The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice
Abstract
1. Introduction
2. Results
2.1. SSd Affects Body Weight and Food Intake in Mice with TAA-Induced Liver Injury
2.2. SSd Affects Liver Weight, Serum ALT, AST, ALP, γ-GT, and Bilirubin in Mice with TAA-Induced Liver Injury
2.3. SSd Reduces Liver Damage in Mice with TAA-Induced Liver Injury
2.4. SSd Affects Serum IL-1β, TNF-α, Fibroblast Growth Factor-21 (FGF21), and C-Reactive Protein (CRP) Levels in Mice with TAA-Induced Liver Injury
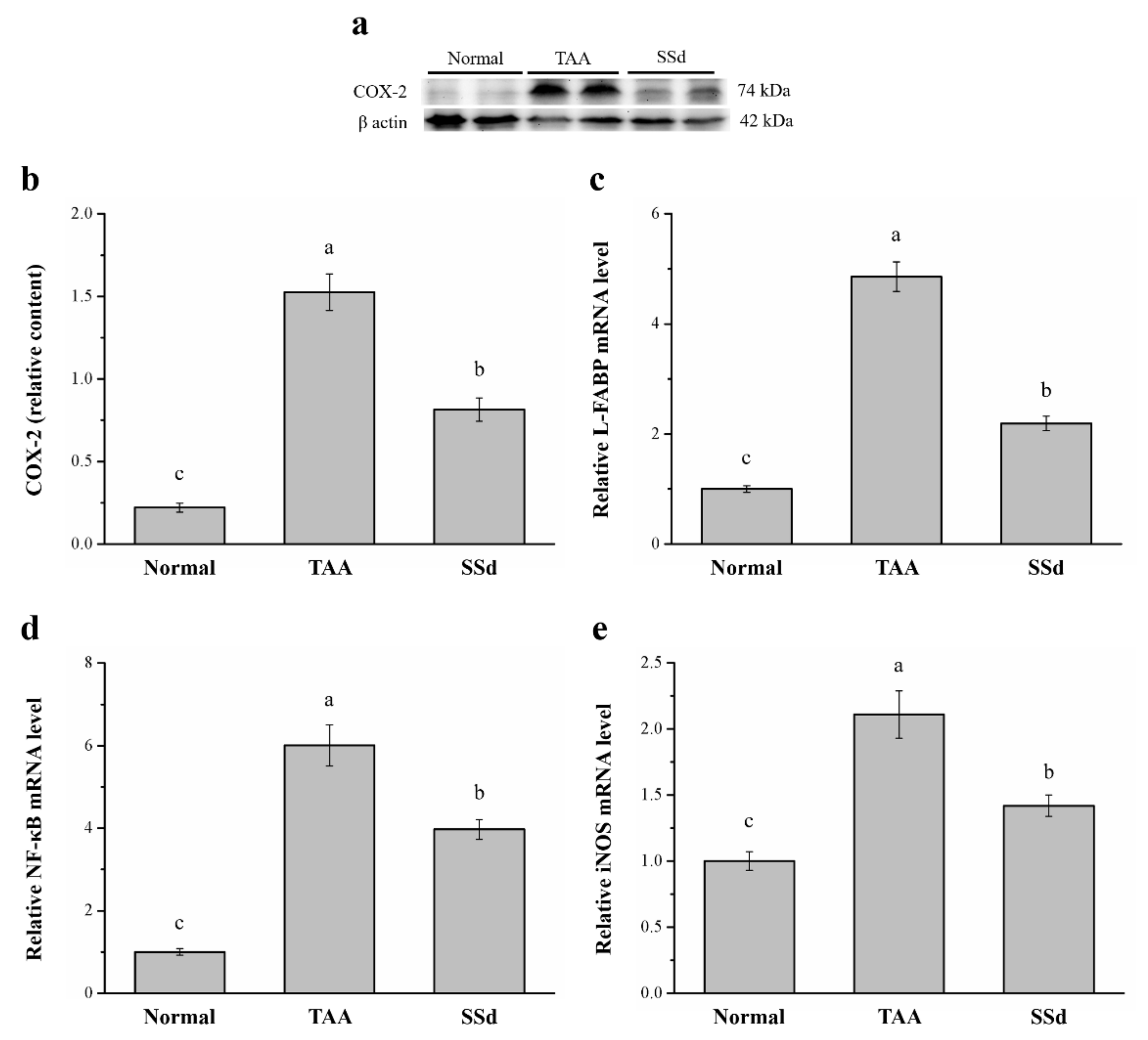
2.5. SSd Aggregates Hepatic Proteins for COX-2 and the mRNA of Liver Fatty Acid–Binding Protein (L-FABP), Inducible Nitric oxide Synthase (iNOS), and NF-κB
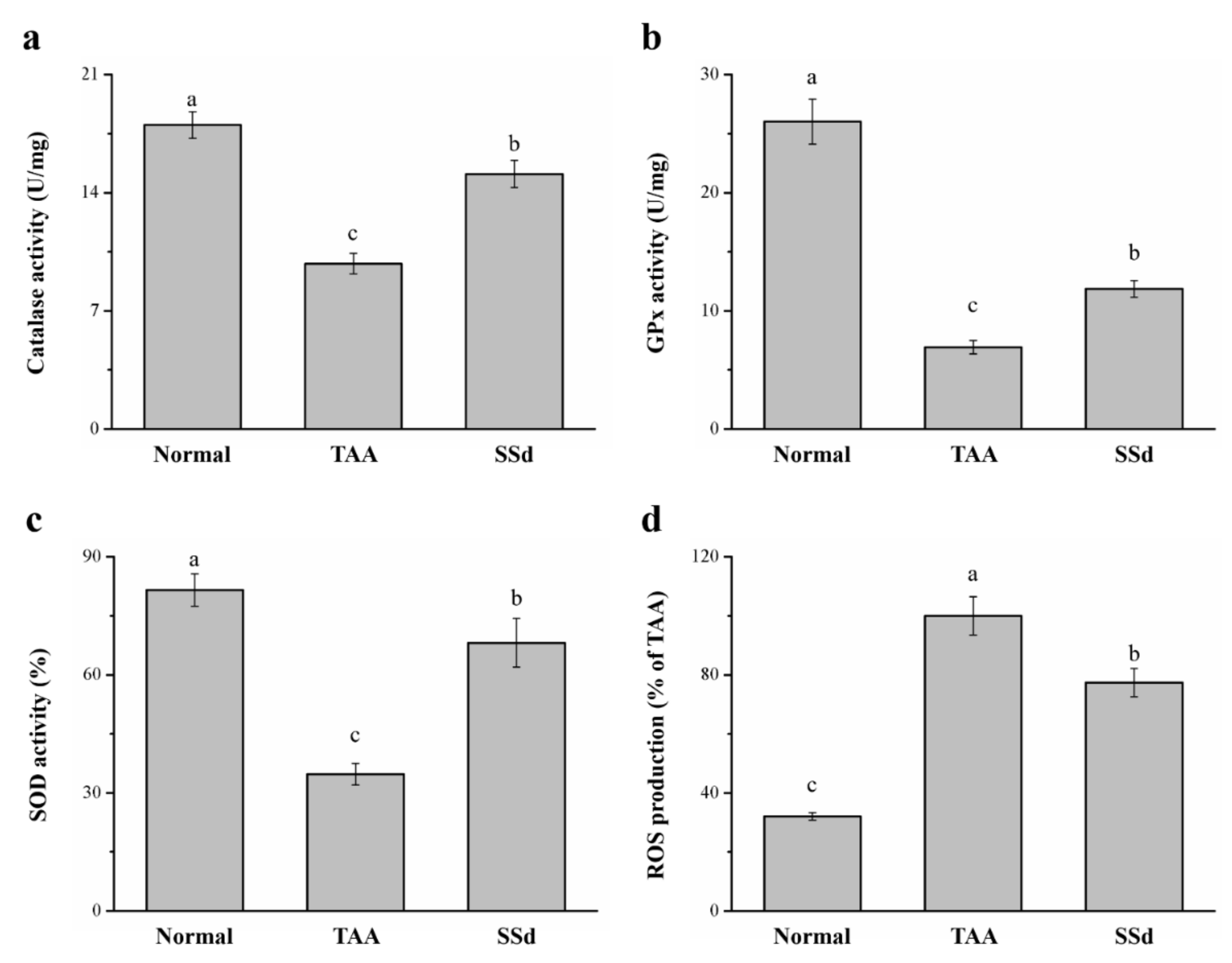
2.6. SSd Increases Antioxidant Enzymes and Reduces Reactive Oxygen Species (ROS) in Mice with TAA-Induced Liver Injury
2.7. SSd Affects Body Weight, Fatty Liver, and Body Weight Gain in HFD-Induced NAFLD Mice
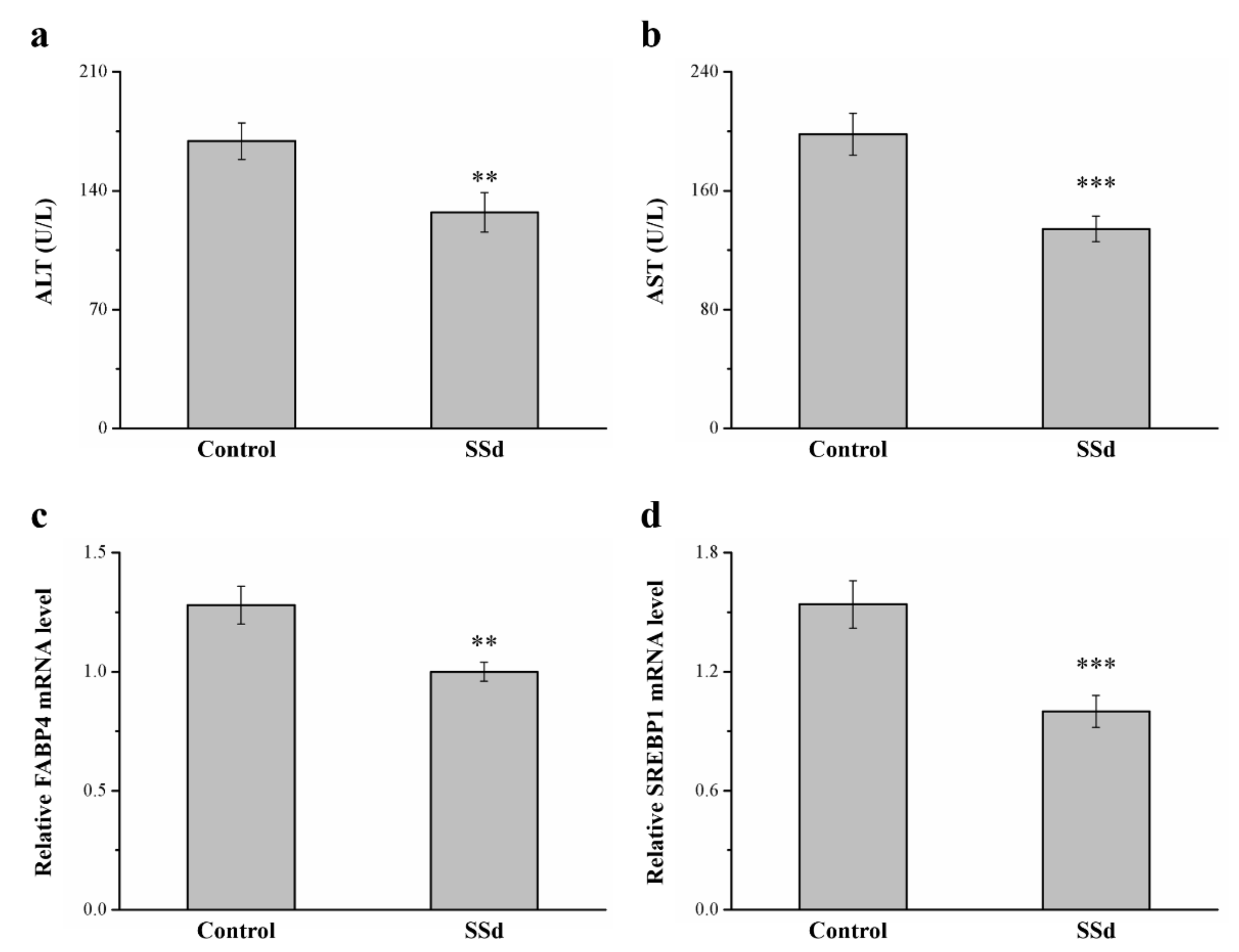
2.8. SSd Affects ALT, AST, and mRNA Levels of FABP4 and Sterol Regulatory Element–Binding Protein 1 (SREBP1) in HFD-Induced NAFLD Mice
2.9. SSd Decreases Serum and Hepatic Triglycerides and the Protein Expression of eIF2α, ATF4, CHOP, and p62
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.1.1. Liver Injury
4.1.2. NAFLD
4.2. Body Weight and Food Intake
4.3. Liver Function Enzyme Tests
4.4. Liver Histopathological Evaluation
4.5. Determination of Serum Inflammatory Cytokines, FGF21, and CRP
4.6. Western Blotting Assay
4.7. RNA Extraction and Real-Time qPCR
4.8. Evaluation of Hepatic CAT, GPx, and SOD
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSd | Saikosaponin d |
| HFD | High fat diet |
| TAA | Thioacetamide |
| CCl4 | Carbon tetrachloride |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor necrosis factor-α |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| ROS | Reactive oxygen species |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible nitric oxide synthase |
| NF-κB | Nuclear factor κB |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| γ-GT | γ-glutamyltransferase |
| RB | Radix Bupleuri |
| FABP4 | Fatty acid-binding protein 4 |
| SREBP1 | Sterol regulatory element–binding protein 1 |
| FASN | Fatty acid synthase |
| FGF-21 | Fibroblast growth factor-21 |
| H&E | Hematoxylin and eosin |
| CRP | C-reactive protein |
| L-FABP | Liver fatty acid binding protein |
| ER | Endoplasmic reticulum |
| eIF2α | Eukaryotic initiation factor 2α subunit |
| ATF4 | Activating transcription factor 4 |
| CHOP | C/EBP homologous protein |
| AUC | Area under the curves |
| UCP1 | Uncoupling protein 1 |
| IHC | Immunohistochemical |
References
- Cullen, J.M. Mechanistic classification of liver injury. Toxicol. Pathol. 2005, 33, 6–8. [Google Scholar] [CrossRef]
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243–287. [Google Scholar] [PubMed]
- de Mingo Pulido, Á.; de Gregorio, E.; Chandra, S.; Colell, A.; Morales, A.; Kronenberg, M.; Marí, M. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Front. Immunol. 2018, 9, 391. [Google Scholar] [CrossRef]
- Ribeiro, P.S.; Cortez-Pinto, H.; Solá, S.; Castro, R.E.; Ramalho, R.M.; Baptista, A.; Rodrigues, C.M. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 2004, 99, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.B.B.; McCullough, A.J. Natural history of nonalcoholic fatty liver disease. Dig. Dis. Sci. 2016, 61, 1226–1233. [Google Scholar] [CrossRef]
- Domenicali, M.; Caraceni, P.; Giannone, F.; Baldassarre, M.; Lucchetti, G.; Quarta, C.; Patti, C.; Catani, L.; Nanni, C.; Lemoli, R.M.; et al. A novel model of CCl4-induced cirrhosis with ascites in the mouse. J. Hepatol. 2009, 51, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kučera, O.; Červinková, Z.; Lotková, H.; Křiváková, P.; Roušar, T.; Mužáková, V.; Hézová, R.; Kandár, R.; Rudolf, E. Protective effect of S-adenosylmethionine against galactosamine-induced injury of rat hepatocytes in primary culture. Physiol. Res. 2006, 55, 551–560. [Google Scholar]
- Chilakapati, J.; Korrapati, M.C.; Hill, R.A.; Warbritton, A.; Latendresse, J.R.; Mehendale, H.M. Toxicokinetics and toxicity of thioacetamide sulfoxide: A metabolite of thioacetamide. Toxicology 2007, 230, 105–116. [Google Scholar] [CrossRef]
- Loh, Z.; Fitzsimmons, R.L.; Reid, R.C.; Ramnath, D.; Clouston, A.; Gupta, P.K.; Irvine, K.M.; Powell, E.E.; Schroder, K.; Stow, J.L.; et al. Inhibitors of class I histone deacetylases attenuate thioacetamide-induced liver fibrosis in mice by suppressing hepatic type 2 inflammation. Br. J. Pharmacol. 2019, 176, 3775–3790. [Google Scholar] [CrossRef]
- Hassan, H.A. Oxidative stress as a crucial factor in liver associated disorders: Potential therapeutic effect of antioxidants. In The Liver; Academic Press: Cambridge, MA, USA, 2018; pp. 121–130. [Google Scholar]
- Ali, N.; Sumon, A.H.; Fariha, K.A.; Asaduzzaman, M.; Kathak, R.R.; Molla, N.H.; Islam, F. Assessment of the relationship of serum liver enzymes activity with general and abdominal obesity in an urban Bangladeshi population. Sci. Rep. 2021, 11, 6640. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Fang, J.Y.; Hong, T.L.; Wang, T.C.; Zhou, Y.E.; Lin, T.C. Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl4-induced liver injury in rats. Int. Immunopharmacol. 2013, 15, 106–113. [Google Scholar] [CrossRef]
- Furtado, K.S.; Prado, M.G.; Aguiar Silva, M.A.; Dias, M.C.; Rivelli, D.P.; Rodrigues, M.A.; Barbisan, L.F. Coffee and caffeine protect against liver injury induced by thioacetamide in male Wistar rats. Basic Clin. Pharmacol. Toxicol. 2012, 111, 339–347. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, X.; Luo, Y.; Fan, W.; Shen, T.; Ding, C.; Yao, M.; Song, S.; Yan, L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019, 71, 110–121. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wei, J.; Nie, J.; Bai, F.; Zhu, X.; Zhuo, L.; Lu, Z.; Huang, Q. Inhibition of RKIP aggravates thioacetamide-induced acute liver failure in mice. Exp. Ther. Med. 2018, 16, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Yang, W.C.; Lin, C.F.; Wang, C.M.; Liu, H.Y.; Lin, C.S.; Lin, J.W.; Lin, W.L.; Lin, T.C.; Fan, P.S.; et al. The ameliorative effects of fucoidan in Thioacetaide-induced liver injury in mice. Molecules 2021, 26, 1937. [Google Scholar] [CrossRef]
- Chang, G.R.; Hsieh, W.T.; Chou, L.S.; Lin, C.S.; Wu, C.F.; Lin, J.W.; Lin, W.L.; Lin, T.C.; Liao, H.J.; Kao, C.Y.; et al. Curcumin improved glucose intolerance, renal injury, and nonalcoholic fatty liver disease and decreased chromium loss through urine in obese mice. Processes 2021, 9, 1132. [Google Scholar] [CrossRef]
- Recena Aydos, L.; Aparecida do Amaral, L.; Serafim de Souza, R.; Jacobowski, A.C.; Freitas dos Santos, E.; Rodrigues Macedo, M.L. Nonalcoholic fatty liver disease induced by high-fat diet in C57BL/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse models of nonalcoholic steatohepatitis: Toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef]
- Ashour, M.L.; Wink, M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef]
- Zheng, L.V.; Rong, S.U.N. Research development on Chemincal compositions in related with efficacy and toxicity. Chin. J. Pharm. 2013, 10, 545. [Google Scholar]
- Dang, S.S.; Wang, B.F.; Cheng, Y.A.; Song, P.; Liu, Z.G.; Li, Z.F. Inhibitory effects of saikosaponin-d on CCl4-induced hepatic fibrogenesis in rats. World J. Gastroenterol. 2007, 13, 557–563. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. The proliferative inhibition and apoptotic mechanism of Saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 1231–1242. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Chiang, L.C.; Lin, C.C. Involvement of p53, nuclear factor κB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett. 2004, 213, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Xiong, W.; Li, J.; An, Y.; Ren, J.; Xie, Y.; Xue, H.; Yan, D.; Li, M.; et al. Saikosaponin-d ameliorates dextran sulfate sodium-induced colitis by suppressing NF-κB activation and modulating the gut microbiota in mice. Int. Immunopharmacol. 2020, 81, 106288. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M. The role of saikosaponins in therapeutic strategies for age-related diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8275256. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; McGowan, E.; Li, Y.; Zhu, X.; Lu, X.; Zhu, Z.; Lin, Y.; He, S. Saikosaponin-d suppresses COX2 through p-STAT3/C/EBPβ signaling pathway in liver cancer: A novel mechanism of action. Front. Pharmacol. 2019, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, M.; Zhong, T.D.; Fang, X.M. Saikosaponin-d attenuates ventilator-induced lung injury in rats. Int. J. Clin. Exp. Med. 2015, 8, 15137–15145. [Google Scholar] [PubMed]
- Wu, K. Antitumor activity and mechanism of saikosaponin D combined with oxaliplatin on A549 cells-bearing nude mice. J. China Pharm. Univ. 2015, 6, 355–358. [Google Scholar]
- Hu, J.; Li, P.; Shi, B.; Tie, J. Effects and Mechanisms of Saikosaponin D Improving the Sensitivity of Human Gastric Cancer Cells to Cisplatin. ACS Omega 2021, 6, 18745–18755. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017, 55, 620–635. [Google Scholar] [CrossRef]
- Tak, E.; Jung, D.H.; Kim, S.H.; Park, G.C.; Jun, D.Y.; Lee, J.; Jung, B.H.; Kirchner, V.A.; Hwang, S.; Song, G.W.; et al. Protective role of hypoxia-inducible factor-1alpha-dependent CD39 and CD73 in fulminant acute liver failure. Toxicol. Appl. Pharmacol. 2017, 314, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Much, C.; Gorzolla, I.C.; Schittenhelm, J.; Kloor, D.; Stahl, G.L.; Eltzschig, H.K. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic. Gastroenterology 2008, 135, 1739–1750.e3. [Google Scholar] [CrossRef] [PubMed]
- Keinicke, H.; Sun, G.; Mentzel, C.M.J.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Hou, P.H.; Yang, W.C.; Wang, C.M.; Fan, P.S.; Liao, H.J.; Chen, T.P. Doxepin exacerbates renal damage, glucose intolerance, nonalcoholic fatty liver disease and urinary chromium loss in obese mice. Pharmaceuticals 2021, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Liu, H.Y.; Yang, W.C.; Wang, C.M.; Wu, C.F.; Lin, J.W.; Lin, W.L.; Wang, Y.C.; Lin, T.C.; Liao, H.J.; et al. Clozapine worsens glucose intolerance, nonalcoholic fatty liver disease, kidney damage and retinal injury and increases renal reactive oxygen species production and chromium loss in obese mice. Int. J. Mol. Sci. 2021, 22, 6680. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, R.; Mollá, B.; Flores, J.M.; Boscá, L.; Casado, M.; Martín-Sanz, P. Constitutive expression of cyclo-oxygenase 2 transgene in hepatocytes protects against liver injury. Biochem. J. 2008, 416, 337–346. [Google Scholar] [CrossRef]
- Salama, S.M.; AlRashdi, A.S.; Abdulla, M.A.; Hassandarvish, P.; Bilgen, M. Protective activity of Panduratin A against thioacetamide-induced oxidative damage: Demonstration with in vitro experiments using WRL-68 liver cell line. BMC Complement. Altern. Med. 2013, 13, 279. [Google Scholar] [CrossRef]
- Sakai, N.; Van Sweringen, H.L.; Belizaire, R.M.; Quillin, R.C.; Schuster, R.; Blanchard, J.; Burns, J.M.; Tevar, A.D.; Edwards, M.J.; Lentsch, A.B. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J. Gastroenterol. Hepatol. 2012, 27, 1609–1616. [Google Scholar] [CrossRef]
- Hou, P.H.; Chang, G.R.; Chen, C.P.; Lin, Y.L.; Chao, I.S.; Shen, T.T.; Mao, F.C. Long-term administration of olanzapine induces adiposity and increases hepatic fatty acid desaturation protein in female C57BL/6J mice. Iran. J. Basic Med. Sci. 2018, 21, 495–501. [Google Scholar]
- Chang, G.R.; Hou, P.H.; Chen, W.K.; Lin, C.T.; Tsai, H.P.; Mao, F.C. Exercise affects blood glucose levels and tissue chromium distribution in high-fat diet-fed C57BL6 mice. Molecules 2020, 25, 1658. [Google Scholar] [CrossRef]
- Chang, G.R.; Chiu, Y.S.; Wu, Y.Y.; Lin, Y.C.; Hou, P.H.; Mao, F.C. Rapamycin impairs HPD-induced beneficial effects on glucose homeostasis. Br. J. Pharmacol. 2015, 172, 3793–3804. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Xin, Y.; Wei, J.; Chunhua, M.; Danhong, Y.; Jianguo, Z.; Zongqi, C.; Jian-An, B. Protective effects of ginsenoside Rg1 against carbon tetrachloride-induced liver injury in mice through suppression of inflammation. Phytomedicine 2016, 23, 583–588. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.; Sookoian, S. The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Gandhi, S.; Loomba, R. Advances in non-invasive biomarkers for the diagnosis and monitoring of non-alcoholic fatty liver disease. Metabolism 2020, 111, 154259. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Roman, J.; Siddiqui, M.S. The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Endocrinol. Diabetes Metab. 2020, 3, e00127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.Y.; Zhang, D.; Zhao, Y.L. Cognitive improvements and reduction in amyloid plaque deposition by saikosaponin D treatment in a murine model of Alzheimer’s disease. Exp. Ther. Med. 2020, 20, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Sheikh, N.; Ali, G.; Tayyeb, A. Fagonia indica repairs hepatic damage through expression regulation of toll-like receptors in a liver injury model. J. Immunol. Res. 2018, 2018, 7967135. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Wu, Y.Y.; Chiu, Y.S.; Chen, W.Y.; Liao, J.W.; Hsu, H.M.; Chao, T.H.; Hung, S.W.; Mao, F.C. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin. Pharmacol. Toxicol. 2009, 105, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Saleem, S.; Shameem, S.; Ahmed, S.P.; Parveen, T.; Haleem, D.J. Is anorexia in thioacetamide-induced cirrhosis related to an altered brain serotonin concentration? Pol. J. Pharmacol. 2004, 56, 73–78. [Google Scholar]
- Singh, V.; Visen, P.K.; Patnaik, G.K.; Kapoor, N.K.; Dhawan, B.N. Effect of picroliv on low density lipoprotein receptor binding of rat hepatocytes in hepatic damage induced by paracetamol. Indian J. Biochem. Biophys. 1992, 29, 428–432. [Google Scholar] [PubMed]
- Lennie, T.A. Relationship of body energy status to inflammation-induced anorexia and weight loss. Physiol. Behav. 1998, 64, 475–481. [Google Scholar] [CrossRef]
- Lennie, T.A.; Wortman, M.D.; Seeley, R.J. Activity of body energy regulatory pathways in inflammation-induced anorexia. Physiol. Behav. 2001, 73, 517–523. [Google Scholar] [CrossRef]
- Czechowska, G.; Celinski, K.; Korolczuk, A.; Wojcicka, G.; Dudka, J.; Bojarska, A.; Reiter, R.J. Protective effects of melatonin against thioacetamide-induced liver fibrosis in rats. J. Physiol. Pharmacol. 2015, 66, 567–579. [Google Scholar]
- Alkiyumi, S.S.; Abdullah, M.A.; Alrashdi, A.S.; Salama, S.M.; Abdelwahab, S.I.; Hadi, A.H.A. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules 2012, 17, 6146–6155. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, Q.; Zhang, K.; Zheng, Y.; Zhao, S. Protective effects of different extracts of Eucommia ulmoides Oliv. against thioacetamide-induced hepatotoxicity in mice. Indian J. Exp. Biol. 2012, 50, 875–882. [Google Scholar]
- Wu, H.T.; Chuang, Y.W.; Huang, C.P.; Chang, M.H. Loss of angiotensin converting enzyme II (ACE2) accelerates the development of liver injury induced by thioacetamide. Exp. Anim. 2018, 67, 41–49. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Rao, X.; Zheng, C.; Peng, X. Saikosaponin a ameliorates lipopolysaccharide and d-galactosamine-induced liver injury via activating LXRα. Int. Immunopharmacol. 2019, 72, 131–137. [Google Scholar] [CrossRef]
- Ajiboye, T.O. Standardized extract of Vitex doniana sweet stalls protein oxidation, lipid peroxidation and DNA fragmention in acetaminophen-induced hepatotoxicity. J. Ethnopharmacol. 2015, 164, 273–282. [Google Scholar] [CrossRef]
- Wu, L.Y.; Hsu, H.P.; Chen, C.C.; Lin, N.N.; Hsiao, Y.J.; Chen, W.C.; Cheng, Z.H.; Shyu, C.L.; Wang, J.S.; Chiu, Y.T. A study of the antifibrotic effects of traditional Chinese herbs modified Chai-Hu-Shu-Gan powder on carbon tetrachloride-induced liver fibrosis in rats. J. Chin. Med. 2004, 15, 257–271. [Google Scholar]
- Chen, I.S.; Chen, Y.C.; Chou, C.H.; Chuang, R.F.; Sheen, L.Y.; Chiu, C.H. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J. Sci. Food Agric. 2012, 92, 1441–1447. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Jena, G.; Kumar, V. Dimethyl fumarate protects thioacetamide-induced liver damage in rats: Studies on Nrf2, NLRP3, and NF-κB. J. Biochem. Mol. Toxicol. 2020, 34, e22476. [Google Scholar] [CrossRef]
- Dasarathy, S. Inflammation and liver. J. Parenter. Enter. Nutr. 2008, 32, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Ben-Ari, Z.; Masoud, R.; Pappo, O.; Harats, D.; Kamari, Y.; Safran, M. Interleukin-1α and interleukin-1β play a central role in the pathogenesis of fulminant hepatic failure in mice. PLoS ONE 2017, 12, e0184084. [Google Scholar] [CrossRef] [PubMed]
- Kamari, Y.; Shaish, A.; Vax, E.; Shemesh, S.; Kandel-Kfir, M.; Arbel, Y.; Olteanu, S.; Barshack, I.; Dotan, S.; Voronov, E.; et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 2011, 55, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.F.; Lu, Z.Y.; Massart, C.; Levon, K. Dynamic immune/inflammation precision medicine: The good and the bad inflammation in infection and cancer. Front. Immunol. 2021, 12, 595722. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Yamada, Y.; Moriwaki, H.; Saito, K.; Seishima, M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 2005, 29, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sakai, T. Animal models for the study of liver fibrosis: New insights from knockout mouse models. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G729–G738. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Gallucci, R.M.; Hulderman, T.; Wilson, R.; Kommineni, C.; Rao, M.; Luster, M.I. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol. Appl. Pharmacol. 2001, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, Y.; Li, H.; Jia, W.; Man, K.; Lo, C.M.; Wang, Y.; Lam, K.S.; Xu, A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 2014, 60, 977–989. [Google Scholar] [CrossRef]
- Liu, W.H.; Liu, T.C.; Yin, M.C. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem. Toxicol. 2008, 46, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Moutachakkir, M.; Lamrani Hanchi, A.; Baraou, A.; Boukhira, A.; Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin. 2017, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Giannitrapani, L.; Ingrao, S.; Soresi, M.; Florena, A.M.; La Spada, E.; Sandonato, L.; D’Alessandro, N.; Cervello, M.; Montalto, G. Cyclooxygenase-2 expression in chronic liver diseases and hepatocellular carcinoma: An immunohistochemical study. Ann. N. Y. Acad. Sci. 2009, 1155, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cakir, O.O.; Toker, A.; Ataseven, H.; Demir, A.; Polat, H. The importance of liver-fatty acid binding protein in diagnosis of liver damage in patients with acute hepatitis. J. Clin. Diagn. Res. 2017, 11, OC17–OC21. [Google Scholar] [CrossRef] [PubMed]
- Özenirler, S.; Degertekin, C.K.; Erkan, G.; Elbeğ, Ş.; Tuncer, C.; Kandilc, U.; Akyol, G. Serum liver fatty acid binding protein shows good correlation with liver histology in NASH. Hepato-Gastroenterology 2013, 60, 1095–1100. [Google Scholar]
- Xiao, C.; Ghosh, S. NF-κB, an evolutionarily conserved mediator of immune and inflammatory responses. Mech. Lymphocyte Act. Immune Regul. 2005, X2005, 41–45. [Google Scholar]
- Inokuchi, S.; Aoyama, T.; Miura, K.; Österreicher, C.H.; Kodama, Y.; Miyai, K.; Akira, S.; Brenner, D.A.; Seki, E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 844–849. [Google Scholar] [CrossRef]
- Wójcik, M.; Ramadori, P.; Blaschke, M.; Sultan, S.; Khan, S.; Malik, I.A.; Naz, N.; Martius, G.; Ramadori, G.; Schultze, F.C. Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma. Histochem. Cell Biol. 2012, 137, 217–233. [Google Scholar] [CrossRef]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Diesen, D.L.; Kuo, P.C. Nitric oxide and redox regulation in the liver: Part II. Redox biology in pathologic hepatocytes and implications for intervention. J. Surg. Res. 2011, 167, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Cressman, D.E.; Greenbaum, L.E.; DeAngelis, R.A.; Ciliberto, G.; Furth, E.E.; Poli, V.; Taub, R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996, 274, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.L.; Leng, S.; Beamer, B.; Walston, J.D. Glutathione peroxidase enzyme activity in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 505–509. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Hu, X.; Liu, Z.; Chen, J.; Lu, Y.; Liu, J.; Liao, S.; Zhang, Y.; Liang, R.; et al. The hepatoprotective role of reduced glutathione and its underlying mechanism in oxaliplatin-induced acute liver injury. Oncol. Lett. 2018, 15, 2266–2272. [Google Scholar] [CrossRef]
- Jones, D.P.; Eklöw, L.; Thor, H.; Orrenius, S. Metabolism of hydrogen peroxide in isolated hepatocytes: Relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch. Biochem. Biophys. 1981, 210, 505–516. [Google Scholar] [CrossRef]
- Verkerk, A.; Jongkind, J.F. Vascular cells under peroxide induced oxidative stress: A balance study on in vitro peroxide handling by vascular endothelial and smooth muscle cells. Arch. Biochem. Biophys. 1992, 17, 121–132. [Google Scholar] [CrossRef]
- Wu, S.J.; Lin, Y.H.; Chu, C.C.; Tsai, Y.H.; Chao, J.C.J. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Med. Food 2008, 11, 224–229. [Google Scholar] [CrossRef]
- Chang, G.R.; Chiu, Y.S.; Wu, Y.Y.; Chen, W.Y.; Liao, J.W.; Chao, T.H.; Mao, F.C. Rapamycin protects against high fat diet–induced obesity in C57BL/6J mice. J. Pharmacol. Sci. 2009, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Hou, P.H.; Mao, F.C.; Chang, C.C.; Yang, W.C.; Wu, C.F.; Liao, H.J.; Lin, T.C.; Chou, L.S.; Hsiao, L.W.; et al. Risperidone exacerbates glucose intolerance, nonalcoholic fatty liver disease, and renal impairment in obese mice. Int. J. Mol. Sci. 2021, 22, 409. [Google Scholar] [CrossRef] [PubMed]
- Kozak, L.P.; Anunciado-Koza, R. UCP1: Its involvement and utility in obesity. Int. J. Obes. 2008, 32 (Suppl. 7), S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Hou, P.H.; Mao, F.C.; Su, Y.C.; Wu, C.Y.; Yang, W.C.; Lin, C.S.; Tsai, H.P.; Liao, H.J.; Chang, G.R. Mirtazapine reduces adipocyte hypertrophy and increases glucose transporter expression in obese mice. Animals 2020, 10, 1423. [Google Scholar] [CrossRef]
- Lescot, T.; Karvellas, C.; Beaussier, M.; Magder, S.; Riou, B. Acquired liver injury in the intensive care unit. Anesthesiology 2012, 117, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Kim, H.S.; Im, J.H.; Kim, J.W.; Jun, D.W.; Lim, S.C.; Lee, K.; Choi, J.M.; Kim, S.K.; Kang, K.W. GPR119: A promising target for nonalcoholic fatty liver disease. FASEB J. 2016, 30, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Wilson, P.W.; Schaefer, E.J. Impact of body mass index on coronary heart disease risk factors in men and women: The Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1509–1515. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Kawasaki, N.; Asada, R.; Saito, A.; Kanemoto, S.; Imaizumi, K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012, 2, 799. [Google Scholar] [CrossRef]
- Kim, H.J.; Joe, Y.; Kim, S.K.; Park, S.U.; Park, J.; Chen, Y.; Kim, J.; Ryu, J.; Cho, G.J.; Surh, Y.J.; et al. Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK-eIF2α-ATF4 pathway. Free Radic. Biol. Med. 2017, 110, 81–91. [Google Scholar] [CrossRef]
- Lauressergues, E.; Bert, E.; Duriez, P.; Hum, D.; Majd, Z.; Staels, B.; Cussac, D. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology 2012, 62, 784–796. [Google Scholar] [CrossRef]
- Ren, L.P.; Yu, X.; Song, G.Y.; Zhang, P.; Sun, L.N.; Chen, S.C.; Hu, Z.J.; Zhang, X.M. Impact of activating transcription factor 4 signaling on lipogenesis in HepG2 cells. Mol. Med. Rep. 2016, 14, 1649–1658. [Google Scholar] [CrossRef][Green Version]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Scheuner, D.; Ron, D.; Pennathur, S.; Kaufman, R.J. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 2008, 118, 3378–3389. [Google Scholar] [CrossRef] [PubMed]
- Czaja, M.J. Function of autophagy in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2016, 61, 1304–1313. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Kassi, E. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE (−/−) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- Niture, S.; Lin, M.; Rios-Colon, L.; Qi, Q.; Moore, J.T.; Kumar, D. Emerging roles of impaired autophagy in fatty liver disease and hepatocellular carcinoma. Int. J. Hepatol. 2021, 2021, 6675762. [Google Scholar] [CrossRef]
- Fukuo, Y.; Yamashina, S.; Sonoue, H.; Arakawa, A.; Nakadera, E.; Aoyama, T.; Uchiyama, A.; Kon, K.; Ikejima, K.; Watanabe, S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol. Res. 2014, 44, 1026–1036. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, Y.; Wang, D.; Cheng, X.; Yang, F.; Zhang, Q.; Xue, Z.; Li, Y.; Zhang, L.; et al. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget 2016, 7, 82864–82875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Que, R.; Lin, L.; Shen, Y.; Liu, J.; Li, Y. Inhibition of oxidative stress and NLRP3 inflammasome by Saikosaponin-d alleviates acute liver injury in carbon tetrachloride-induced hepatitis in mice. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950593. [Google Scholar] [CrossRef]
- Liu, A.; Tanaka, N.; Sun, L.; Guo, B.; Kim, J.H.; Krausz, K.W.; Fang, Z.; Jiang, C.; Yang, J.; Gonzalez, F.J. Saikosaponin D protects against acetaminophen-induced hepatotoxicity by inhibiting NF-κB and STAT3 signaling. Chem. Biol. Interact. 2014, 223, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhou, M.; Que, R.; Chen, Y.; Liu, X.; Zhang, K.; Shi, Z.; Li, Y. Saikosaponin-d protects against liver fibrosis by regulating estrogen receptor-β/NLRP3 inflammasome pathway. Biochem. Cell Biol. 2021, 99, 561. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, G.-R.; Lin, W.-L.; Lin, T.-C.; Liao, H.-J.; Lu, Y.-W. The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice. Int. J. Mol. Sci. 2021, 22, 11383. https://doi.org/10.3390/ijms222111383
Chang G-R, Lin W-L, Lin T-C, Liao H-J, Lu Y-W. The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice. International Journal of Molecular Sciences. 2021; 22(21):11383. https://doi.org/10.3390/ijms222111383
Chicago/Turabian StyleChang, Geng-Ruei, Wei-Li Lin, Tzu-Chun Lin, Huei-Jyuan Liao, and Yu-Wen Lu. 2021. "The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice" International Journal of Molecular Sciences 22, no. 21: 11383. https://doi.org/10.3390/ijms222111383
APA StyleChang, G.-R., Lin, W.-L., Lin, T.-C., Liao, H.-J., & Lu, Y.-W. (2021). The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice. International Journal of Molecular Sciences, 22(21), 11383. https://doi.org/10.3390/ijms222111383




