757–765. [Google Scholar] [CrossRef]
- Mat Yusuf, S.N.A.; Che Mood, C.N.A.; Ahmad, N.H.; Sandai, D.; Lee, C.K.; Lim, V. Optimization of biogenic synthesis of silver nanoparticles from flavonoid-rich Clinacanthus nutans leaf and stem aqueous extracts. R. Soc. Open Sci. 2020, 7, 200065. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Nayagam, V.; Gabriel, M.; Palanisamy, K. Green synthesis of silver nanoparticles mediated by Coccinia grandis and Phyllanthus emblica: A comparative comprehension. Appl. Nanosci. 2018, 8, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Al-Asady, Z.M.; Al-Hamdani, A.H.; Hussein, M.A. Study the optical and morphology properties of zinc oxide nanoparticles. Proc. AIP Conf. Proc. 2021, 2213, 020061. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Khalil, A.T.; Ali, M.; Numan, M.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine 2019, 14, 655–673. [Google Scholar] [CrossRef]
- Ehsan, M.; Raja, N.I.; Mashwani, Z.u.R.; Ikram, M.; Zohra, E.; Zehra, S.S.; Abasi, F.; Hussain, M.; Iqbal, M.; Mustafa, N. Responses of bimetallic Ag/ZnO alloy nanoparticles and urea on morphological and physiological attributes of wheat. IET Nanobiotechnol. 2021, 15, 602–610. [Google Scholar] [CrossRef]
- Suica-Bunghez, I.-R.; Dumitrescu, O.; Somoghi, R.; Ionita, I.; Ion, R.-M. Silver nanoparticles via Morus Nigra Extract: Synthesis and Antioxidant Activity. Rev. Chim. Buchar. Orig. Ed. 2015, 66, 1112–1115. [Google Scholar]
- Some, S.; Bulut, O.; Biswas, K.; Kumar, A.; Roy, A.; Sen, I.; Mandal, A.; Franco, O.; Ince, I.A.; Neog, K.; et al. Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L. (Lepidoptera: Bombycidae). Sci. Rep. 2019, 9, 14839. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lafta, A.; Alsultani, A.; Ali, H.; Ismael, H.; Jameel, A.; Mohammed, A.; Mubarak, I. Study Self-cleaning of Congo Red from Cotton Fabric Loaded by Zno-Ag. Int. J. Chem. 2015, 7, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Rheima, A.M.; Mohammed, M.A.; Jaber, S.H.; Hameed, S.A. Synthesis of silver nanoparticles using the UV-irradiation technique in an antibacterial application. J. Southwest Jiaotong Univ. 2019, 54, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Nguyen, T.D.; Cao, M.T.; Pham, V.V. Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124659. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Rustaiyan, A.; Ibrahim, N.A.; Zargar, M.; Abdollahi, Y. Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV irradiation method and evaluation of antibacterial activity. Int. J. Nanomed. 2010, 5, 875. [Google Scholar] [CrossRef] [Green Version]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, A.; Naz, S.; Ali, J.S.; Mannan, A.; Zia, M. Synthesis, characterization and biological activities of monometallic and bimetallic nanoparticles using Mirabilis jalapa leaf extract. Biotechnol. Rep. 2019, 22, e00338. [Google Scholar] [CrossRef] [PubMed]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Taghavi Fardood, S. Biosynthesis of Ag, ZnO and bimetallic Ag/ZnO alloy nanoparticles by aqueous extract of oak fruit hull (Jaft) and investigation of photocatalytic activity of ZnO and bimetallic Ag/ZnO for degradation of basic violet 3 dye. J. Mater. Sci. Mater. Electron. 2018, 29, 2806–2814. [Google Scholar] [CrossRef]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef] [Green Version]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alghamdi, A.A.; Al-Zaqri, N.; Alanazi, H.S.; Alsyahi, A.A.; Marghany, A.E.; Ahmad, N. Facile one-pot green synthesis of Ag–ZnO Nanocomposites using potato peeland their Ag concentration dependent photocatalytic properties. Sci. Rep. 2020, 10, 20229. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pan, Y.; Li, N.; Wang, Q.; Chen, Y.; Phisalaphong, M.; Chen, H. Antibacterial and cytotoxic activities of a green synthesized silver nanoparticles using corn silk aqueous extract. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 598, 124827. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Rajabi, S.; Ramazani, A.; Hamidi, M.; Naji, T. Artemia salina as a model organism in toxicity assessment of nanoparticles. DARU J. Pharm. Sci. 2015, 23, 20. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green bio-assisted synthesis, characterization and biological evaluation of biocompatible ZnO NPs synthesized from different tissues of milk thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943. [Google Scholar] [CrossRef]
- Paul, R.; Roy, A.; Rajeshkumar, S.; Thangavelu, D.L. Cytotoxic Effect and Antibacterial Activity of Coriander Oleoresin Mediated Zinc Oxide Nanoparticles. Int. J. Pharm. Res. 2020, 12, 3057–3062. [Google Scholar] [CrossRef]
- Kumar, N.H.; Murali, M.; Satish, A.; Singh, S.B.; Gowtham, H.G.; Mahesh, H.M.; Lakshmeesha, T.R.; Amruthesh, K.N.; Jagannath, S. Bioactive and Biocompatible Nature of Green Synthesized Zinc Oxide Nanoparticles from Simarouba glauca DC.: An Endemic Plant to Western Ghats, India. J. Clust. Sci. 2020, 31, 523–534. [Google Scholar] [CrossRef]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Pugazhendhi, A.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102289. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Chandrashekar, B.N.; Ramakrishna, S.; et al. Novel Green Biomimetic Approach for Synthesis of ZnO-Ag Nanocomposite; Antimicrobial Activity against Food-borne Pathogen, Biocompatibility and Solar Photocatalysis. Sci. Rep. 2019, 9, 8303. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Li, M.; Chang, R.; Xiong, L.; Sun, Q. In vitro inhibition of pancreatic α-amylase by spherical and polygonal starch nanoparticles. Food Funct. 2018, 9, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Arvanag, F.; Bayrami, A.; Habibi-Yangjeh, A.; Rahim Pouran, S. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L. seed extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Bakur, A.; Elshaarani, T.; Niu, Y.; Chen, Q. Comparative study of antidiabetic, bactericidal, and antitumor activities of MEL@ AgNPs, MEL@ ZnONPs, and Ag–ZnO/MEL/GA nanocomposites prepared by using MEL and gum arabic. RSC Adv. 2019, 9, 9745–9754. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Fatma, S.; Abdullah, S.M.; Iqbal, J.; Madkhali, A.; Hamali, A.H.; Ahmad, S.; Jerah, A.; Echeverria, V. Inhibiting effect of zinc oxide nanoparticles on advanced glycation products and oxidative modifications: A potential tool to counteract oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2018, 55, 7438–7452. [Google Scholar] [CrossRef]
- Zgura, I.; Enculescu, M.; Istrate, C.; Negrea, R.; Bacalum, M.; Nedelcu, L.; Barbinta-Patrascu, M.E. Performant Composite Materials Based on Oxide Semiconductors and Metallic Nanoparticles Generated from Cloves and Mandarin Peel Extracts. Nanomaterials 2020, 10, 2146. [Google Scholar] [CrossRef]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Al Ali, A.A.A.L.A. Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Guo, D.; Bi, H.; Liu, B.; Wu, Q.; Wang, D.; Cui, Y. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol. Vitr. 2013, 27, 731–738. [Google Scholar] [CrossRef]
- Ghaemi, B.; Shaabani, E.; Najafi-Taher, R.; Jafari Nodooshan, S.; Sadeghpour, A.; Kharrazi, S.; Amani, A. Intracellular ROS induction by Ag@ ZnO core–shell nanoparticles: Frontiers of permanent optically active holes in breast cancer theranostic. ACS Appl. Mater. Interfaces 2018, 10, 24370–24381. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. Vitr. 2013, 27, 330–338. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, X.; Xu, L.; Liu, X.; Zhu, X.; Song, E.; Song, Y. Zinc oxide nanoparticles effectively regulate autophagic cell death by activating autophagosome formation and interfering with their maturation. Part. Fibre Toxicol. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, X.; Hao, X.; Ren, Q.; Gao, J.; Wang, Y.; Chang, N.; Qiu, Y.; Song, G. Sodium Fluoride Induces Apoptosis in H9c2 Cardiomyocytes by Altering Mitochondrial Membrane Potential and Intracellular ROS Level. Biol. Trace Elem. Res. 2015, 166, 210–215. [Google Scholar] [CrossRef]
- Li, J.-H.; Liu, X.-R.; Zhang, Y.; Tian, F.-F.; Zhao, G.-Y.; Yu, Q.-L.-Y.; Jiang, F.-L.; Liu, Y. Toxicity of nano zinc oxide to mitochondria. Toxicol. Res. 2012, 1, 137–144. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, J.; Huang, Y.; Gao, X.; Kong, L.; Zhang, T.; Tang, M. Comparative cytotoxicity and apoptotic pathways induced by nanosilver in human liver HepG2 and L02 cells. Hum. Exp. Toxicol. 2018, 37, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Lafuente, D.; Gómez, M.; García, T.; Domingo, J.L.; Sánchez, D.J. Polyvinyl pyrrolidone-coated silver nanoparticles in a human lung cancer cells: Time- and dose-dependent influence over p53 and caspase-3 protein expression and epigenetic effects. Arch. Toxicol. 2017, 91, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.-J.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Phulukdaree, A.; Gengan, R.M.; Anand, K.; Chuturgoon, A.A. Silver nanoparticles of Albizia adianthifolia: The induction of apoptosis in human lung carcinoma cell line. J. Nanobiotechnol. 2013, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-W.; Lee, C.-H.; Lin, M.-S.; Chi, C.-W.; Chen, Y.-J.; Wang, G.-S.; Liao, K.-W.; Chiu, L.-P.; Wu, S.-H.; Huang, D.-M. ZnO nanoparticles induced caspase-dependent apoptosis in gingival squamous cell carcinoma through mitochondrial dysfunction and p70S6K signaling pathway. Int. J. Mol. Sci. 2020, 21, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.-J.; Jeong, M.-S.; Choi, D.-M.; Kim, K.-N.; Wie, M.-B. Zinc Oxide Nanoparticles Induce Autophagy and Apoptosis via Oxidative Injury and Pro-Inflammatory Cytokines in Primary Astrocyte Cultures. Nanomaterials 2019, 9, 1043. [Google Scholar] [CrossRef] [Green Version]
- Bratton, S.B.; Walker, G.; Srinivasula, S.M.; Sun, X.M.; Butterworth, M.; Alnemri, E.S.; Cohen, G.M. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001, 20, 998–1009. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 208–217. [Google Scholar] [CrossRef]
- Kang, B.; Austin, L.A.; El-Sayed, M.A. Observing real-time molecular event dynamics of apoptosis in living cancer cells using nuclear-targeted plasmonically enhanced Raman nanoprobes. ACS Nano 2014, 8, 4883–4892. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Yuan, S.; Yu, X.; Topf, M.; Ludtke, S.J.; Wang, X.; Akey, C.W. Structure of an apoptosome-procaspase-9 CARD complex. Structure 2010, 18, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Kamtekar, S.; Keer, V.; Patil, V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharm. Sci. 2014, 4, 61. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.; Abbasi, B.H.; Hano, C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2017, 129, 73–87. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar]
- Abbasi, B.H.; Anjum, S.; Hano, C. Differential effects of in vitro cultures of Linum usitatissimum L. (Flax) on biosynthesis, stability, antibacterial and antileishmanial activities of zinc oxide nanoparticles: A mechanistic approach. RSC Adv. 2017, 7, 15931–15943. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.-P.; Hano, C. A critical cross-species comparison of pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for authentication of Thai medicinal herbal tea. Plants 2020, 9, 921. [Google Scholar] [CrossRef]
- Ahmed, M.; Fatima, H.; Qasim, M.; Gul, B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complement. Altern. Med. 2017, 17, 386. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M. Sageretia thea (Osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications. Nanomedicine 2017, 12, 1767–1789. [Google Scholar] [CrossRef]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorg. Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef] [PubMed]














| Nanoparticles Type | Average Diameter (nm) |
|---|---|
| Control AgNPs | 147.23 |
| UV-C mediated AgNPs | 22.93 |
| Control ZnONPs | 53.21 |
| UV-C mediated ZnONPs | 37.03 |
| Control Ag–ZnONPs (0.1/0.1) | 24. 21 |
| UV-C mediated Ag–ZnONPs (0.1/0.1) | 21.69 |
| Control Ag–ZnONPs (0.1/0.5) | 40.11 |
| UV-C mediated Ag–ZnONPs (0.1/0.5) | 23.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, S.; Khan, A.K.; Qamar, A.; Fatima, N.; Drouet, S.; Renouard, S.; Blondeau, J.P.; Abbasi, B.H.; Hano, C. Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles. Int. J. Mol. Sci. 2021, 22, 11294. https://doi.org/10.3390/ijms222011294
Anjum S, Khan AK, Qamar A, Fatima N, Drouet S, Renouard S, Blondeau JP, Abbasi BH, Hano C. Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles. International Journal of Molecular Sciences. 2021; 22(20):11294. https://doi.org/10.3390/ijms222011294
Chicago/Turabian StyleAnjum, Sumaira, Amna Komal Khan, Anza Qamar, Noor Fatima, Samantha Drouet, Sullivan Renouard, Jean Philippe Blondeau, Bilal Haider Abbasi, and Christophe Hano. 2021. "Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles" International Journal of Molecular Sciences 22, no. 20: 11294. https://doi.org/10.3390/ijms222011294
APA StyleAnjum, S., Khan, A. K., Qamar, A., Fatima, N., Drouet, S., Renouard, S., Blondeau, J. P., Abbasi, B. H., & Hano, C. (2021). Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles. International Journal of Molecular Sciences, 22(20), 11294. https://doi.org/10.3390/ijms222011294








