Simultaneous Inhibition of PD-1 and Stimulation of CD40 Signaling Pathways by Anti-PD-L1/CD40L Bispecific Fusion Protein Synergistically Activate Target and Effector Cells
Abstract
1. Introduction
2. Results
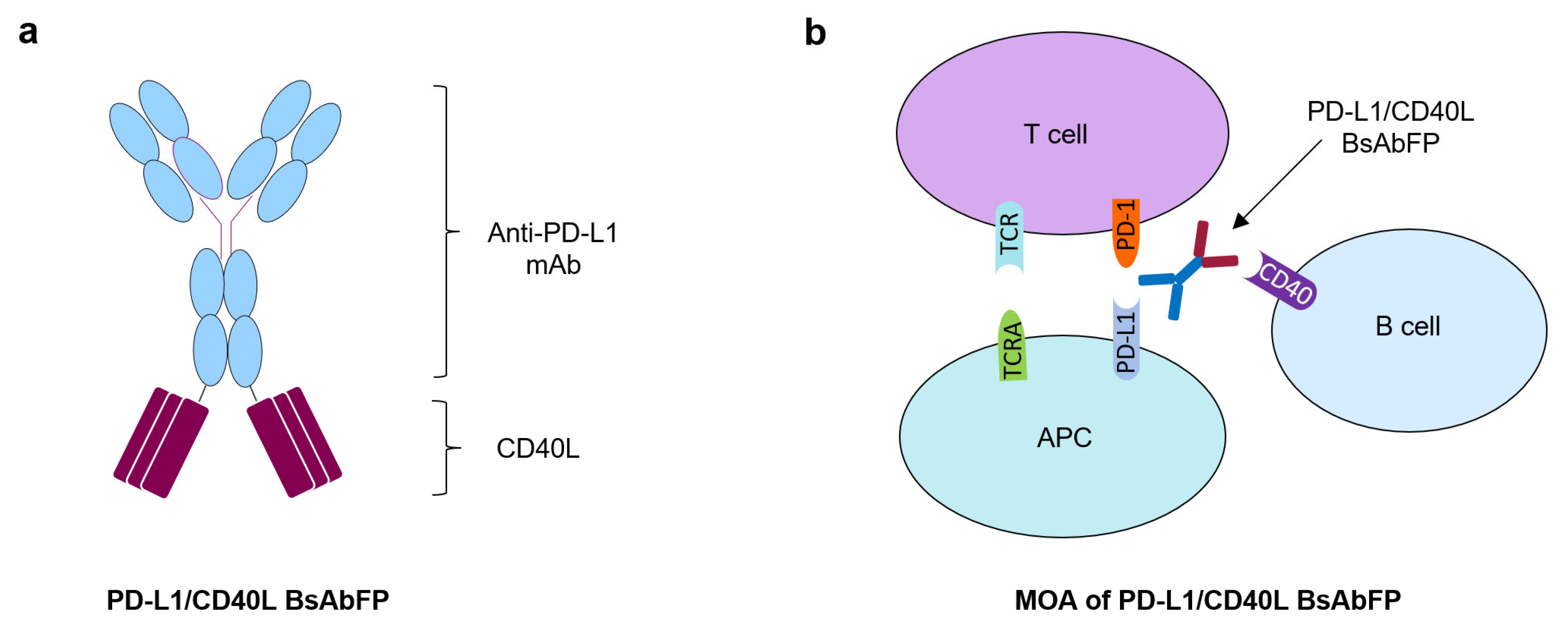
2.1. Generation of Anti-PD-L1/CD40L Bispecific Fusion Protein
2.2. Assessment of Anti-PD-L1 Activity of Anti-PD-L1/CD40L BsAbFP
2.3. Assessment of CD40L Activity of Anti-PD-L1/CD40L BsAbFP
2.4. Design of “Three-Cell-in-One” Dual Target Reporter Gene Bioassay for Anti-PD-L1/CD40L BsAbFP
2.5. Potency Assessment of Anti-PD-L1/CD40L BsAbFP by Dual Target Reporter Gene Bioassay
2.6. Characterization of PD-L1/CD40 Dual Target Reporter Bioassay
2.6.1. Assessment of the Role of Raji/NFkB-Luc Cells on the PD-L1/CD40L Dual Target Reporter Bioassay
2.6.2. Assessment of the Role of Jurkat/PD-1/NFAT-Luc Cells on the PD-L1/CD40L Dual Target Reporter Bioassay
2.7. Stability and Specificity Assessment of PD-L1/CD40 Dual Target Reporter Bioassay
2.8. Linearity Assessment of PD-L1/CD40 Dual Target Reporter Assay
2.9. Qualification of PD-L1/CD40L Dual Target Reporter Bioassay
2.10. Evaluation of Structure-Function Attributes Using Different Bioassays and Analytical Methods
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. PD-1 Bioassay
4.3. CD40 Bioassay
4.4. PD-L1/CD40L Dual-Target Reporter Gene Bioassay
4.5. Size Exclusion Chromatography (SEC)
4.6. Peptide Mapping for PTM Measurements
5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BsAb | Bispecific antibody |
| BsAbFP | Bispecific antibody fusion protein |
| mAb | Monoclonal antibody |
| MOA | Mechanism of action |
| APC | Antigen presenting cell |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
References
- Dahlén, E.; Veitonmäki, N.; Norlén, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2018, 6, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Kontermann, R.E. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 2010, 24, 89–98. [Google Scholar] [CrossRef]
- May, C.; Sapra, P.; Gerber, H.-P. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem. Pharmacol. 2012, 84, 1105–1112. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Khong, A.; Nelson, D.J.; Nowak, A.K.; Lake, R.A.; Robinson, B.W.S. The Use of Agonistic Anti-CD40 Therapy in Treatments for Cancer. Int. Rev. Immunol. 2012, 31, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. CD40/CD154 Interactions at the Interface of Tolerance and Immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Matsushima, M.; Hashimoto, N.; Imaizumi, K.; Hasegawa, Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011, 73, 69–78. [Google Scholar]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Chand Dakal, T.; Agarwal, D.; Gupta, R.; Nagda, G.; Meena, A.R.; Dhakar, R.; Menon, A.; Mathur, R.; Mona, S.; Yadav, V.; et al. Mechanistic basis of co-stimulatory CD40-CD40L ligation mediated regulation of im-mune responses in cancer and autoimmune disorders. Immunobiology 2019, 225, 151899. [Google Scholar] [CrossRef] [PubMed]
- Homig-Holzel, C.; Hojer, C.; Rastelli, J.; Casola, S.; Strobl, L.J.; Müller, W.; Quintanilla-Martinez, L.; Gewies, A.; Ruland, J.; Rajewsky, K.; et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J. Exp. Med. 2008, 205, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Cohen, D.; Belmar, N.A.; Choi, D.; Tan, S.S.; Sho, M.; Akamatsu, Y.; Kim, H.; Iyer, R.; Cabel, J.M.; et al. A Bispecific Molecule Targeting CD40 and Tumor Antigen Mesothelin Enhances Tumor-Specific Immunity. Cancer Immunol. Res. 2019, 7, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Luheshi, N.M.; Coates-Ulrichsen, J.; Harper, J.; Mullins, S.; Sulikowski, M.G.; Martin, P.; Brown, L.; Lewis, A.; Davies, G.; Morrow, M.; et al. Transformation of the tumour microenvironment by a CD40 agonist antibody corre-lates with improved responses to PD-L1 blockade in a mouse orthotopic pancreatic tumour model. Oncotarget 2016, 7, 18508–18520. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Challis, R.; Chowdhury, F.; Gao, Y.; Harvey, M.; Geldart, T.; Kerr, P.; Chan, C.; Smith, A.; Steven, N.; et al. Clinical and Biological Effects of an Agonist Anti-CD40 Antibody: A Cancer Research UK Phase I Study. Clin. Cancer Res. 2015, 21, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, G.; Li, W.; Li, X.; Zhao, C.; Jiang, T.; Jia, Y.; He, Y.; Li, A.; Su, C.; et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019, 130, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Myers, L.; Muralimohan, G.; Dai, J.; Qiao, Y.; Li, Z.; Mittler, R.S.; Vella, A.T. 4-1BB and OX40 Dual Costimulation Synergistically Stimulate Primary Specific CD8 T Cells for Robust Effector Function. J. Immunol. 2004, 173, 3002–3012. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Cheng, D.; Xia, Z.; Luan, M.; Zhang, S. PD-1 Blockade and OX40 Triggering Synergistically Protects against Tumor Growth in a Murine Model of Ovarian Cancer. PLoS ONE 2014, 9, e89350. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Vianden, C.; Cantwell, M.J.; Dai, Z.; Xiao, Z.; Sharma, M.; Khong, H.; Jaiswal, A.; Faak, F.; Hailemichael, Y.; et al. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M. PD-1/PD-L1 blockage in cancer treatment—From basic research to clinical application. Int. J. Clin. Oncol. 2016, 21, 447. [Google Scholar] [CrossRef][Green Version]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed]
- Mineva, N.D.; Rothstein, T.L.; Meyers, J.A.; Lerner, A.; Sonenshein, G.E. CD40 ligand-mediated activation of the de novo RelB NF-kappaB synthesis pathway in transformed B cells promotes rescue from apoptosis. J. Biol. Chem. 2007, 282, 17475–17485. [Google Scholar] [CrossRef]
- Attanasio, J.; Wherry, E.J. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity 2016, 44, 1052–1068. [Google Scholar] [CrossRef]
- Chen, W.; Pandey, M.; Sun, H.; Rolong, A.; Cao, M.; Liu, D.; Wang, J.; Zeng, L.; Hunter, A.; Lin, S. Development of a mechanism of action-reflective, dual target cell-based reporter bioassay for a bispecific monoclonal antibody targeting human CTLA-4 and PD-1. mAbs 2021, 13, 1914359. [Google Scholar] [CrossRef]
- Sotomayor, E.M.; Borrello, I.; Tubb, E.; Rattis, F.-M.; Bien, H.; Lu, Z.; Fein, S.; Schoenberger, S.; Levitsky, H.I. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 1999, 5, 780–787. [Google Scholar] [CrossRef]
- Bhadra, R.; Cobb, D.A.; Khan, I.A. CD40 Signaling to the Rescue: A CD8 Exhaustion Perspective in Chronic Infectious Diseases. Crit. Rev. Immunol. 2013, 33, 361–378. [Google Scholar] [CrossRef]
- Ma, H.S.; Poudel, B.; Torres, E.R.; Sidhom, J.-W.; Robinson, T.M.; Christmas, B.; Scott, B.; Cruz, K.; Woolman, S.; Wall, V.Z.; et al. A CD40 Agonist and PD-1 Antagonist Antibody Reprogram the Microenvironment of Nonimmunogenic Tumors to Allow T-cell–Mediated Anticancer Activity. Cancer Immunol. Res. 2019, 7, 428–442. [Google Scholar] [CrossRef]
- Yaya Wang, S.T.; Malhotra, D.; Gilbreth, R.; Wang, Y. Abstract 5178: MEDI7526: A novel bispecif-ic antibody that activates the CD40 pathway and down-regulates cell surface PD-L1 expression. In Proceedings of the AACR Annual Meeting, Philadelphia, PA, USA, 27–28 April 2020. [Google Scholar]
- Morrison, A.H.; Diamond, M.S.; Hay, C.A.; Byrne, K.T.; Vonderheide, R.H. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc. Natl. Acad. Sci. USA 2020, 117, 8022–8031. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Acceptance Criteria | Results |
|---|---|---|
| Linearity Assay range | R2 ≥ 0.970 60–167% relative potency | R2 = 0.996 60–167% relative potency |
| Accuracy | 80–125% across assay range 60–167% relative potency | Accuracy: 92.0–109.8% |
| Precision | Repeatability: %GCV: ≤15% Intermediate precision: %GCV: ≤20% | Repeatability: %GCV = 7.7% Intermediate Precision: %GCV = 14.2% |
| Specificity | No concentration-response to formulation buffer and structurally similar but functionally irrelevant antibody | No concentration-response for formulation buffer and irrelevant antibody |
| Stability Indicating | Detects a decrease in potency of stressed samples | Demonstrated a decrease in potency of UV- and thermal-stressed samples |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, M.S.; Wang, C.; Umlauf, S.; Lin, S. Simultaneous Inhibition of PD-1 and Stimulation of CD40 Signaling Pathways by Anti-PD-L1/CD40L Bispecific Fusion Protein Synergistically Activate Target and Effector Cells. Int. J. Mol. Sci. 2021, 22, 11302. https://doi.org/10.3390/ijms222111302
Pandey MS, Wang C, Umlauf S, Lin S. Simultaneous Inhibition of PD-1 and Stimulation of CD40 Signaling Pathways by Anti-PD-L1/CD40L Bispecific Fusion Protein Synergistically Activate Target and Effector Cells. International Journal of Molecular Sciences. 2021; 22(21):11302. https://doi.org/10.3390/ijms222111302
Chicago/Turabian StylePandey, Madhu S., Chunlei Wang, Scott Umlauf, and Shihua Lin. 2021. "Simultaneous Inhibition of PD-1 and Stimulation of CD40 Signaling Pathways by Anti-PD-L1/CD40L Bispecific Fusion Protein Synergistically Activate Target and Effector Cells" International Journal of Molecular Sciences 22, no. 21: 11302. https://doi.org/10.3390/ijms222111302
APA StylePandey, M. S., Wang, C., Umlauf, S., & Lin, S. (2021). Simultaneous Inhibition of PD-1 and Stimulation of CD40 Signaling Pathways by Anti-PD-L1/CD40L Bispecific Fusion Protein Synergistically Activate Target and Effector Cells. International Journal of Molecular Sciences, 22(21), 11302. https://doi.org/10.3390/ijms222111302





