MUG Mel3 Cell Lines Reflect Heterogeneity in Melanoma and Represent a Robust Model for Melanoma in Pregnancy
Abstract
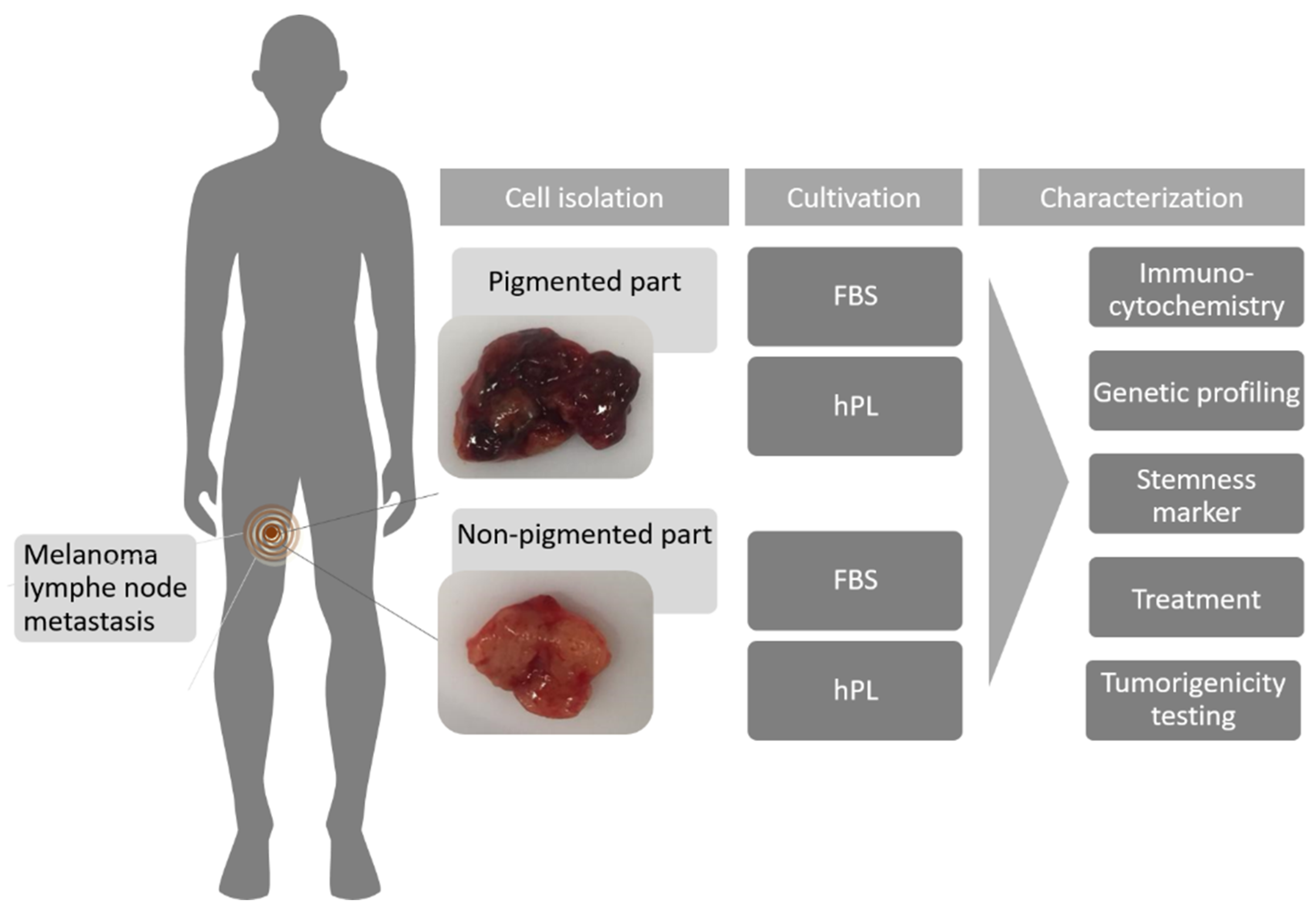
:1. Introduction
2. Results
2.1. Cell Growth and Classical Melanoma Markers Confirm the Heterogenic Character of MUG Mel3 Cell Lines
2.2. Measurement of Growth Factors and Chemokines with Luminex Technology
2.3. Copy Number Profiling
2.4. BRAF Mutation Analysis
2.5. Assessment of Tumorigenicity
2.6. PS Exposure and Peptide Treatment
3. Discussion
4. Material and Methods
4.1. Patient History
4.2. Pooled Human Platelet Lysate (hPL)
4.3. Cell Culture
4.4. Cell Line Authentication
4.5. CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS)
4.6. Immunocytochemistry (ICC)
4.7. qPCR
4.8. FACS Analysis
4.9. Growth Factors xMAP® Technology
4.10. DNA Extraction
4.11. Copy Number Profiling
4.12. Mutation Analysis Using AmpliSeq (BRAF)
4.13. Tumorigenicity Study
4.14. Ultrasound Imaging
4.15. PS Exposure
4.16. Peptides
4.17. PI Uptake Toxicity Assay
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and intertumor heterogeneity in melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef]
- Andor, N.; Graham, T.A.; Jansen, M.; Xia, L.C.; Aktipis, C.A.; Petritsch, C.; Ji, H.P.; Maley, C.C. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat. Med. 2016, 22, 105–113. [Google Scholar] [CrossRef]
- Pavlidis, N.A. Coexistence of pregnancy and malignancy. Oncologist 2002, 7, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Cottreau, C.M.; Dashevsky, I.; Andrade, S.E.; Li, D.-K.; Nekhlyudov, L.; Raebel, M.A.; Ritzwoller, D.P.; Partridge, A.H.; Pawloski, P.; Toh, S. Pregnancy-associated cancer: A U.S. population-based study. J. Women’s Health 2019, 28, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nat. Cell Biol. 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, B.; Bai, X.; Cohen, C.; Zhong, H.; Kilroy, S.; Louis, G.; Moses, M.; Arbiser, J.L. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J. Biol. Chem. 2003, 278, 9790–9795. [Google Scholar] [CrossRef] [Green Version]
- Richtig, G.; Hoeller, C.; Kashofer, K.; Aigelsreiter, A.; Heinemann, A.; Kwong, L.N.; Pichler, M.; Richtig, E. Beyond the BRAFV600E hotspot: Biology and clinical implications of rare BRAF gene mutations in melanoma patients. Br. J. Dermatol. 2017, 177, 936–944. [Google Scholar] [CrossRef]
- Vanni, I.; Tanda, E.T.; Spagnolo, F.; Andreotti, V.; Bruno, W.; Ghiorzo, P. The current state of molecular testing in the BRAF-mutated melanoma landscape. Front. Mol. Biosci. 2020, 7, 113. [Google Scholar] [CrossRef]
- Seidel, J.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.K.; Wolchok, J.D. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Winder, M.; Virós, A. Mechanisms of drug resistance in melanoma. In New Approaches to Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2017; Volume 249, pp. 91–108. [Google Scholar] [CrossRef]
- Cheng, L.; López-Beltrán, A.; Massari, F.; MacLennan, G.T.; Montironi, R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod. Pathol. 2018, 31, 24–38. [Google Scholar] [CrossRef]
- Reuben, A.; Spencer, C.N.; Prieto, P.A.; Gopalakrishnan, V.; Reddy, S.; Miller, J.P.; Mao, X.; De Macedo, M.P.; Chen, J.; Song, X.; et al. Genomic and immune heterogeneity are associated with differential responses to therapy in melanoma. NPJ Genom. Med. 2017, 2, 10. [Google Scholar] [CrossRef]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K.; Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef]
- Laner-Plamberger, S.; Lener, T.; Schmid, D.; Streif, D.A.; Salzer, T.; Öller, M.; Hauser-Kronberger, C.; Fischer, T.; Jacobs, V.R.; Schallmoser, K.; et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J. Transl. Med. 2015, 13, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schallmoser, K.; Strunk, D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J. Vis. Exp. 2009, 32, e1523. [Google Scholar] [CrossRef] [Green Version]
- Riedl, S.; Leber, R.; Rinner, B.; Schaider, H.; Lohner, K.; Zweytick, D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2918–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedl, S.; Rinner, B.; Schaider, H.; Liegl-Atzwanger, B.; Meditz, K.; Preishuber-Pflügl, J.; Grissenberger, S.; Lohner, K.; Zweytick, D. In Vitro and In Vivo cytotoxic activity of human lactoferricin derived antitumor peptide R-DIM-P-LF11-334 on human malignant melanoma. Oncotarget 2017, 8, 71817–71832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitzer, E.; Groenewoud, A.; Meditz, K.; Lohberger, B.; Liegl-Atzwanger, B.; Prokesch, A.; Kashofer, K.; Behrens, D.; Haybaeck, J.; Kolb-Lenz, D.; et al. Human melanoma brain metastases cell line MUG-Mel1, isolated clones and their detailed characterization. Sci. Rep. 2019, 9, 4096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wodlej, C.; Riedl, S.; Rinner, B.; Leber, R.; Drechsler, C.; Voelker, D.R.; Choi, J.-Y.; Lohner, K.; Zweytick, D. Interaction of two antitumor peptides with membrane lipids—influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS ONE 2019, 14, e0211187. [Google Scholar] [CrossRef] [PubMed]
- Grissenberger, S.; Riedl, S.; Rinner, B.; Leber, R.; Zweytick, D. Design of human lactoferricin derived antitumor peptides-activity and specificity against malignant melanoma in 2D and 3D model studies. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183264. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef]
- Riedl, S.; Rinner, B.; Asslaber, M.; Schaider, H.; Walzer, S.M.; Novak, A.; Lohner, K.; Zweytick, D. In search of a novel target—phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2638–2645. [Google Scholar] [CrossRef] [Green Version]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides—challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, B.C.; Johnson, G.; Wang, J.; Cohen, C. SOX10: A useful marker for identifying metastatic melanoma in sentinel lymph nodes. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Shannan, B.; Perego, M.; Somasundaram, R.; Herlyn, M. Heterogeneity in melanoma. In Melanoma; Cancer Treatment and Research; Springer: Cham, Switzerland, 2016; Volume 167, pp. 1–15. [Google Scholar]
- Kyrgidis, A.; Lallas, A.; Moscarella, E.; Longo, C.; Alfano, R.; Argenziano, G. Does pregnancy influence melanoma prognosis? A meta-analysis. Melanoma Res. 2017, 27, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, M.B.; Driscoll, M.S.; Grant-Kels, J.M. Melanoma in pregnancy. Clin. Obstet. Gynecol. 2011, 54, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer during pregnancy: The oncologist overview. World J. Oncol. 2019, 10, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riker, A.I. Melanoma: A Modern Multidisciplinary Approach; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Tyler, K.H. Physiological skin changes during pregnancy. Clin. Obstet. Gynecol. 2015, 58, 119–124. [Google Scholar] [CrossRef]
- Ding, L.; Kim, M.; Kanchi, K.L.; Dees, N.D.; Lu, C.; Griffith, M.; Fenstermacher, D.; Sung, H.; Miller, C.A.; Goetz, B.; et al. Clonal architectures and driver mutations in metastatic melanomas. PLoS ONE 2014, 9, e111153. [Google Scholar] [CrossRef]
- Sanborn, J.Z.; Chung, J.; Purdom, E.; Wang, N.J.; Kakavand, H.; Wilmott, J.S.; Butler, T.; Thompson, J.F.; Mann, G.J.; Haydu, L.E.; et al. Phylogenetic analyses of melanoma reveal complex patterns of metastatic dissemination. Proc. Natl. Acad. Sci. USA 2015, 112, 10995–11000. [Google Scholar] [CrossRef] [Green Version]
- Iida, J.; Pei, D.; Kang, T.; Simpson, M.A.; Herlyn, M.; Furcht, L.T.; McCarthy, J.B. Melanoma chondroitin sulfate proteoglycan regulates matrix metalloproteinase-dependent human melanoma invasion into type I collagen. J. Biol. Chem. 2001, 276, 18786–18794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Price, M.A.; Neudauer, C.L.; Wilson, C.; Ferrone, S.; Xia, H.; Iida, J.; Simpson, M.A.; McCarthy, J.B. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J. Cell Biol. 2004, 165, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Schwenk, U.; Maune, S.; Bartels, J.; Küpper, M.; Fichtner, I.; Schröder, J.-M.; Schadendorf, D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br. J. Cancer 1999, 79, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.S.; Cornelius, L.A. The role of chemokines in melanoma tumor growth and metastasis. J. Investig. Dermatol. 2002, 118, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Tudrej, K.B.; Czepielewska, E.; Kozłowska-Wojciechowska, M. SOX10-MITF pathway activity in melanoma cells. Arch. Med. Sci. 2017, 13, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redmer, T.; Welte, Y.; Behrens, D.; Fichtner, I.; Przybilla, D.; Wruck, W.; Yaspo, M.-L.; Lehrach, H.; Schäfer, R.; Regenbrecht, C.R.A. The nerve growth factor receptor CD271 is crucial to maintain tumorigenicity and stem-like properties of melanoma cells. PLoS ONE 2014, 9, e92596. [Google Scholar] [CrossRef] [Green Version]
- Filipp, F.V.; Li, C.; Boiko, A.D. CD271 is a molecular switch with divergent roles in melanoma and melanocyte development. Sci. Rep. 2019, 9, 7696. [Google Scholar] [CrossRef] [Green Version]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef] [Green Version]
- Manzano, J.L.; Layos, L.; Bugés, C.; de Los Llanos Gil, M.; Vila, L.; Martínez-Balibrea, E.; Martinez-Cardus, A. Resistant mechanisms to BRAF inhibitors in melanoma. Ann. Transl. Med. 2016, 4, 237. [Google Scholar] [CrossRef] [Green Version]
- Somasundaram, R.; Villanueva, J.; Herlyn, M. Intratumoral heterogeneity as a therapy resistance mechanism: Role of melanoma subpopulations. Adv. Pharmacol. 2012, 65, 335–359. [Google Scholar] [PubMed] [Green Version]
- Omholt, K.; Platz, A.; Kanter, L.; Ringborg, U.; Hansson, J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin. Cancer Res. 2003, 9, 6483–6488. [Google Scholar] [PubMed]
- Harbst, K.; Lauss, M.; Cirenajwis, H.; Isaksson, K.; Rosengren, F.; Törngren, T.; Kvist, A.; Johansson, M.C.; Vallon-Christersson, J.; Baldetorp, B.; et al. Multiregion whole-exome sequencing uncovers the genetic evolution and mutational heterogeneity of early-stage metastatic melanoma. Cancer Res. 2016, 76, 4765–4774. [Google Scholar] [CrossRef] [Green Version]
- Chiappetta, C.; Proietti, I.; Soccodato, V.; Puggioni, C.; Zaralli, R.; Pacini, L.; Porta, N.; Skroza, N.; Petrozza, V.; Potenza, C.; et al. BRAF and NRAS mutations are heterogeneous and not mutually exclusive in nodular melanoma. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Ziogas, D.C.; Diamantopoulos, P.; Benopoulou, O.; Anastasopoulou, A.; Bafaloukos, D.; Stratigos, A.J.; Kirkwood, J.M.; Gogas, H. Prognosis and management of BRAF V600E-mutated pregnancy-associated melanoma. Oncologist 2020, 25, e1209–e1220. [Google Scholar] [CrossRef] [Green Version]
- Marcé, D.; Cornillier, H.; Denis, C.; Jonville-Bera, A.-P.; Machet, L. Partial response of metastatic melanoma to BRAF-inhibitor-monotherapy in a pregnant patient with no fetal toxicity. Melanoma Res. 2019, 29, 446–447. [Google Scholar] [CrossRef]
- Schallmoser, K.; Bartmann, C.; Rohde, E.; Reinisch, A.; Kashofer, K.; Stadelmeyer, E.; Drexler, C.; Lanzer, G.; Linkesch, W.; Strunk, D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 2007, 47, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Ulz, P.; Belic, J.; Gutschi, S.; Quehenberger, F.; Fischereder, K.; Benezeder, T.; Auer, M.; Pischler, C.; Mannweiler, S.; et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2012, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Pigmented | Non-Pigmented | |||
|---|---|---|---|---|
| FBS | hPL | FBS | hPL | |
| Growth Behavior | Fast | Slow | Fast | Slow |
| Adhesion | ++ | Semi-adherent | ++ | Semi-adherent |
| ICC-HMB45 | − | + | ++ | ++ |
| ICC-Melan-A | − | + | ++ | ++ |
| ICC-tyrosinase | − | − | + | ++ |
| ICC-MCSP | ++ | ++ | ++ | ++ |
| qPCR-Sox10 | + | ++ | + | ++ |
| qPCR-Melan-A | − | ++ | + | ++ |
| CD271 | 13.94% ± 4.22 | 20.09% ± 6.71 | 3.66% ± 1.67 | 2.91% ± 1.40 |
| CR ATH HO | MUG Mel3 PF | MUG Mel3 Ph | MUG Mel3 NPF | MUG Mel3 NPh |
|---|---|---|---|---|
| HMB45 | ++ | ++ | ++ | ++ |
| Melan-A | + | ++ | ++ | ++ |
| Tyrosinase | − | + | − | + |
| NXG | MUG Mel3 PF | MUG Mel3 Ph | MUG Mel3 NPF | MUG Mel3 NPh |
| HMB45 | + | ++ | ++ | ++ |
| Melan-A | + | ++ | ++ | ++ |
| Tyrosinase | − | + | − | − |
| IC50 (PI) [µM] | |||
|---|---|---|---|
| R-DIM-P-LF11-322 | R-DIM-P-LF11-334 | p-Value | |
| MUG Mel 3 PF (10% FBS) | 14.4 ± 0.4 | 21.9 ± 1.7 | 0.0017 |
| MUG Mel 3 Ph (2.5% hPL) | 4.3 ± 0.3 | 6.6 ± 0.7 | 0.0064 |
| MUG Mel 3 NPF (10% FBS) | 22.1 ± 1.2 | 28.1 ± 1.3 | 0.0042 |
| MUG Mel 3 NPh (2.5% hPL) | 16.0 ± 0.9 | 26.9 ± 0.7 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrom, S.; Hebesberger, T.; Wallner, S.A.; Anders, I.; Richtig, E.; Brandl, W.; Hirschmugl, B.; Garofalo, M.; Bernecker, C.; Schlenke, P.; et al. MUG Mel3 Cell Lines Reflect Heterogeneity in Melanoma and Represent a Robust Model for Melanoma in Pregnancy. Int. J. Mol. Sci. 2021, 22, 11318. https://doi.org/10.3390/ijms222111318
Schrom S, Hebesberger T, Wallner SA, Anders I, Richtig E, Brandl W, Hirschmugl B, Garofalo M, Bernecker C, Schlenke P, et al. MUG Mel3 Cell Lines Reflect Heterogeneity in Melanoma and Represent a Robust Model for Melanoma in Pregnancy. International Journal of Molecular Sciences. 2021; 22(21):11318. https://doi.org/10.3390/ijms222111318
Chicago/Turabian StyleSchrom, Silke, Thomas Hebesberger, Stefanie Angela Wallner, Ines Anders, Erika Richtig, Waltraud Brandl, Birgit Hirschmugl, Mariangela Garofalo, Claudia Bernecker, Peter Schlenke, and et al. 2021. "MUG Mel3 Cell Lines Reflect Heterogeneity in Melanoma and Represent a Robust Model for Melanoma in Pregnancy" International Journal of Molecular Sciences 22, no. 21: 11318. https://doi.org/10.3390/ijms222111318
APA StyleSchrom, S., Hebesberger, T., Wallner, S. A., Anders, I., Richtig, E., Brandl, W., Hirschmugl, B., Garofalo, M., Bernecker, C., Schlenke, P., Kashofer, K., Wadsack, C., Aigelsreiter, A., Heitzer, E., Riedl, S., Zweytick, D., Kretschmer, N., Richtig, G., & Rinner, B. (2021). MUG Mel3 Cell Lines Reflect Heterogeneity in Melanoma and Represent a Robust Model for Melanoma in Pregnancy. International Journal of Molecular Sciences, 22(21), 11318. https://doi.org/10.3390/ijms222111318









