Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening
Abstract
:1. Introduction
2. Results
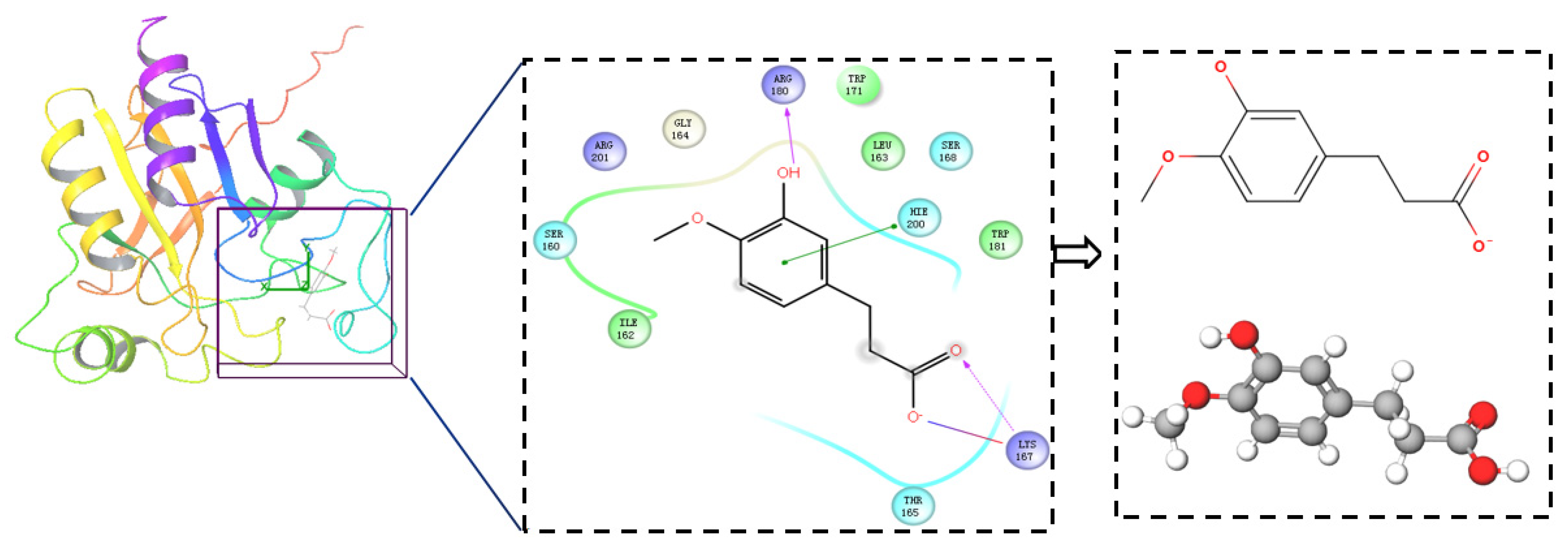
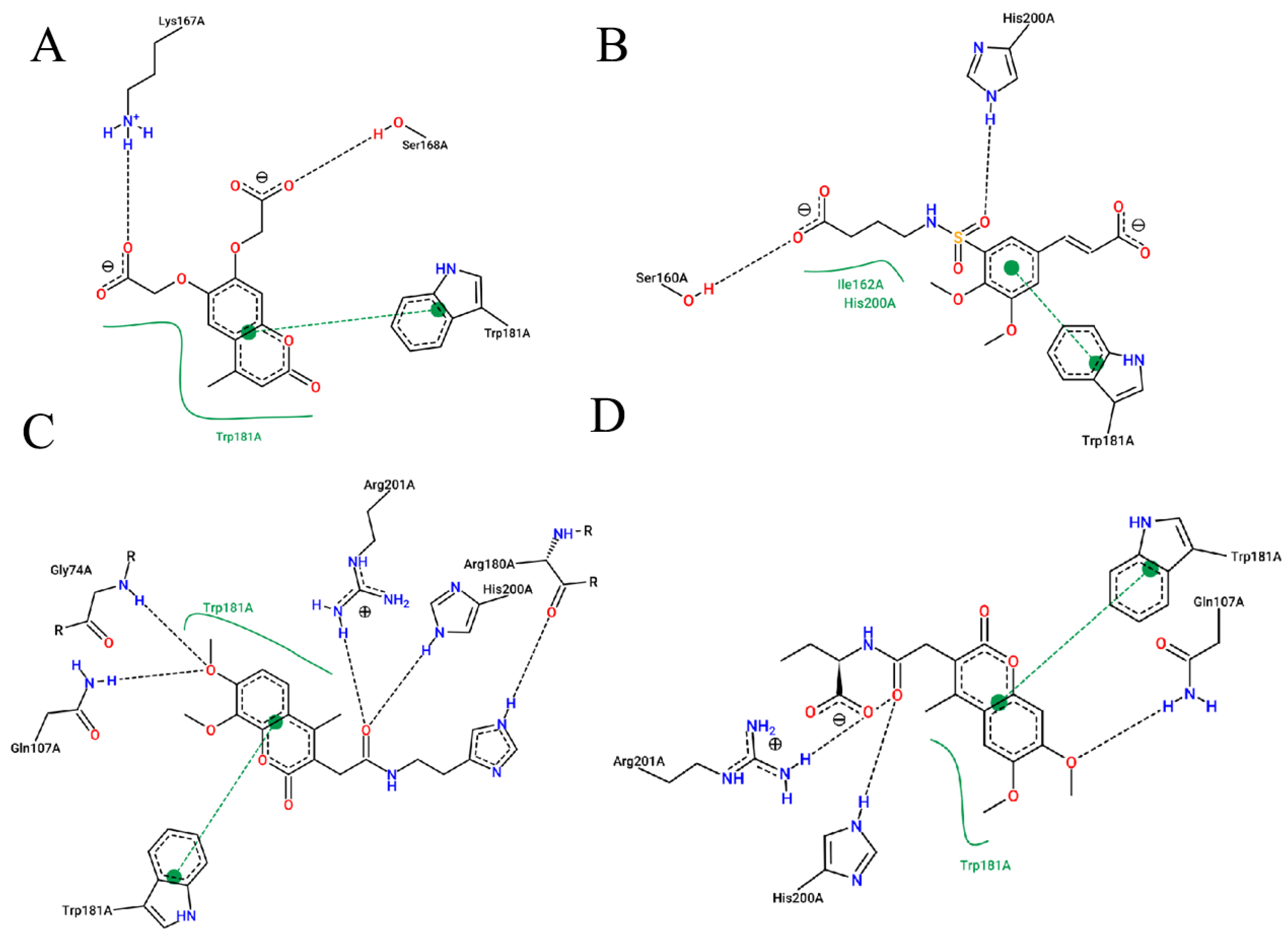
2.1. Virtual Screening
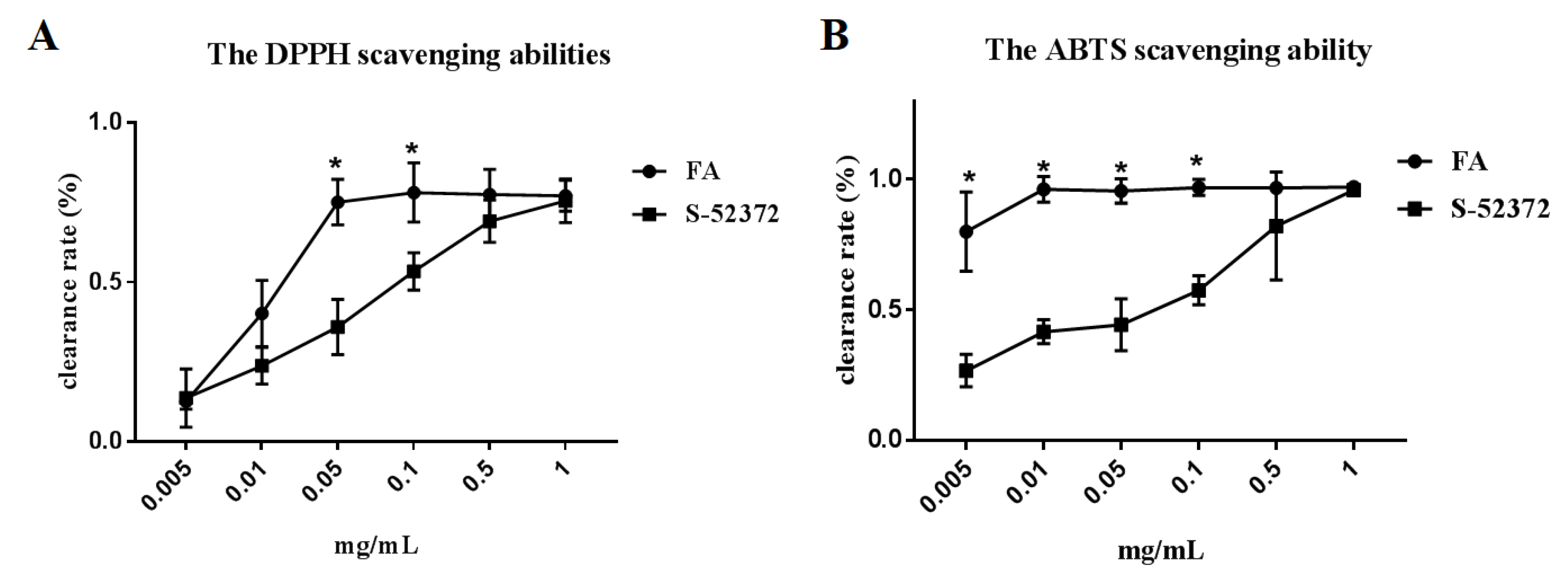
2.2. Extracellular Antioxidant Capacity Test
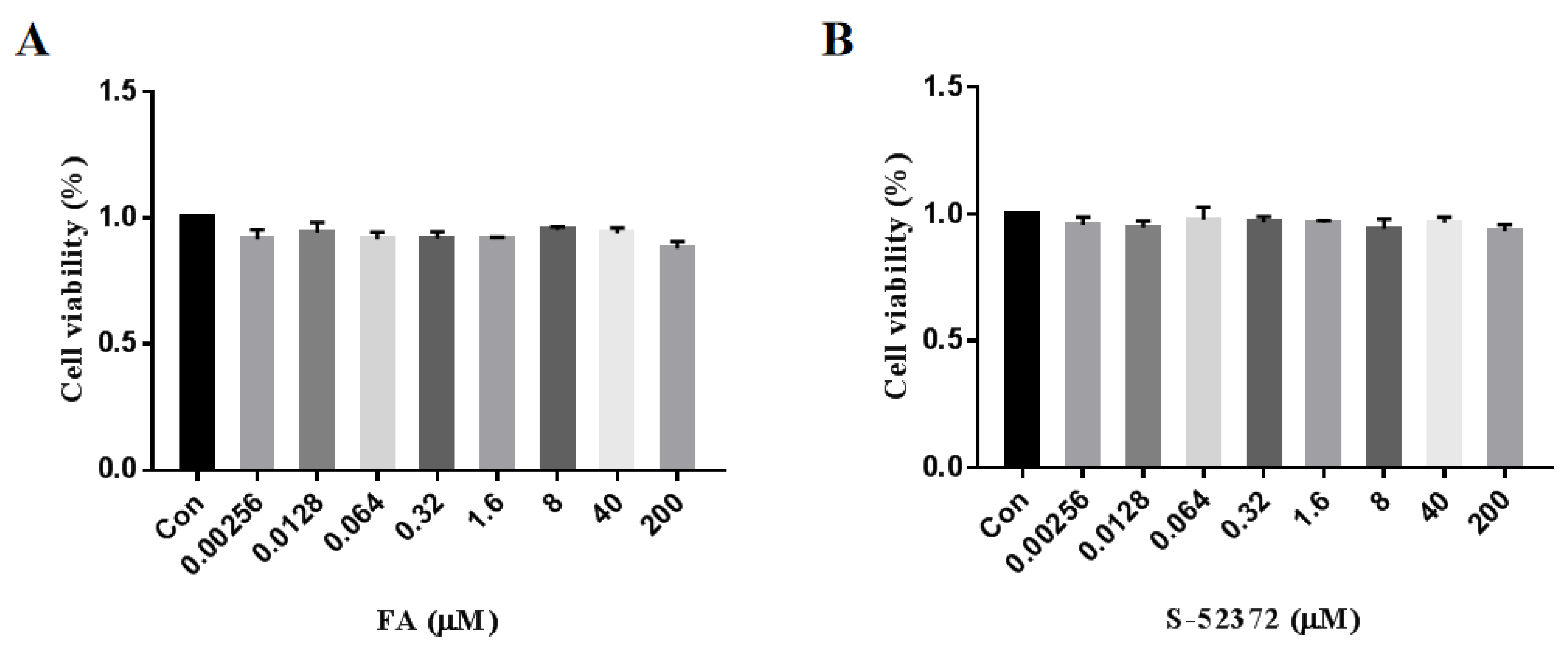
2.3. Cytotoxicity Measurement of FA and Its Derivative
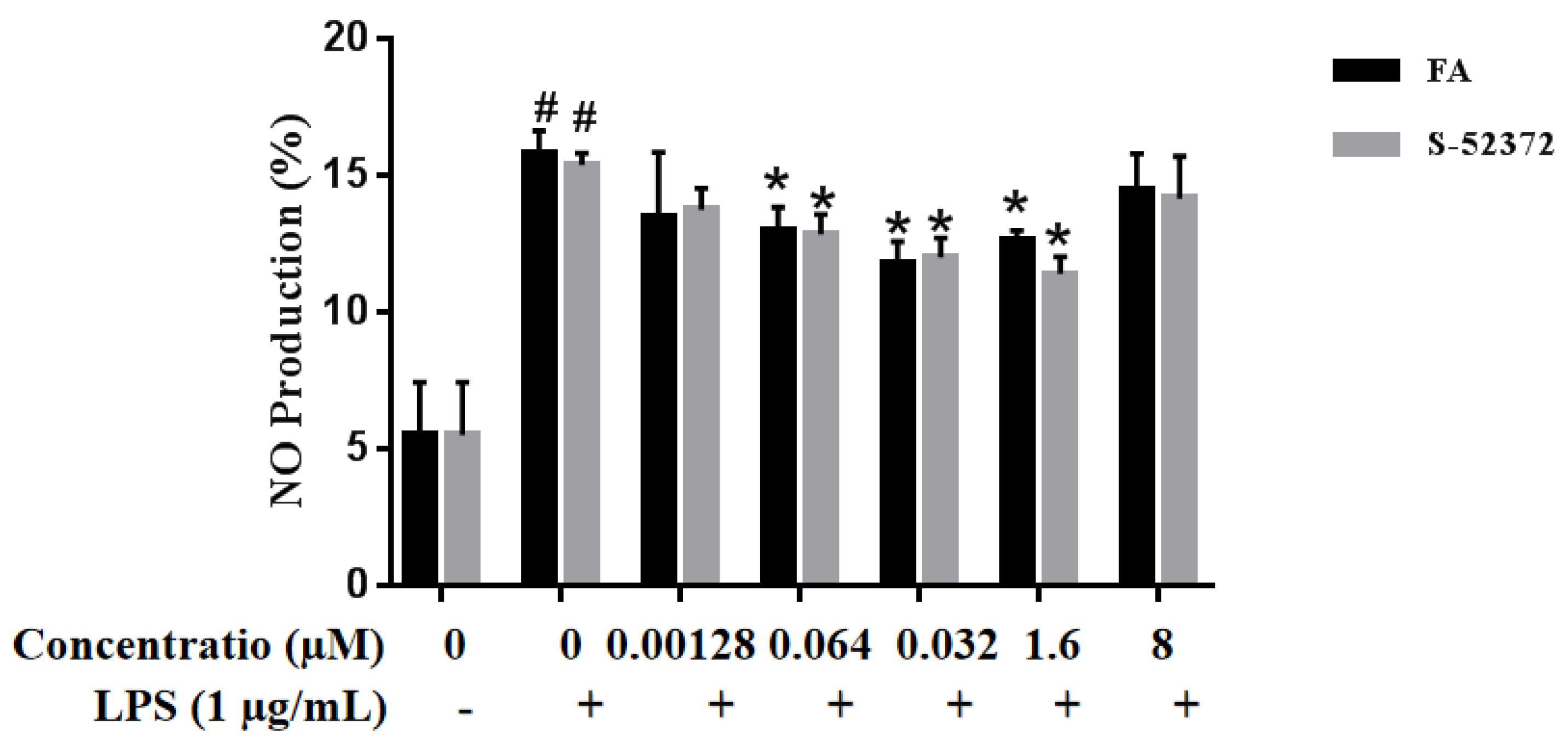
2.4. Effects on the Suppression of NO by FA and its Derivative
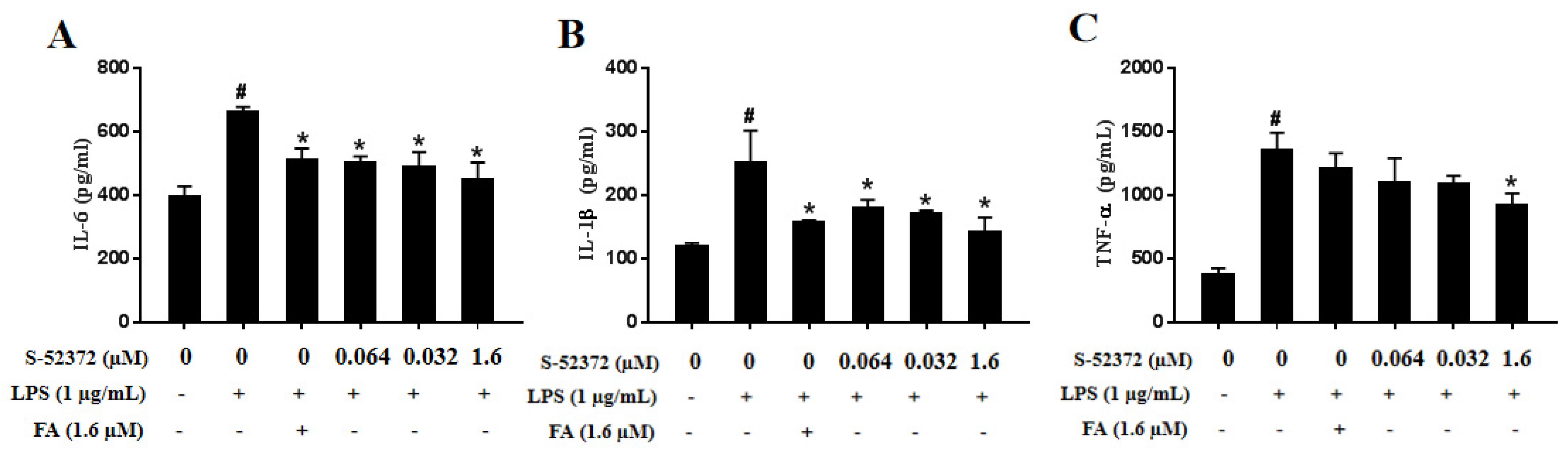
2.5. Inhibitory Effect of FA and S-52372 on IL-6, IL-1β, and TNF-α Production
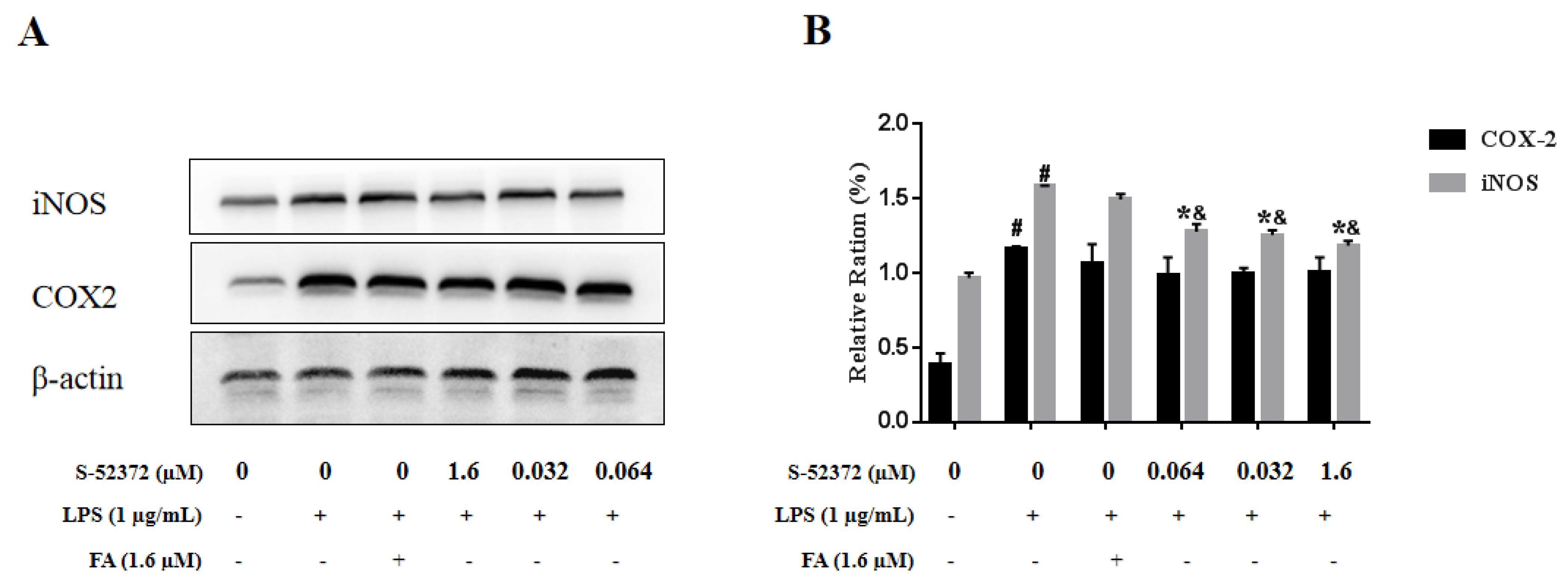
2.6. Effect of FA and S-52372 on LPS−Induced Protein Expression of iNOS and COX-2
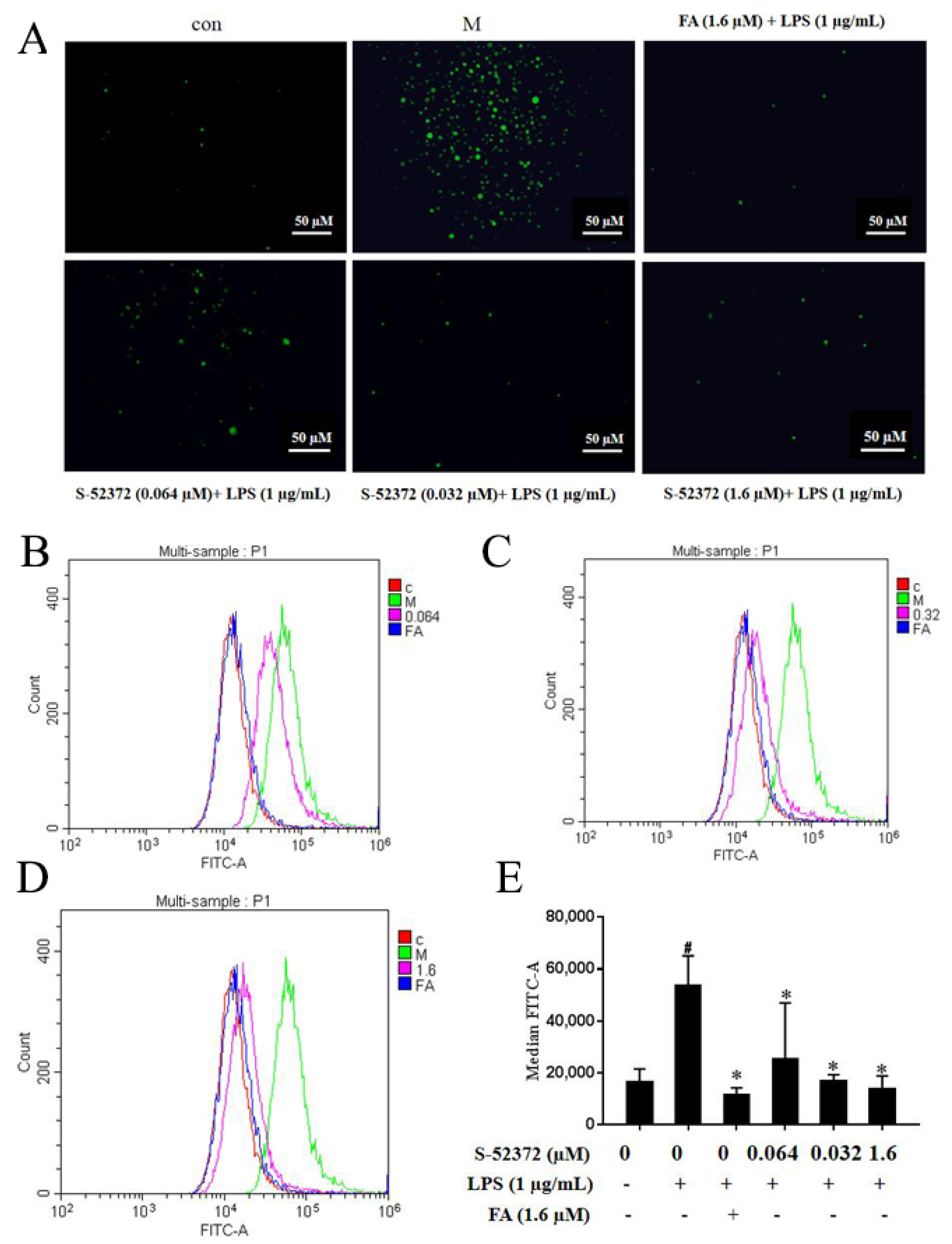
2.7. Inhibitory Effect of FA and S-52372 on the ROS Production of LPS-induced RAW264.7 Cells
2.8. Computer-Aided Prediction of the Related Properties of S-52372 and FA
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Computer-Aided Virtual Screening
4.2.1. Obtaining FA Derivatives and Primary Docking Screening
4.2.2. Drug Likeness Evaluation
4.2.3. High Precision Screening
4.3. Antioxidant Capacity Evaluation
4.3.1. DPPH Radical Scavenging Activity
4.3.2. ABTS Radical Scavenging Activity
4.4. Cell Culture and Stimulation
4.4.1. Cell Viability Assay
4.4.2. Measurement of NO
4.4.3. Determinations of IL-6, TNF-α, and IL-1β Levels
4.5. Western Blot Analysis
4.6. Determination of Intracellular ROS Content
4.7. Prediction of ADMET Properties
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, P.; Floridi, L.; Boraschi, D.; Cuadrado, A.; Manda, G.; Levic, S.; D’Acquisto, F.; Hamilton, A.; Athersuch, T.J.; Selley, L. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. Antioxid. Redox Signal 2018, 28, 852–872. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [Green Version]
- Akashi, S.; Saitoh, S.; Wakabayashi, Y.; Kikuchi, T.; Takamura, N.; Nagai, Y.; Kusumoto, Y.; Fukase, K.; Kusumoto, S.; Adachi, Y.; et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: Higher affinity than that with MD-2 or CD14. J. Exp. Med. 2003, 198, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Feng, D.; Ling, W.H.; Duan, R.D. Lycopene suppresses proinflammatory response in lipopolysaccharide-stimulated macrophages by inhibiting ROS-induced trafficking of TLR4 to lipid raft-like domains. J. Nutr. Biochem. 2013, 24, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-alpha, and IL-6 via AP-1, NF-kappaB, and JAK-STAT inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 49, 21–29. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Busselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, S.; Kanimozhi, G.; Prasad, N.R.; Mahalakshmi, R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol. In Vitro 2011, 25, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.Y.; Kim, S.M.; Park, J.; Lee, J.; Cho, H.; Lee, H.; Jeon, C.; Park, J.H.; Han, I.O. A caffeic acid-ferulic acid hybrid compound attenuates lipopolysaccharide-mediated inflammation in BV2 and RAW264.7 cells. Biochem. Biophys. Res. Commun. 2019, 515, 565–571. [Google Scholar] [CrossRef]
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid beta-peptide(1-42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758. [Google Scholar] [CrossRef]
- Panneerselvam, L.; Subbiah, K.; Arumugam, A.; Senapathy, J.G. Ferulic acid modulates fluoride-induced oxidative hepatotoxicity in male Wistar rats. Biol. Trace Elem. Res. 2013, 151, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Cosme, F.; Jordão, A.M. Anthocyanin profile and antioxidant activity from grape varieties cultivated in two Portuguese wine regions. J. Int. Des Sci. 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug. Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Willett, P. Similarity-based virtual screening using 2D fingerprints. Drug Discov. Today 2006, 11, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Szulc-Kielbik, I.; Kielbik, M.; Klink, M. Ferulic acid but not alpha-lipoic acid effectively protects THP-1-derived macrophages from oxidant and pro-inflammatory response to LPS. Immunopharmacol. Immunotoxicol. 2017, 39, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Thyagaraju, B.M. Muralidhara, Ferulic acid supplements abrogate oxidative impairments in liver and testis in the streptozotocin-diabetic rat. Zoolog. Sci. 2008, 25, 854–860. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P. Bioactive 530 compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: 531 Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Beutler, B.; Jiang, Z.; Georgel, P.; Crozat, K.; Croker, B.; Rutschmann, S.; Du, X.; Hoebe, K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev. Immunol. 2006, 24, 353–389. [Google Scholar] [CrossRef] [Green Version]
- Palsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Piazza, M.; Calabrese, V.; Baruffa, C.; Gioannini, T.; Weiss, J.; Peri, F. The cationic amphiphile 3,4-bis(tetradecyloxy)benzylamine inhibits LPS signaling by competing with endotoxin for CD14 binding. Biochem Pharmacol 2010, 80, 2050–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.; Shi, Y.; Su, J.; Zhao, Z.; Wang, X.; Li, J.; Liu, H. Virtual Screening and Bioactivity Evaluation of Novel Androgen Receptor Antagonists From Anti-PCa Traditional Chinese Medicine Prescriptions. Front Chem. 2020, 8, 582861. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase Family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Accelrys. Accelrys Discovery Studio, Version 2.5; Accelrys: San Diego, CA, USA, 2010. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Drug Deliv. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that inflfluence the oral bioavailability of drug candidates. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. | Database | Structure | Docking Score |
|---|---|---|---|
| FA | 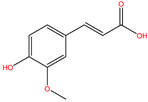 | −4.608 | |
| S-69904 | InterBioScreen |  | −4.985 |
| S-52372 | InterBioScreen |  | −5.024 |
| S-11593 | InterBioScreen | 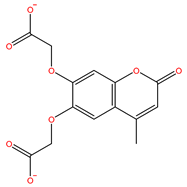 | −5.841 |
| S-25207 | InterBioScreen | 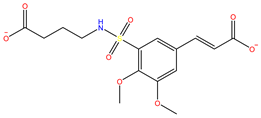 | −4.379 |
| Model | FA | s-52372 | FA | s-52372 |
|---|---|---|---|---|
| Absorbtion | ||||
| Blood–Brain Barrier | BBB- | BBB- | 0.5305 | 0.9526 |
| Human Intestinal Absorption | HIA+ | HIA+ | 0.9614 | 0.8187 |
| Aqueous Solubility | −2.4766 | −1.5911 | LogS | |
| Caco-2 Permeability | Caco2+ | Caco2- | 0.7183 | 0.7275 |
| Metabolism | ||||
| CYP450 2C9 Substrate | Non-substrate | Non-substrate | 0.7464 | 0.8111 |
| CYP450 2D6 Substrate | Non-substrate | Non-substrate | 0.8922 | 0.8404 |
| CYP450 1A2 Inhibitor | Non-inhibitor | Non-inhibitor | 0.7513 | 0.6462 |
| CYP450 2D6 Inhibitor | Non-inhibitor | Non-inhibitor | 0.9588 | 0.9165 |
| CYP450 3A4 Inhibitor | Non-inhibitor | Non-inhibitor | 0.924 | 0.8916 |
| Toxicity | ||||
| AMES Toxicity | Non-AMES-toxic | Non-AMES-toxic | 0.9132 | 0.6156 |
| Carcinogens | Non-carcinogens | Non-carcinogens | 0.9076 | 0.9523 |
| Acute Oral Toxicity | IV | III | 0.6265 | 0.5929 |
| Rat Acute Toxicity | 1.4314 | 2.8767 | LD50, moL/kg | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Chen, X.; Qiang, S.; Su, J.; Li, J. Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening. Int. J. Mol. Sci. 2021, 22, 11305. https://doi.org/10.3390/ijms222111305
Shi Y, Chen X, Qiang S, Su J, Li J. Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening. International Journal of Molecular Sciences. 2021; 22(21):11305. https://doi.org/10.3390/ijms222111305
Chicago/Turabian StyleShi, Yuqi, Xuelian Chen, Shaojia Qiang, Jie Su, and Jiazhong Li. 2021. "Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening" International Journal of Molecular Sciences 22, no. 21: 11305. https://doi.org/10.3390/ijms222111305
APA StyleShi, Y., Chen, X., Qiang, S., Su, J., & Li, J. (2021). Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening. International Journal of Molecular Sciences, 22(21), 11305. https://doi.org/10.3390/ijms222111305





