The Lack of Amyloidogenic Activity Is Persistent in Old WT and APPswe/PS1ΔE9 Mouse Retinae
Abstract
:1. Introduction
2. Results
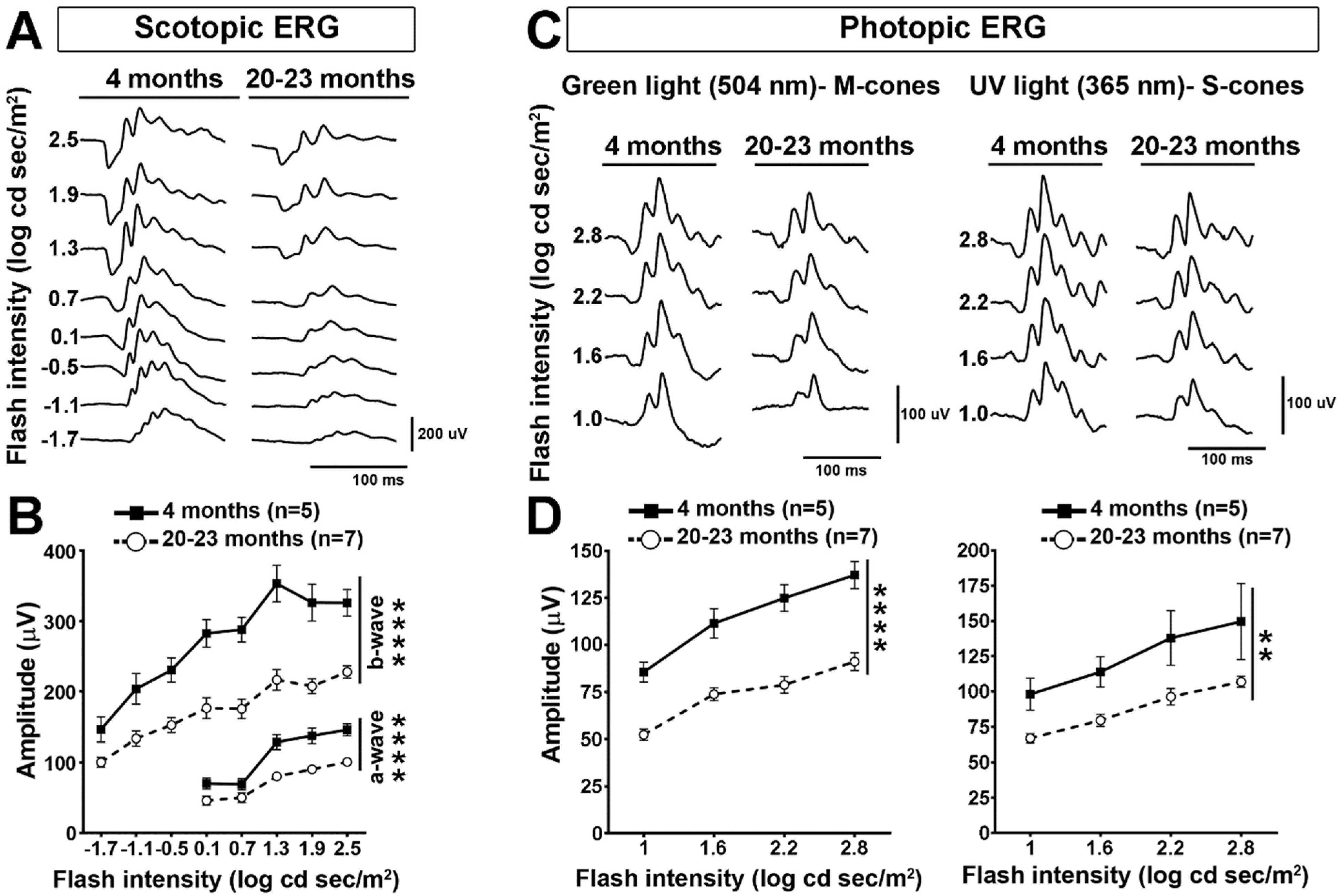
2.1. Retinal Cell Activity Decrease in Old Mice
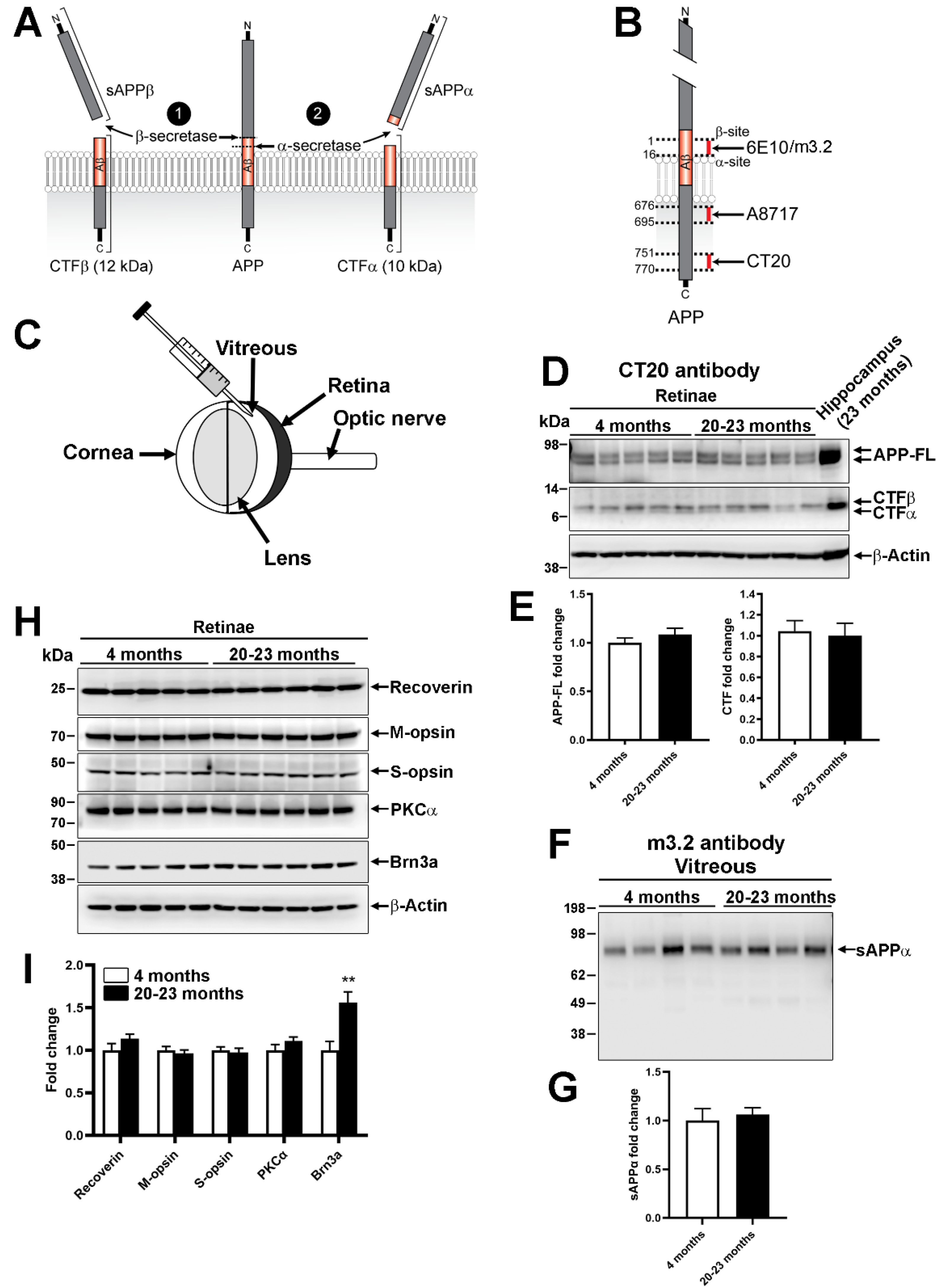
2.2. Endogenous Processing of Mouse Amyloid β Precursor Protein in Aged Animals
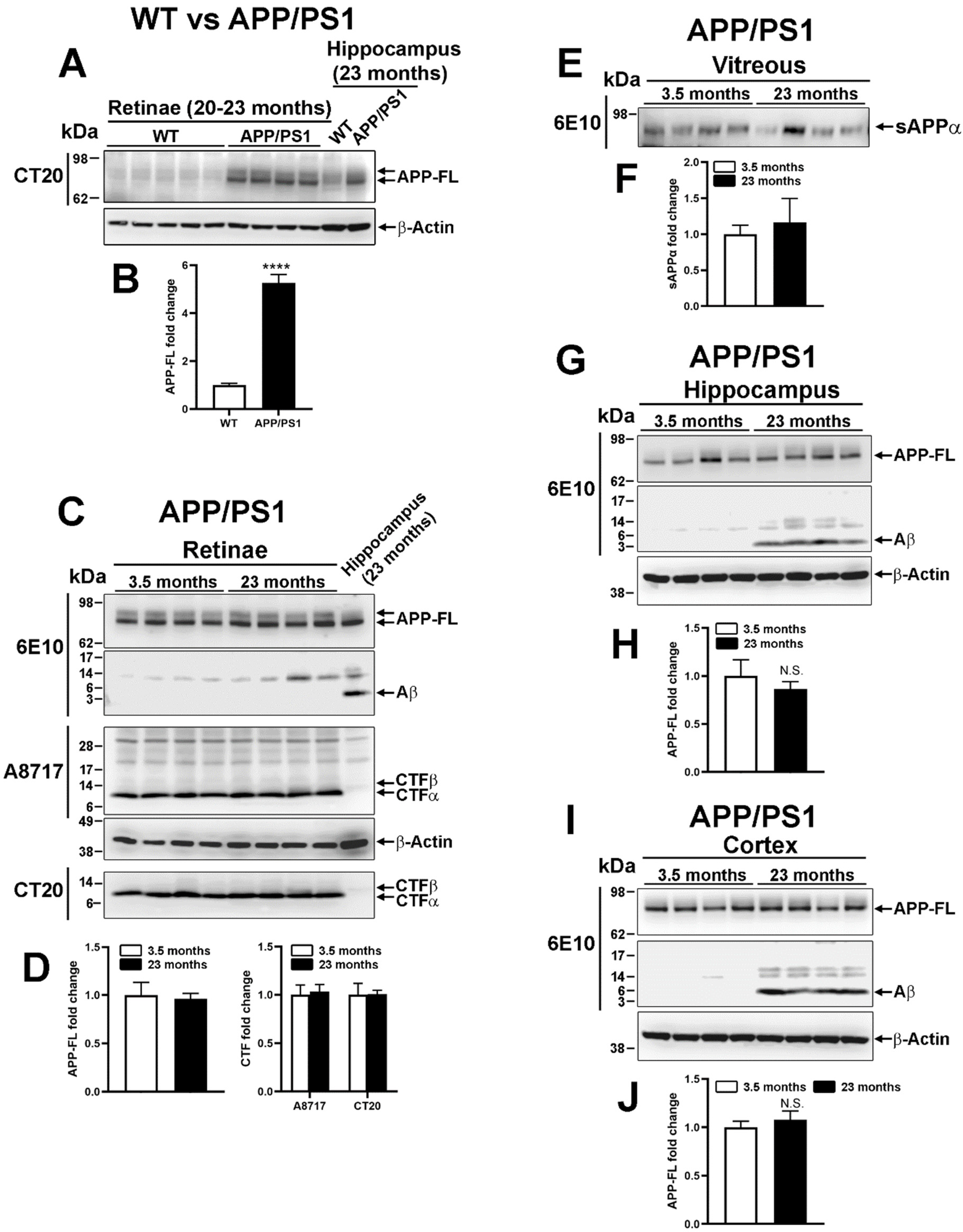
2.3. Human Amyloid β Precursor Protein Transformation in Transgenic APP/PS1
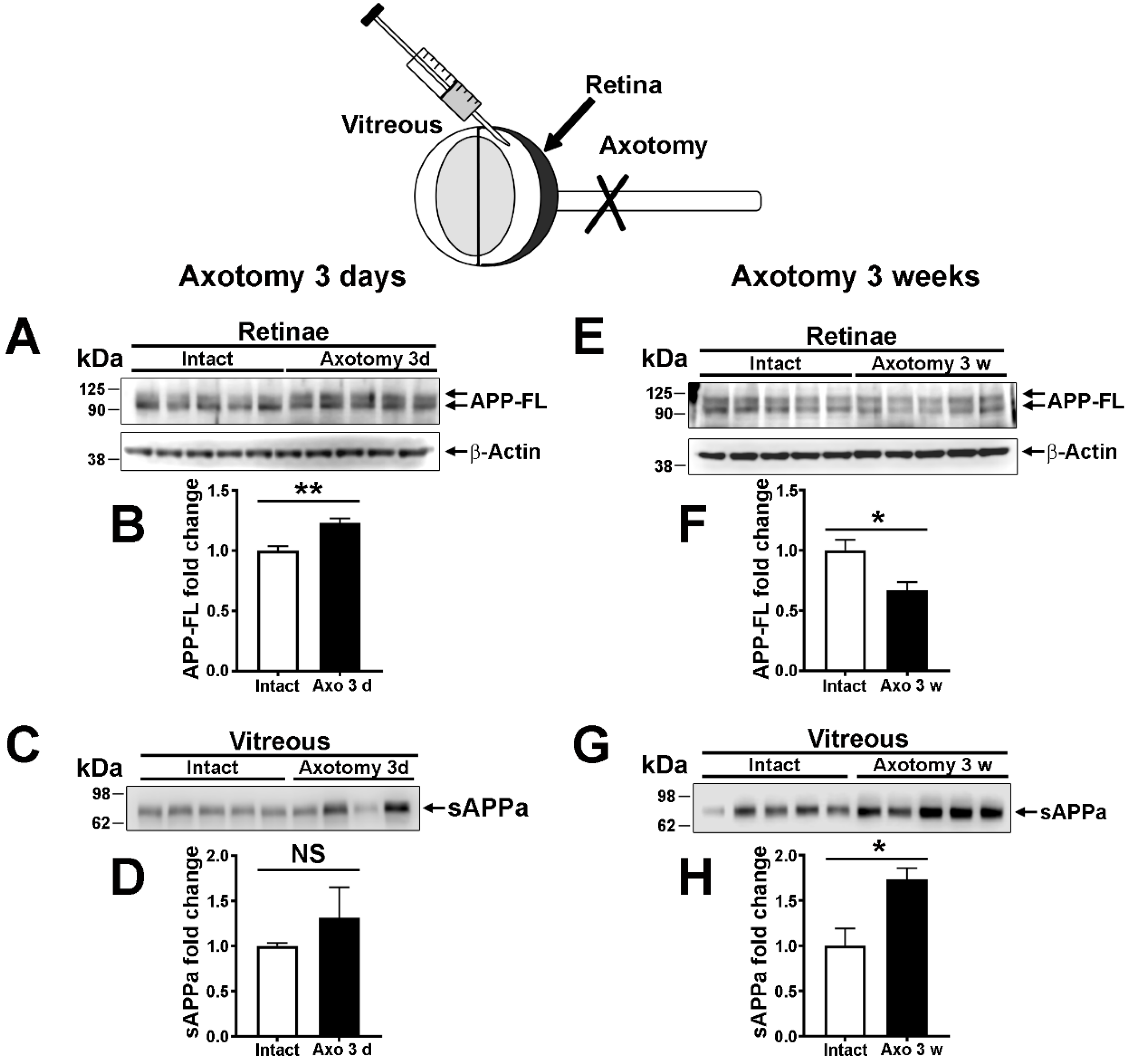
2.4. The Expression and the Nonamyloidogenic Processing of APP Are Influenced by Optic Nerve Injury
3. Discussion
3.1. Visual Function Decrease in Old Mice
3.2. Amyloidogenic vs. Nonamyloidogenic Processing in the Mouse Retina
3.3. Modulation of Retinal APP Expression and Secretion of sAPPα in the Vitreous after Lesion
4. Materials and Methods
4.1. Animals
4.2. Electroretinography
4.3. Western Blot Analysis
4.4. Optic Nerve Injury
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Haegerstrom-Portnoy, G. The Glenn A. Fry Award Lecture 2003: Vision in elders—Summary of findings of the SKI study. Optom. Vis. Sci. 2005, 82, 87–93. [Google Scholar] [CrossRef]
- Birch, D.G.; Anderson, J.L. Standardized full-field electroretinography. Normal values and their variation with age. Arch. Ophthalmol. 1992, 110, 1571–1576. [Google Scholar] [CrossRef]
- Freund, P.R.; Watson, J.; Gilmour, G.S.; Gaillard, F.; Sauve, Y. Differential changes in retina function with normal aging in humans. Doc. Ophthalmol. 2011, 122, 177–190. [Google Scholar] [CrossRef]
- Weleber, R.G. The effect of age on human cone and rod ganzfeld electroretinograms. Investig. Ophthalmol. Vis. Sci. 1981, 20, 392–399. [Google Scholar]
- Li, C.; Cheng, M.; Yang, H.; Peachey, N.S.; Naash, A.M.I. Age-Related Changes in the Mouse Outer Retina. Optom. Vis. Sci. 2001, 78, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Trachimowicz, R.A.; Fisher, L.J.; Hinds, J.W. Preservation of retinal structure in aged pigmented mice. Neurobiol. Aging 1981, 2, 133–141. [Google Scholar] [CrossRef]
- Gresh, J.; Goletz, P.W.; Crouch, R.K.; Rohrer, B. Structure–function analysis of rods and cones in juvenile, adult, and aged C57BL/6 and Balb/c mice. Vis. Neurosci. 2003, 20, 211–220. [Google Scholar] [CrossRef]
- Joly, S.; Lamoureux, S.; Pernet, V. Nonamyloidogenic processing of amyloid beta precursor protein is associated with retinal function improvement in aging male APP swe /PS1ΔE9 mice. Neurobiol. Aging 2017, 53, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-Related Alterations in Neurons of the Mouse Retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef] [Green Version]
- Cunea, A.; Powner, M.B.; Jeffery, G. Death by color: Differential cone loss in the aging mouse retina. Neurobiol. Aging 2014, 35, 2584–2591. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.A.; Voinescu, P.E.; Lilley, B.N.; de Cabo, R.; Foretz, M.; Viollet, B.; Pawlyk, B.; Sandberg, M.A.; Vavvas, D.; Sanes, J.R. LKB1 and AMPK regulate synaptic remodeling in old age. Nat. Neurosci. 2014, 17, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Liets, L.C.; Eliasieh, K.; van der List, D.A.; Chalupa, L.M. Dendrites of rod bipolar cells sprout in normal aging retina. Proc. Natl. Acad. Sci. USA 2006, 103, 12156–12160. [Google Scholar] [CrossRef] [Green Version]
- Terzibasi, E.; Calamusa, M.; Novelli, E.; Domenici, L.; Strettoi, E.; Cellerino, A. Age-dependent remodelling of retinal circuitry. Neurobiol. Aging 2009, 30, 819–828. [Google Scholar] [CrossRef]
- La Morgia, C. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol. Ther. 2014, 142, 244–257. [Google Scholar] [CrossRef]
- Prakasam, A. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J. Alzheimer’s Dis. 2010, 20, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Fol, R. Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016, 131, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Hick, M. Acute function of secreted amyloid precursor protein fragment APPsalpha in synaptic plasticity. Acta Neuropathol. 2015, 129, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Chasseigneaux, S.; Allinquant, B. Functions of Abeta, sAPPalpha and sAPPbeta: Similarities and differences. J. Neurochem. 2012, 120 (Suppl. 1), 99–108. [Google Scholar] [CrossRef]
- Radulescu, C.I.; Cerar, V.; Haslehurst, P.; Kopanitsa, M.; Barnes, S.J. The aging mouse brain: Cognition, connectivity and calcium. Cell Calcium 2021, 94, 102358. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative Memory Deficits, Aβ Elevation, and Amyloid Plaques in Transgenic Mice. Science 1996, 274, 99–103. [Google Scholar] [CrossRef]
- Jankowsky, J.L. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: Evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcomb, L.A.; Gordon, M.N.; McGowan, E.; Yu, X.; Benkovic, S.; Jantzen, P.T.; Wright, K.; Saad, I.; Mueller, R.; Morgan, D.; et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998, 4, 97–100. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Berkelaar, M.; Clarke, D.; Wang, Y.; Bray, G.; Aguayo, A. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 1994, 14, 4368–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas-Pérez, M.-P.; Vidal-Sanz, M.; Rasminsky, M.; Bray, G.M.; Aguayo, A.J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J. Neurobiol. 1993, 24, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; López, M.J.; Sobrado-Calvo, P.; Nieto-Lo´pez, L.; Ca´novas-Marti´nez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Brn3a as a Marker of Retinal Ganglion Cells: Qualitative and Quantitative Time Course Studies in Naiíve and Optic Nerve–Injured Retinas. Investig. Opthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Wang, J.; Zhang, Y.; He, J.; Kong, J.; Wang, J.-F.; Li, X.-M. The role of neuroinflammation and amyloid in cognitive impairment in an APP/PS1 transgenic mouse model of Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 310–320. [Google Scholar] [CrossRef]
- Rodriguez, L.; Joly, S.; Zine-Eddine, F.; Mdzomba, J.B.; Pernet, V. Tau modulates visual plasticity in adult and old mice. Neurobiol. Aging 2020, 95, 214–224. [Google Scholar] [CrossRef]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular Reorganization in the Human Retina during Normal Aging. Investig. Opthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef]
- Glaschke, A.; Weiland, J.; Del Turco, D.; Steiner, M.; Peichl, L.; Glösmann, M. Thyroid Hormone Controls Cone Opsin Expression in the Retina of Adult Rodents. J. Neurosci. 2011, 31, 4844–4851. [Google Scholar] [CrossRef]
- Puzzo, D. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef]
- Galanis, C.; Fellenz, M.; Becker, D.; Bold, C.; Lichtenthaler, S.F.; Müller, U.C.; Deller, T.; Vlachos, A. Amyloid-Beta Mediates Homeostatic Synaptic Plasticity. J. Neurosci. 2021, 41, 5157–5172. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Förstera, B.; Peng, S.; Shi, M.; Ladrón-De-Guevara, A.; et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef]
- Ring, S. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007, 27, 7817–7826. [Google Scholar] [CrossRef]
- Taylor, C.J.; Ireland, D.R.; Ballagh, I.; Bourne, K.; Marechal, N.M.; Turner, P.R.; Bilkey, D.K.; Tate, W.P.; Abraham, W.C. Endogenous secreted amyloid precursor protein-α regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 2008, 31, 250–260. [Google Scholar] [CrossRef]
- Araki, W.; Kitaguchi, N.; Tokushima, Y.; Ishii, K.; Aratake, H.; Shimohama, S.; Nakamura, S.; Kimura, J. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem. Biophys. Res. Commun. 1991, 181, 265–271. [Google Scholar] [CrossRef]
- Young-Pearse, T.L.; Chen, A.C.; Chang, R.; Marquez, C.; Selkoe, D.J. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Zhang, C.-W.; Zhou, Y.; Wong, W.Q.; Lee, L.C.; Kah-Leong, L.; Yoon, S.O.; Hong, W.; Fu, X.-Y.; Soong, T.W.; et al. APP upregulation contributes to retinal ganglion cell degeneration via JNK3. Cell Death Differ. 2018, 25, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Thornton, E.; Vink, R.; Blumbergs, P.C.; Heuvel, C.V.D. Soluble amyloid precursor protein α reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006, 1094, 38–46. [Google Scholar] [CrossRef]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006, 49, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, S.; Pernet, V.; Dorfman, A.L.; Chemtob, S.; Lachapelle, P. Light-Induced Retinopathy: Comparing Adult and Juvenile Rats. Investig. Opthalmol. Vis. Sci. 2006, 47, 3202–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | Species | Dilution | Source | Catalog Number |
|---|---|---|---|---|
| β-Actin | mouse | 1:5000 | Sigma, Oakville, ON, Canada | A5441 |
| β-Amyloid (m3.2) | mouse | 1:1000 | BioLegend, San Diego, CA, USA | 805701 |
| β-Amyloid (6E10) | mouse | 1:2000 | BioLegend | 803001 |
| APP C-Term (CT20) | rabbit | 1:10,000 | Millipore, Etobicoke, ON, Canada | 171610 |
| Amyloid Precursor Protein, C-Term | rabbit | 1:5000 | Sigma | A8717 |
| Brn3a | mouse | 1:200 | Santa Cruz | sc-8429 |
| M-opsin | rabbit | 1:5000 | Millipore | AB5405 |
| PKCα | mouse | 1:200 | Santa Cruz | sc-17769 |
| Recoverin | rabbit | 1:2000 | Millipore | AB5585 |
| S-opsin | goat | 1:2000 | Santa Cruz | sc-14363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joly, S.; Rodriguez, L.; Pernet, V. The Lack of Amyloidogenic Activity Is Persistent in Old WT and APPswe/PS1ΔE9 Mouse Retinae. Int. J. Mol. Sci. 2021, 22, 11344. https://doi.org/10.3390/ijms222111344
Joly S, Rodriguez L, Pernet V. The Lack of Amyloidogenic Activity Is Persistent in Old WT and APPswe/PS1ΔE9 Mouse Retinae. International Journal of Molecular Sciences. 2021; 22(21):11344. https://doi.org/10.3390/ijms222111344
Chicago/Turabian StyleJoly, Sandrine, Léa Rodriguez, and Vincent Pernet. 2021. "The Lack of Amyloidogenic Activity Is Persistent in Old WT and APPswe/PS1ΔE9 Mouse Retinae" International Journal of Molecular Sciences 22, no. 21: 11344. https://doi.org/10.3390/ijms222111344





