Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films
Abstract
:1. Introduction
2. Results and Discussion
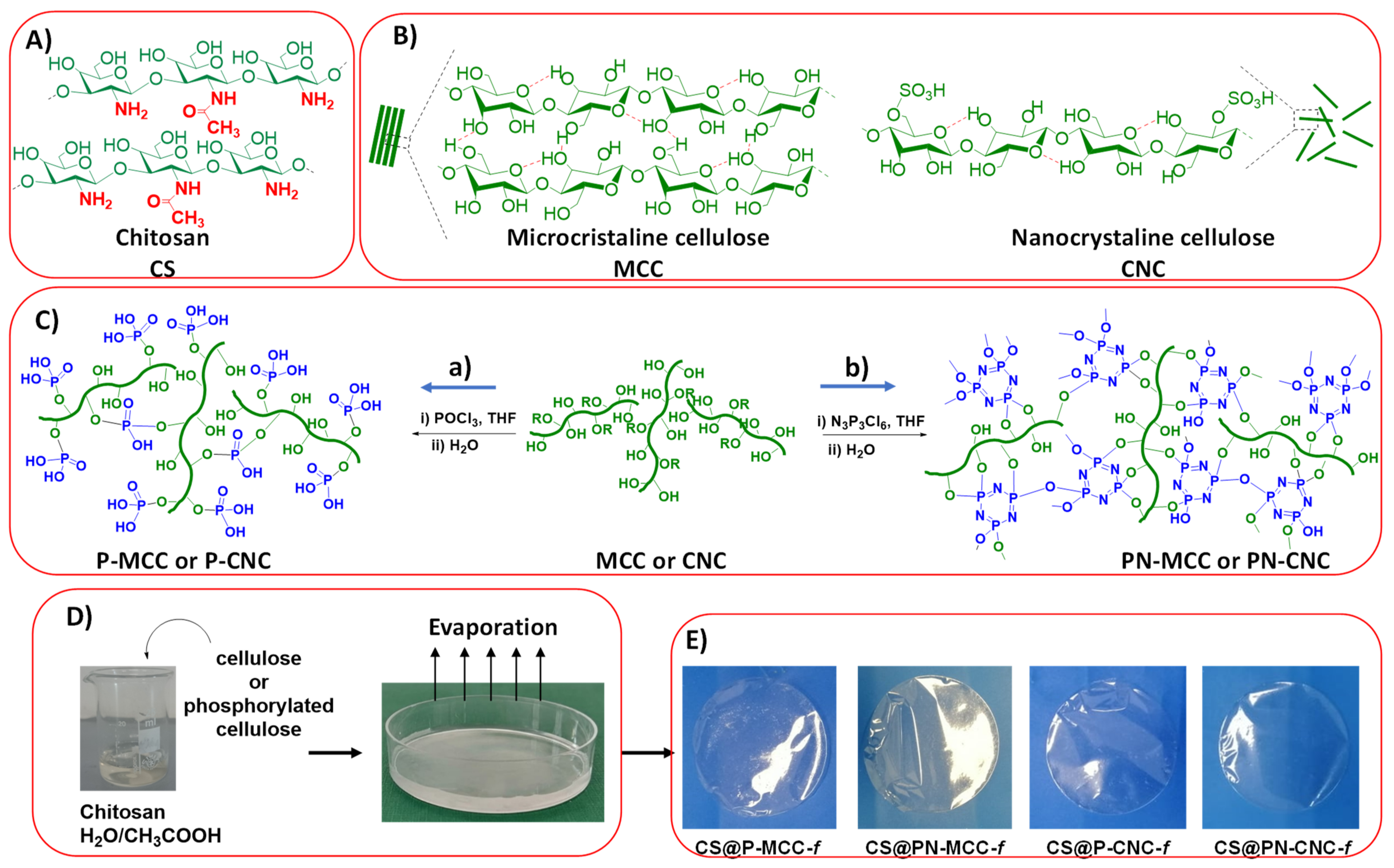
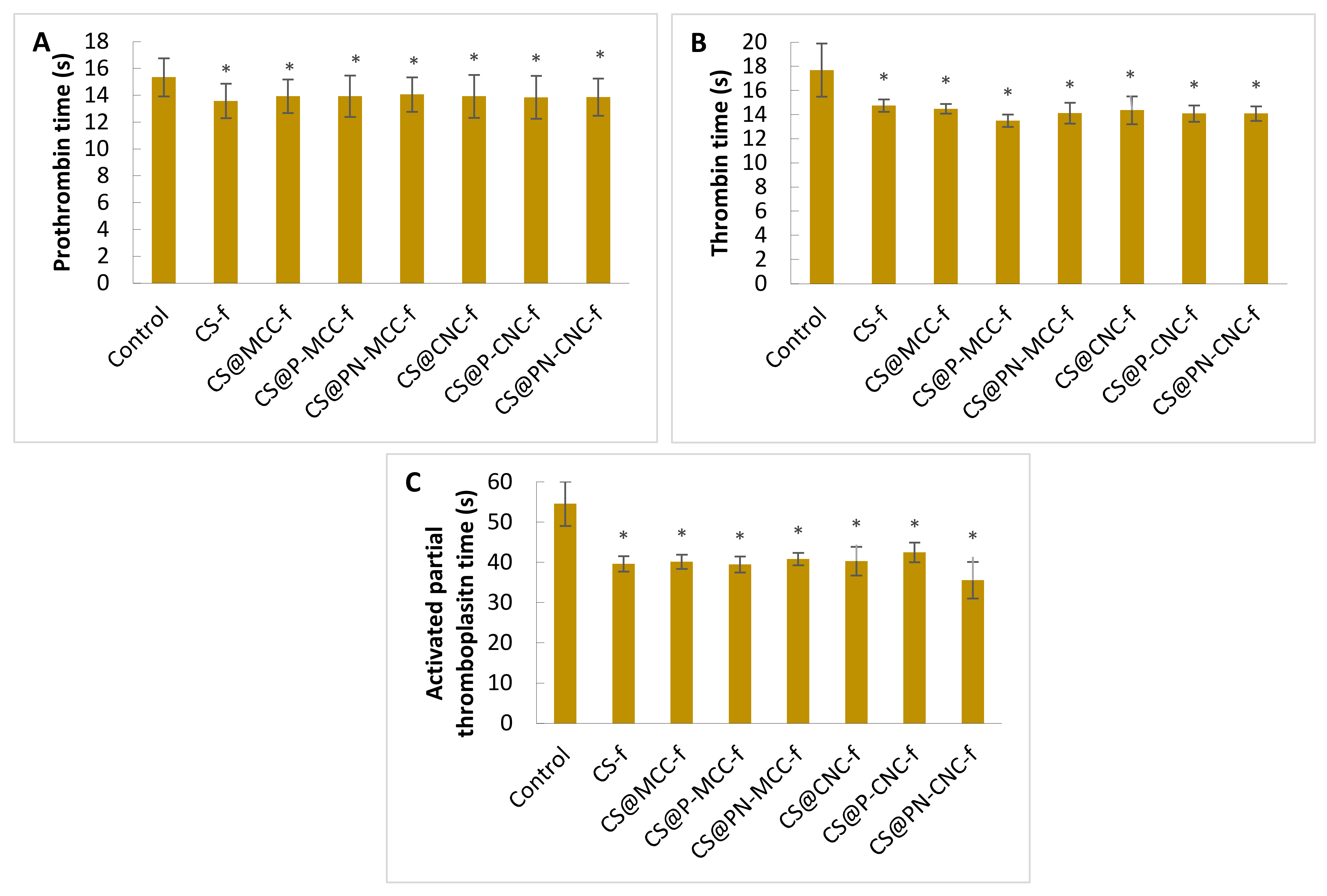
2.1. Hemostatic Properties
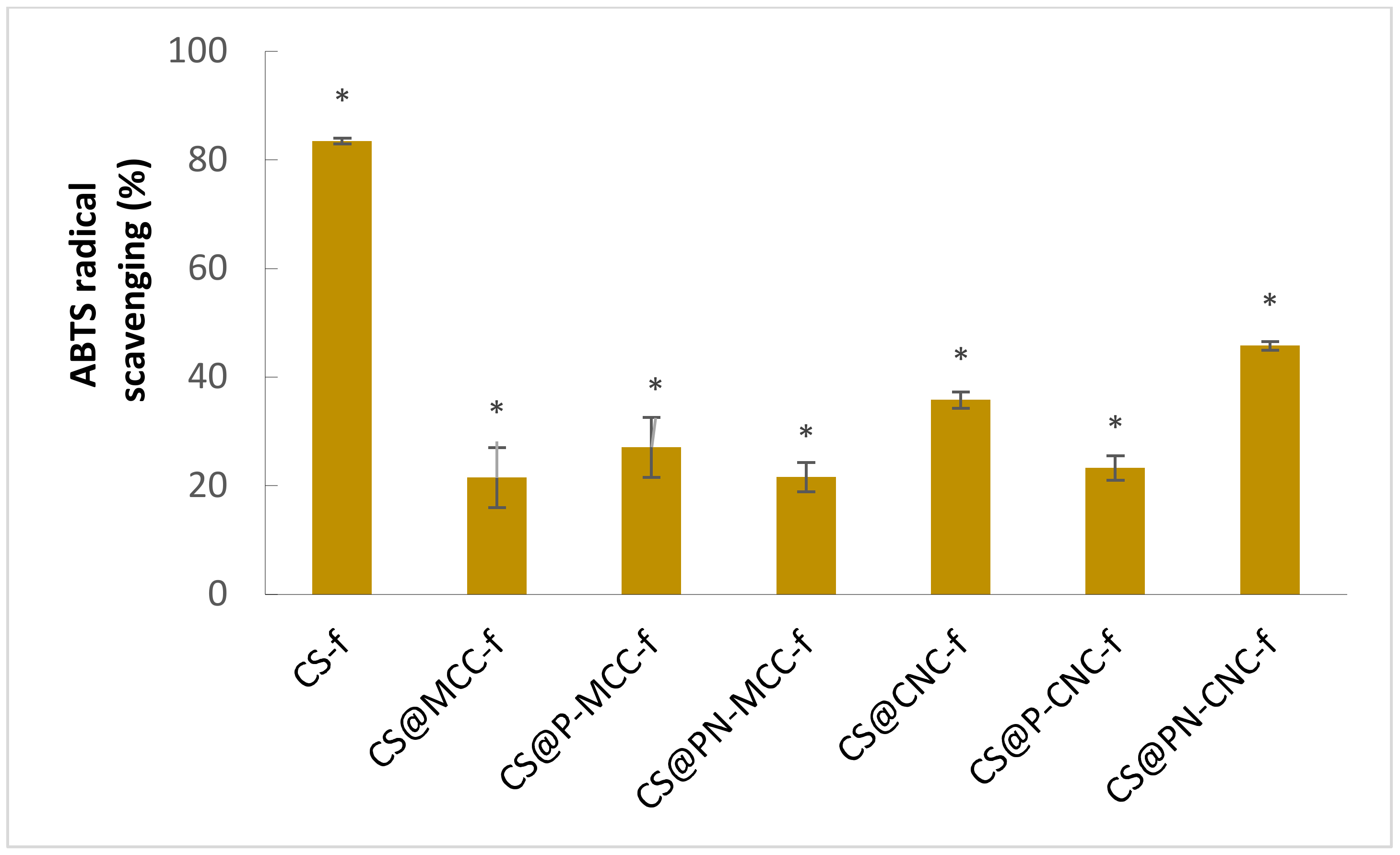
2.2. Measurement of the Total Antioxidant Capacity
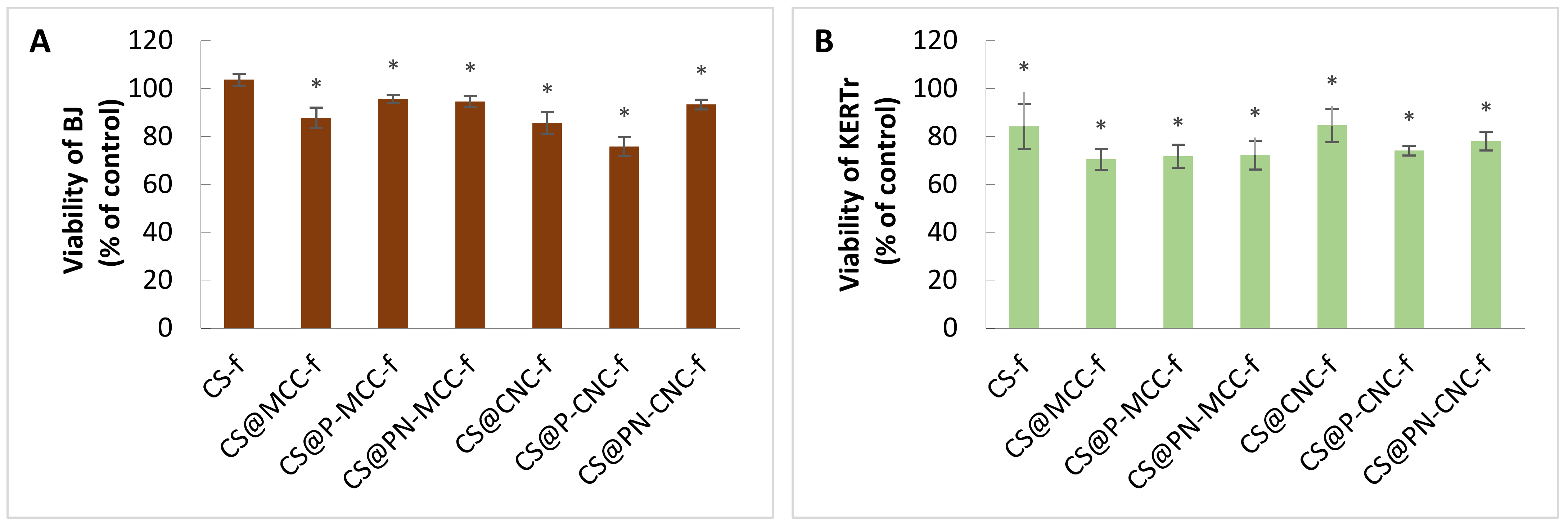
2.3. Cell Viability
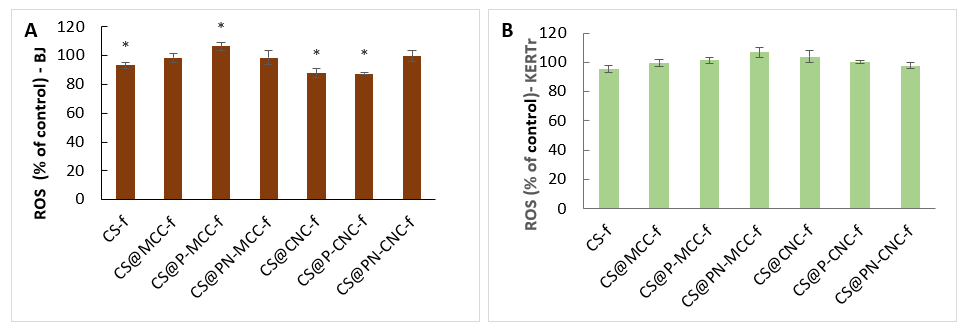
2.4. Generation of Reactive Oxygen Species
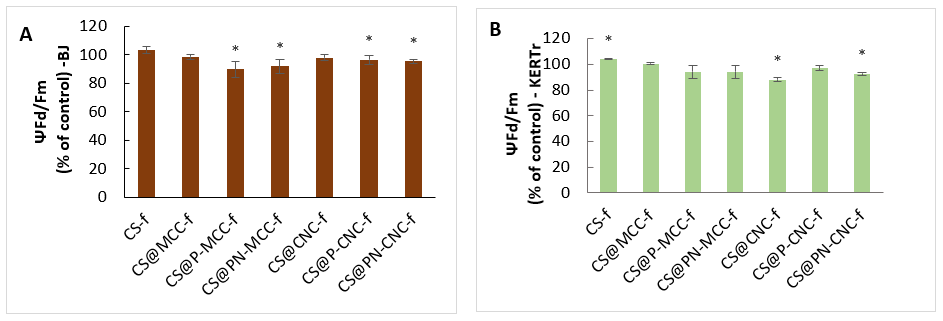
2.5. Assessment of Mitochondrial Membrane Potential (ΔΨm)
2.6. Migration of Fibroblasts and Keratinocytes
2.7. Genotoxicity
3. Materials and Methods
3.1. Materials
3.2. Measurements of Prothrombin Time (PT)
3.3. Measurements of the Thrombin Time (TT)
3.4. Activated Partial Thromboplastin Time (aPTT)
3.5. Measurement of the Total Antioxidant Capacity
3.6. Cell Culture
3.7. Cell Viability Assay (MTT Assay)
3.8. Generation of Reactive Oxygen Species
3.9. Assessment of the Mitochondrial Membrane Potential (ΔΨm)
3.10. Cell Migration
3.11. Comet Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atala, A.; Lanza, R.; Thomson, J.A.; Nerem, R. Principles of Regenerative Medicine; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Lanza, R.; Langer, R.; Vacanti, J. Principles of Tissue Engineering; Elsevier: Burlington, MA, USA, 2007; pp. 421–429. [Google Scholar]
- Roubelakis, M.G.; Trohatou, O.; Roubelakis, A.; Mili, E.; Kalaitzopoulos, I.; Papazoglou, G.; Pappa, K.I.; Anagnou, N.P. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. 2014, 10, 417–428. [Google Scholar] [CrossRef]
- Kalinin, A.E.; Kajava, A.V.; Steinert, P.M. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. Bioessays 2002, 24, 789–800. [Google Scholar] [CrossRef]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar] [PubMed]
- Harris, T.R.; Bransford, J.D.; Brophy, S.P. Roles for learning sciences and learning technologies in biomedical engineering education: A review of recent advances. Annu. Rev. Biomed. Eng. 2002, 4, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Pittman, J. Effect of aging on wound healing: Current concepts. J. Wound Ostomy Cont. Nurs. 2007, 34, 412–417. [Google Scholar] [CrossRef]
- Pikuła, M.; Langa, P.; Kosikowska, P.; Trzonkowski, P. Komórki macierzyste i czynniki wzrostu w gojeniu ran. Postepy Hig. Med. Dosw. 2015, 69, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L. A comparative study of the mechanism of wound healing. J. Med. Res. 1920, 41, 247–281. [Google Scholar]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Andrea, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chatterjee, M.P. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Cheng, H.; Li, C.; Jiang, Y.; Wang, B.; Wang, F.; Mao, Z.; Xu, H.; Wang, L.; Sui, X. Facile preparation of polysaccharide-based sponges and their potential application in wound dressing. J. Mater. Chem. B. 2018, 6, 634–640. [Google Scholar] [CrossRef]
- Jing, X.; Sun, Y.; Ma, X.; Hu, H. Marine polysaccharides: Green and recyclable resources as wound dressings. Mater. Chem. Front. 2021, 5, 5595–5616. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- El Kadib, A. Green and Functional Aerogels by Macromolecular and Textural Engineering of Chitosan Microspheres. Chem. Rec. 2020, 20, 753–772. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100–102. [Google Scholar]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Chabbi, J.; Aqil, A.; Katir, N.; Vertruyen, B.; Jerome, C.; Lahcini, M.; El Kadib, A. Aldehyde-conjugated chitosan-graphene oxide glucodynamers: Ternary cooperative assembly and controlled chemical release. Carbohydr. Polym 2020, 230, 115634. [Google Scholar] [CrossRef]
- Wrońska, N.; Katir, N.; Miłowska, K.; Hammi, N.; Nowak, M.; Kędzierska, M.; Anouar, A.; Zawadzka, K.; Bryszewska, M.; El Kadib, A.; et al. Antimicrobial effect of chitosan films on food spoilage bacteria. Int. J. Mol. Sci. 2021, 22, 5839. [Google Scholar] [CrossRef]
- Wronska, N.; Anouar, A.; El Achaby, M.; Zawadzka, K.; Kedzierska, M.; Miłowska, K.; Katir, N.; Draoui, K.; Rózalska, S.; Piwonski, I.; et al. Chitosan-Functionalized Graphene Nanocomposite Films: Interfacial Interplay and Biological Activity. Materials 2020, 13, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammi, N.; Wrońska, N.; Katir, N.; Lisowska, K.; Marcotte, N.; Cacciaguerra, T.; Bryszewska, M.; El Kadib, A. Supramolecular chemistry driven preparation of nanostructured, transformable and biologically-active chitosan-clustered single, binary and ternary metal oxide bioplastics. ACS Appl. Bio Mater. 2019, 2, 61–69. [Google Scholar]
- Chabbi, J.; Jennah, O.; Katir, N.; Lahcini, M.; Bousmina, M.; El Kadib, A. Aldehyde-functionalized chitosan-montmorillonite films as dynamically-assembled, switchable-chemical release bioplastics. Carbohydr. Polym. 2018, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Blilid, S.; Kędzierska, M.; Wrońska, N.; El Achaby, M.; Katir, N.; Belamie, E.; Alonso, B.; Lisowska, K.; Lahcini, M.; Bryszewska, M.; et al. Phosphorylated Micro- and Nanocellulose-filled chitosan nanocomposites as fully sustainable, biologically active bioplastics. ACS Sustain. Chem. Eng. 2020, 8, 18354–18365. [Google Scholar] [CrossRef]
- Blilid, S.; Katir, N.; El Haskouri, J.; Lachini, M.; Royer, S.; El Kadib, A. Phosphorylated micro- vs. nano-cellulose: A comparative study on their surface functionalisation, growth of titanium-oxo-phosphate clusters and removal of chemical pollutants. New J. Chem. 2019, 43, 15555. [Google Scholar] [CrossRef]
- Li, H.; Cheng, W.; Liua, K.; Chen, L.; Huang, Y.; Wanga, X.; Lva, Z.; He, J.; Li, C. Reinforced collagen with oxidized microcrystalline cellulose shows improved hemostatic effects. Carbohydr. Polym. 2017, 165, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; He, J.; Chen, M.; Li, D.; Li, H.; Chen, L.; Cao, Y.; Wang, J.; Huang, H. Preparation, Functional Characterization and Hemostatic Mechanism Discussion for Oxidized Microcrystalline Cellulose and Its Composites. Fibers Polym. 2016, 17, 1277–1286. [Google Scholar] [CrossRef]
- Schonauer, C.; Tessitore, E.; Barbagallo, G.; Albanese, V.; Moraci, A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur. Spine J. 2004, 13, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomat. 2014, 10, 1502–1514. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Han, L.; Liu, C.; Du, Y.; Hu, X.; Du, G.; Shan, C.; Yang, K.; Wang, C.; Li, M.; et al. A rapid hemostatic sponge based on large, mesoporous silica nanoparticles and N alkylated chitosan. Nanoscale 2018, 10, 20234–22024. [Google Scholar] [CrossRef]
- Kheirabadi, B.S.; Scherer, M.R.; Estep, J.S.; Dubick, M.A.; Holcomb, J.B. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J. Trauma 2009, 67, 450–460. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhou, Z. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Omer, A.M.; Temer, T.M. Evaluation of antimicrobial and antioxidant activities for cellulose aceteate films incorporated with Rosemary and Aloe Vera essential oils. J. Food Sci. Technol. 2019, 56, 1510–1518. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Kim, J.W.; Zhai, L.; Zhu, Q.Y.; Kim, J. Incorporation of melanin nanoparticles improves UV-shielding, mechanical and antioxidant properties of cellulose nanofiber based nanocomposite films. Mater. Today Commun. 2020, 24, 100984. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Khaleel Basha, S.; Sugantha Kumari, V. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Mathew, A.P.; Girandon, L. Porous electrospun nanocomposite mats based on chitosan–cellulose nanocrystals for wound dressing: Effect of surface characteristics of nanocrystals. Cellulose 2015, 22, 521–534. [Google Scholar] [CrossRef]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and Cellular Uptake of Cellulose Nanocrystals. Nano Life 2012, 2, 1–11. [Google Scholar] [CrossRef]
- Rodríguez, K.; Gatenholm, P.; Renneckar, S. Electrospinning cellulosic nanofibers for biomedical applications: Structure and in vitro biocompatibility. Cellulose 2012, 19, 1583–1598. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Karim, Z.; Liu, P.; Khan, S.A.; Naseri, N. Process scale up and characterization of wood cellulose nanocrystals hydrolysed using bioethanol pilot plant. Ind. Crop. Prod. 2014, 58, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Rokstad, A.M.; Brekke, O.L.; Steinkjer, B.; Ryan, L.; Kolláriková, G.; Strand, B.L.; Skjåk-Bræk, G.; Lambris, J.D.; Lacík, I.; Mollnes, T.E.; et al. The induction of cytokines by polycation containing microspheres by a complement dependent mechanism. Biomaterials 2013, 34, 621–630. [Google Scholar] [CrossRef]
- Burd, A.; Kwok, C.H.; Hung, S.C.; Chan, H.S.; Gu, H.; Lam, W.K.; Huang, L. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen. 2007, 15, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Čolid, M.; Mihajlovid, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar]
- Paddle-Ledinek, J.E.; Nasa, Z.; Cleland, H.J. Effect of Different Wound Dressings on Cell Viability and Proliferation. Plast. Reconstr. Surg. 2006, 117, 110–118. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Aimonen, K.; Suhonen, S.; Hartikainen, M.; Lopes, V.R.; Norppa, H.; Ferraz, N.; Catalán, J. Role of Surface Chemistry in the In Vitro Lung Response to Nanofibrillated Cellulose. Nanomaterials 2021, 11, 389. [Google Scholar] [CrossRef]
- Jabaut, J.; Ather, J.L.; Taracanova, A.; Poynter, M.E.; Ckless, K. Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1beta secretion in association with alterations in cellular redox and energy status. Free Radic. Biol. Med. 2013, 60, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAP). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Araoye, E.; Pyram, D.; Usha, D.; Hemraz, D.; Boluk, Y.; Ckless, K. Cellulose nanocrystal cationic derivative induces NLRP3 inflammasome-dependent IL-1β secretion associated with mitochondrial ROS production. Biochem. Biophys. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Tyagi, N.; Ovechkin, A.V.; Lominadze, D.; Moshal, K.S.; Tyagi, S.C. Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J. Cell Biochem. 2006, 98, 1150–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxic 2012, 33, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mao, L.; Qi, F.; Li, X.; Ullah, W.M.; Zhon, M.; Shi, Z.; Yan, G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- Bacakova, M.; Pajorova, J.; Sopuch, T.; Bacakova, L. Fibrin-Modified Cellulose as a Promising Dressing for Accelerated Wound Healing. Materials 2018, 11, 2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorankova, B.; Smetana, K., Jr.; Vacik, J.; Jelinkova, M. Cultivation of keratinocytes on poly HEMA and their migration after inversion. Folia Biol. 1996, 42, 83–86. [Google Scholar]
- Collins, A.R.; Dobson, V.L.; Dusinska, M.; Kennedy, G.; Stetina, R. The comet assay: What can it really tell us? Mutat. Res. 1997, 375, 183–193. [Google Scholar] [CrossRef]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivão, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagen. Adv. 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.; Medeiros, A. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar]
- Coelho, F.; do Vale Braido, G.V.; Cavicchioli, M.; Mendes, L.S.; Specian, S.S.; Franchi, L.P. Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency. Contact Lens Anterior Eye 2019, 42, 512–519. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, N.B.; Almeida-Lima, J.; Rocha, H.A.O.; Medeiros, S.R.B.; Alves, C.; Gama, F.M. BC nanofibres: In vitro study of genotoxicity and cell proliferation. Toxicol. Lett. 2009, 189, 235–241. [Google Scholar]
- Prasa, D. The ability of thrombin inhibitors to reduce the thrombin activity generated in plasma on extrinsic and intrinsic activation. Thromb Haemost. 1997, 77, 498–503. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Sieradzka, M.; Moniuszko-Szajwaj, B.; Nowak, P.; Oleszek, W.; Stochmal, A. Phenolic fractions from nine Trifolium species modulate the coagulant properties of blood plasma in vitro without cytotoxicity towards blood cells. J. Pharm. Pharmacol. 2018, 70, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Ponczek, M.; Sady-Janczak, M.; Pilarski, R.; Bukowska, B. Extracts from Uncaria tomentosa as antiplatelet agents and thrombin inhibitors—The in vitro and in silico study. J. Ethnopharmacol. 2020, 113494. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yoo, D.J.; Ravichandiran, P.; Subramaniyan, S.A.; Kim, S.-Y.; Kim, J.-S.; Park, B.-H.; Shim, K.S. Synthesis and anticancer evaluation of novel 1,4-naphthoquinone derivatives containing a phenylamino-sulfanyl moiety. ChemMedChem 2019, 14, 512. [Google Scholar]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. ChemistryOpen 2019, 8, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldkamp, T.; Kribben, A.; Weinberg, J.M. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am. J. Physiol. Ren. Physiol. 2005, 288, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entschladen, F.; Drell, T.L.; Lang, K.; Masur, K.; Palm, D.; Bastian, P.; Niggemann, B.; Zaenker, K.S. Analysis methods of human cell migration. Exp. Cell Res. 2005, 307, 418–426. [Google Scholar] [CrossRef]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Kowalik, J. A comparison of the in vitro genotoxicity of tri-and hexavalent chromium. Mutat. Res. 2000, 469, 135–145. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierska, M.; Blilid, S.; Miłowska, K.; Kołodziejczyk-Czepas, J.; Katir, N.; Lahcini, M.; El Kadib, A.; Bryszewska, M. Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films. Int. J. Mol. Sci. 2021, 22, 11386. https://doi.org/10.3390/ijms222111386
Kędzierska M, Blilid S, Miłowska K, Kołodziejczyk-Czepas J, Katir N, Lahcini M, El Kadib A, Bryszewska M. Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films. International Journal of Molecular Sciences. 2021; 22(21):11386. https://doi.org/10.3390/ijms222111386
Chicago/Turabian StyleKędzierska, Marta, Sara Blilid, Katarzyna Miłowska, Joanna Kołodziejczyk-Czepas, Nadia Katir, Mohammed Lahcini, Abdelkrim El Kadib, and Maria Bryszewska. 2021. "Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films" International Journal of Molecular Sciences 22, no. 21: 11386. https://doi.org/10.3390/ijms222111386
APA StyleKędzierska, M., Blilid, S., Miłowska, K., Kołodziejczyk-Czepas, J., Katir, N., Lahcini, M., El Kadib, A., & Bryszewska, M. (2021). Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films. International Journal of Molecular Sciences, 22(21), 11386. https://doi.org/10.3390/ijms222111386





