Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application
Abstract
:1. Introduction
2. Mechanisms of Autophagy Induction
3. Sensing Mechanisms of Amino Acid Regulation Autophagy
3.1. mTORC1 Pathway and Autophagy
3.2. GCN2 Pathway and Autophagy
4. The mTORC1-Mediated Signal Transduction from Replete Functional Amino Acids to Autophagy
4.1. Multiple Arginine Sensors Signal Arginine to mTORC1
4.2. Leucine Transmits Signals to mTORC1 in a Similar Manner to Arginine
4.3. Glutamine Activates mTORC1 in a RagA/B-Independent Fashion
4.4. Methionine Also Activates mTORC1 as a Sulfur-Containing Functional Amino Acid
5. FAAs Starvation Induce Autophagy
5.1. Arginine and Autophagy
5.2. Leucine and Autophagy
5.3. Glutamine and Autophagy
5.4. Methionine and Autophagy
6. FAAs as Potential Treatments for Autophagy-Related Diseases
6.1. Cancer
6.2. Aging
6.3. Obesity
6.4. Immune Metabolism Disorder
6.5. Neurodegeneration
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATF4 | activating transcription factor 4 |
| ATGL | adipose triglyceride lipase |
| AS | aminosuccinate synthase |
| ASS1 | aminosuccinate synthase1 |
| ADI | arginine deiminase |
| As2O3 | arsenic trioxide |
| ASNS | asparagine synthetase |
| ACT | aspartate kinases, chorismate mutase and TyrA |
| Atgs | autophagy-related genes |
| BMECs | bovine mammary epithelial cells |
| BCAAs | branched chain amino acids |
| CHOP | C/EBP-homologous protein |
| CR | caloric restriction |
| CASTOR1 | cellular arginine sensor for mammalian target of rapamycin complex 1 |
| CMA | chaperon-mediated autophagy |
| CRM | CR mimetics |
| Dvl2 | dishevelled-2 |
| ESCRT | endosomal sorting complexes required for transport |
| ETEC | Escherichia coli |
| EAAs | essential amino acids |
| eIF2α | eukaryotic translation initiator factor 2α |
| EAAT | excitatory amino acid transporter |
| EAE | experimental autoimmune encephalomyelitis |
| FOX | forkhead box |
| FAAs | functional amino acids |
| GAAC | general amino acid control |
| GCN2 | general control nonderepressible 2 |
| GLUD | glutamate dehydrogenase |
| GDH | glutamate dehydrogenase |
| GS | glutamate synthetase |
| GLS | glutaminase |
| GLS1 | glutaminase 1 |
| GLS2 | glutaminase 2 |
| GS1 | glutamine synthetase 1 |
| GEF | guanine nucleotide exchange factor |
| HCC | hepatocellular carcinoma |
| HFD | high-fat diet |
| HMGB1 | high-mobility group box-1 |
| Hcy | homocysteine |
| HOPS | homotypic fusion and protein sorting |
| HSL | hormone-sensitive lipase |
| HGPS | Hutchinson–Gilford progeria syndrome |
| H2S | hydrogen sulfide |
| Islr | immunoglobulin superfamily containing leucine-rich repeat |
| IRE1α | inositol-requiring enzyme1α |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFN-γ | interferon-gamma |
| LRS | leucyl tRNA synthetase |
| LC3-I | light chain 3-I |
| LPS | lip-polysaccharide |
| LEL | long extracellular loop |
| LAMP1 | lysosomal-associated membrane protein 1 |
| mLST8 | mammalian lethal with SEC13 protein 8 |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MET | methionine |
| MR | methionine restriction |
| MsrA | methionine sulfoxide reductase A |
| Msrs | methionine sulfoxide reductases |
| MAPK8 | mitogen protein kinases 8 |
| MEF | mouse embryonic fibroblast |
| MEFs | mouse embryonic fibroblasts |
| NO | nitric oxide |
| NNS-autophagy | non-nitrogen-starvation-induced autophagy |
| NFκB1 | nuclear factor kappa B1 |
| NRBF2 | nuclear receptor binding factor 2 |
| PE | phosphatidylethanolamine |
| PtdIns(3)K | phosphatidylinositol 3-kinase |
| PtdIns3P | phosphatidylinositol 3-phosphate |
| PLD1 | phospholipase D1 |
| PAS | pre-autophagosome structures |
| PRAS40 | proline-rich Akt substrate of 40 kDa |
| PP2A | protein phosphatase 2A |
| PPD | protopanaxadiol |
| Raptor | ragulatory-associated protein of mTOR |
| ROS | reactive oxygen species |
| S6K1 | ribosomal protein S6 kinase, polypeptide 1 |
| PACER | rubicon like autophagy enhancer |
| SAM | S-adenosylmethionine |
| SA-β-gal | senescence-associated beta-galactosidase |
| SQSTM1/p62 | sequestosome 1 |
| siRNA | small interfering RNA |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SNAP29 | soluble NSF attachment protein 29 |
| SLC38A9 | solute carrier family 38, member 9 |
| SREBP-1c | sterol regulatory element binding protein-1c |
| STX17 | syntaxin 17 |
| SLE | systemic lupus erythematosus |
| TFEB | transcription factor EB |
| TM4SF5 | transmembrane 4 L Six Family Member 5 |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-α |
| T2DM | type 2 diabetic mellitus |
| ULK1 | unc-51-like kinase 1 |
| UVRAG | UV radiation resistance-associated gene |
| VPS34 | vacuolar protein sorting 34 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VAMP8 | vesicle-associated membrane protein 8 |
| WIPI | WD-repeat protein interacting with phosphoinositides |
| WAT | white adipose tissue |
| HUWE1, HECT, UBA | WWE domain containing 1 |
References
- Radewa, J. Observations on autophagocytosis phenomena in the blood. Z. Rheumaforsch. 1963, 22, 36–46. [Google Scholar]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Levine, B.; Green, D.R.; Kroemer, G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017, 7, 487–511. [Google Scholar] [CrossRef] [Green Version]
- Mortimore, G.E.; Schworer, C.M. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 1977, 5633, 174–176. [Google Scholar] [CrossRef]
- He, L.; Zhang, J.; Zhao, J.; Ma, N.; Kim, S.W.; Qiao, S.; Ma, X. Autophagy: The Last Defense against Cellular Nutritional Stress. Adv. Nutr. 2018, 4, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 3, 521–534. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Eslamfam, S.; Ma, X.; Li, D. Autophagy and the nutritional signaling pathway. Front Agr. Sci. Eng. 2016, 3, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 2, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal 2014, 3, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 1, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 7, 226. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 7, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsumi, Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001, 3, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 2, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wang, Y.; Abi Saab, W.F.; Yang, F.; Pessin, J.E.; Backer, J.M. NRBF2 regulates macroautophagy as a component of Vps34 Complex I. Biochem. J. 2014, 2, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Klionsky, D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: Beyond the usual suspects’ review series. EMBO Rep. 2008, 9, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Noda, T.; Kim, J.; Huang, W.P.; Baba, M.; Tokunaga, C.; Ohsumi, Y.; Klionsky, D.J. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J. Cell Biol. 2000, 3, 465–480. [Google Scholar] [CrossRef]
- Proikas-Cezanne, T.; Takacs, Z.; Dönnes, P.; Kohlbacher, O. WIPI proteins: Essential PtdIns3P effectors at the nascent autophagosome. J. Cell Sci. 2015, 2, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.J.; Lee, J.A. The roles of phosphoinositides in mammalian autophagy. Arch. Pharm. Res. 2016, 8, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.; Colf, L.A.; Sundquist, W.I. Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 2013, 82, 663–692. [Google Scholar] [CrossRef] [Green Version]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 6, 1256–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, J.; Liu, R.; Rong, Y.; Zhao, M.; Zhang, J.; Lai, Y.; Zhou, Q.; Wilz, L.M.; Li, J.; Vivona, S.; et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 2015, 7548, 563–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnell, A.M.; Subramaniam, A.R.; O’Shea, E.K. Translational Control through Differential Ribosome Pausing during Amino Acid Limitation in Mammalian Cells. Mol. Cell 2018, 2, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 2017, 3, 642–654. [Google Scholar] [CrossRef]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012, 2, 410–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsun, Z.Y.; Bar-Peled, L.; Chantranupong, L.; Zoncu, R.; Wang, T.; Kim, C.; Spooner, E.; Sabatini, D.M. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell 2013, 4, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Kamada, Y.; Yoshino, K.; Kondo, C.; Kawamata, T.; Oshiro, N.; Yonezawa, K.; Ohsumi, Y. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell Biol. 2010, 4, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.X.; Russell, R.C.; Guan, K.L. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy 2013, 12, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhang, S.; He, L.; Rong, Y.; Brier, L.W.; Sun, Q.; Liu, R.; Fan, W.; Chen, S.; Yue, Z.; et al. MTORC1-mediated NRBF2 phosphorylation functions as a switch for the class III PtdIns3K and autophagy. Autophagy 2017, 3, 592–607. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.M.; Feng, Y.; Wang, J.; Shi, R.; Jiang, X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat. Commun. 2015, 6, 8048. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; You, Z.; Zhou, L.; Xu, Y.; Peng, C.; Zhou, T.; Yi, C.; Shi, Y.; Liu, W. mTORC1-Regulated and HUWE1-Mediated WIPI2 Degradation Controls Autophagy Flux. Mol. Cell 2018, 2, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Ma, X.; Zhu, Q.; Song, D.; Ding, X.; Li, L.; Jiang, X.; Wang, X.; Tian, R.; Su, H.; et al. Pacer Is a Mediator of mTORC1 and GSK3-TIP60 Signaling in Regulation of Autophagosome Maturation and Lipid Metabolism. Mol. Cell 2019, 4, 788–802. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Jung, C.H.; Seo, M.; Kim, E.K.; Park, J.M.; Bae, S.S.; Kim, D.H. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol. Cell 2015, 2, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012, 6, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Goraksha-Hicks, P.; Li, L.; Neufeld, T.P.; Guan, K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008, 8, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 5882, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Qiu, H.; Garcia-Barrio, M.; Anderson, J.; Hinnebusch, A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 2000, 2, 269–279. [Google Scholar] [CrossRef] [Green Version]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 16, 7683–7699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibenhener, M.L.; Babu, J.R.; Geetha, T.; Wong, H.C.; Krishna, N.R.; Wooten, M.W. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol. Cell Biol. 2004, 18, 8055–8068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.J.; Gao, Y.Y.; Zhang, J.; Wang, L.; Zhao, S.; Che, Y.Y.; Ao, C.J.; Yang, H.J.; Wang, J.Q.; Lei, L.C. Autophagy mediated by arginine depletion activation of the nutrient sensor GCN2 contributes to interferon-γ-induced malignant transformation of primary bovine mammary epithelial cells. Cell Death Discov. 2016, 2, 15065. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.R.; Du, C.H.; Wang, C.Z.; Yuan, C.S.; Du, W. Ginseng metabolite Protopanaxadiol induces Sestrin2 expression and AMPK activation through GCN2 and PERK. Cell Death Dis. 2019, 4, 311. [Google Scholar] [CrossRef]
- Ye, J.; Palm, W.; Peng, M.; King, B.; Lindsten, T.; Li, M.O.; Koumenis, C.; Thompson, C.B. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015, 22, 2331–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wengrod, J.; Wang, D.; Weiss, S.; Zhong, H.; Osman, I.; Gardner, L.B. Phosphorylation of eIF2α triggered by mTORC1 inhibition and PP6C activation is required for autophagy and is aberrant in PP6C-mutated melanoma. Sci. Signal. 2015, 367, 27. [Google Scholar]
- Tan, P.; Liu, H.; Zhao, J.; Gu, X.; Wei, X.; Zhang, X.; Ma, N.; Johnston, L.J.; Bai, Y.; Zhang, W.; et al. Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci. Total Environ. 2021, 800, 149596. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Wyant, G.A.; Gu, X.; Orozco, J.M.; Shen, K.; Condon, K.J.; Petri, S.; Kedir, J.; Scaria, S.M.; et al. KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature 2017, 7645, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 4, 771–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 12, 2579s–2586s. [Google Scholar] [CrossRef]
- Saxton, R.A.; Chantranupong, L.; Knockenhauer, K.E.; Schwartz, T.U.; Sabatini, D.M. Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature 2016, 7615, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Yin, Y.L.; Chu, W.; Liu, Z.; Deng, D.; Li, T.; Huang, R.; Zhang, J.; Tan, B.; Wang, W.; et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 2008, 5, 867–872. [Google Scholar] [CrossRef]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Genau, H.M.; Behrends, C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol. Cell Biol. 2015, 14, 2479–2494. [Google Scholar] [CrossRef] [Green Version]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araújo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 7544, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 6218, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.W.; Macalino, S.J.Y.; Cui, M.; Kim, J.E.; Kim, H.J.; Song, D.G.; Nam, S.H.; Kim, S.; Choi, S.; Lee, J.W. Transmembrane 4 L Six Family Member 5 Senses Arginine for mTORC1 Signaling. Cell Metab. 2019, 6, 1306–1319. [Google Scholar] [CrossRef]
- Kim, J.S.; Ro, S.H.; Kim, M.; Park, H.W.; Semple, I.A.; Park, H.; Cho, U.S.; Wang, W.; Guan, K.L.; Karin, M.; et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci. Rep. 2015, 5, 9502. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K. How mTORC1 senses leucine. Nat. Rev. Mol. Cell Biol. 2015, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Hallett, J.E.H.; Manning, B.D. CASTORing New Light on Amino Acid Sensing. Cell 2016, 1, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S.; Du, G.; Backer, J.M.; Frohman, M.A.; Chen, J. Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J. Cell Biol. 2011, 3, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kim, J.H.; Yoon, I.; Lee, C.; Fallahi Sichani, M.; Kang, J.S.; Kang, J.; Guo, M.; Lee, K.Y.; Han, G.; et al. Coordination of the leucine-sensing Rag GTPase cycle by leucyl-tRNA synthetase in the mTORC1 signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 23, E5279–E5288. [Google Scholar] [CrossRef] [Green Version]
- Lorin, S.; Tol, M.J.; Bauvy, C.; Strijland, A.; Poüs, C.; Verhoeven, A.J.; Codogno, P.; Meijer, A.J. Glutamate dehydrogenase contributes to leucine sensing in the regulation of autophagy. Autophagy 2013, 6, 850–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.L. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frigerio, F.; Karaca, M.; De Roo, M.; Mlynárik, V.; Skytt, D.M.; Carobbio, S.; Pajęcka, K.; Waagepetersen, H.S.; Gruetter, R.; Muller, D.; et al. Deletion of glutamate dehydrogenase 1 (Glud1) in the central nervous system affects glutamate handling without altering synaptic transmission. J. Neurochem. 2012, 3, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ren, J.; Song, T.; Peng, J.; Wei, H. Methionine Regulates mTORC1 via the T1R1/T1R3-PLCβ-Ca (2+)-ERK1/2 Signal Transduction Process in C2C12 Cells. Int. J. Mol. Sci. 2016, 10, 1684. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Luo, C.; Huang, X.; Chen, D.; Li, S.; Qi, H.; Gao, X. Amino acids stimulate glycyl-tRNA synthetase nuclear localization for mammalian target of rapamycin expression in bovine mammary epithelial cells. J. Cell Physiol. 2019, 5, 7608–7621. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H(2)S Production. Cell 2018, 1, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Konrad, C.; Wek, R.C.; Sullivan, W.J., Jr. GCN2-like eIF2α kinase manages the amino acid starvation response in Toxoplasma gondii. Int. J. Parasitol. 2014, 2, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzymski, T.; Milani, M.; Pike, L.; Buffa, F.; Mellor, H.R.; Winchester, L.; Pires, I.; Hammond, E.; Ragoussis, I.; Harris, A.L. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 2010, 31, 4424–4435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Navas, R.; Munder, M.; Mollinedo, F. Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy 2012, 11, 1557–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savaraj, N.; You, M.; Wu, C.; Wangpaichitr, M.; Kuo, M.T.; Feun, L.G. Arginine deprivation, autophagy, apoptosis (AAA) for the treatment of melanoma. Curr. Mol. Med. 2010, 4, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Chen, Y.R.; Liu, X.; Chu, C.Y.; Shen, L.J.; Xu, J.; Gaur, S.; Forman, H.J.; Zhang, H.; Zheng, S.; et al. Arginine starvation impairs mitochondrial respiratory function in ASS1-deficient breast cancer cells. Sci. Signal. 2014, 319, ra31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jin, L.; Tian, Z.; Wang, J.; Yang, Y.; Liu, J.; Chen, Y.; Hu, C.; Chen, T.; Zhao, Y.; et al. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci. 2019, 3, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacchiotti, A.; Corsetti, G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 989. [Google Scholar] [CrossRef]
- Sivangala Thandi, R.; Radhakrishnan, R.K.; Tripathi, D.; Paidipally, P.; Azad, A.K.; Schlesinger, L.S.; Samten, B.; Mulik, S.; Vankayalapati, R. Ornithine-A urea cycle metabolite enhances autophagy and controls Mycobacterium tuberculosis infection. Nat. Commun. 2020, 1, 3535. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.I.; Ando, M.; Aoyama, S.; Nakamura, K.; Shibata, S. Leucine restores murine hepatic triglyceride accumulation induced by a low-protein diet by suppressing autophagy and excessive endoplasmic reticulum stress. Amino Acids 2016, 4, 1013–1021. [Google Scholar] [CrossRef]
- Yan, X.; Sun, Q.; Ji, J.; Zhu, Y.; Liu, Z.; Zhong, Q. Reconstitution of leucine-mediated autophagy via the mTORC1-Barkor pathway in vitro. Autophagy 2012, 2, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wang, F.; Hu, S.; Yin, C.; Li, X.; Zhao, S.; Wang, J.; Yan, X. MiR-20a and miR-106b negatively regulate autophagy induced by leucine deprivation via suppression of ULK1 expression in C2C12 myoblasts. Cell Signal. 2012, 11, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.H.; Zoncu, R.; Kim, D.; Sabatini, D.M. Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell 2011, 5, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, S.A.; Caplan, A.B. Autophagy proteins play cytoprotective and cytocidal roles in leucine starvation-induced cell death in Saccharomyces cerevisiae. Autophagy 2012, 5, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 11, 1564. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.W.S.; Sim, A.Y.L.; Long, Y.C. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat. Commun. 2017, 1, 338. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Chen, Y.R.; Kensicki, E.; Li, A.Y.; Kong, M.; Li, Y.; Mohney, R.P.; Shen, H.M.; Stiles, B.; Mizushima, N.; et al. Autophagy: Resetting glutamine-dependent metabolism and oxygen consumption. Autophagy 2012, 10, 1477–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Zou, Y.; Mao, D.; Sun, D.; Gao, G.; Shi, J.; Liu, X.; Zhu, C.; Yang, M.; Ye, W.; et al. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J. Cell Biol. 2014, 2, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, M. FOXOphagy path to inducing stress resistance and cell survival. Nat. Cell Biol. 2012, 8, 786–788. [Google Scholar] [CrossRef]
- Berlicki, Ł. Inhibitors of glutamine synthetase and their potential application in medicine. Mini Rev. Med. Chem. 2008, 9, 869–878. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Eliasson, P.; Proikas-Cezanne, T.; Vervoort, S.J.; van Boxtel, R.; Putker, M.; van Zutphen, I.J.; Mauthe, M.; Zellmer, S.; Pals, C.; et al. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat. Cell Biol. 2012, 8, 829–837. [Google Scholar] [CrossRef]
- Durán, R.V.; Oppliger, W.; Robitaille, A.M.; Heiserich, L.; Skendaj, R.; Gottlieb, E.; Hall, M.N. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 2012, 3, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, M.S. Autophagy—a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 6, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.H.; Yu, K.; Lucas, J.; White, E.; Abraham, R.T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal. 2010, 119, 31. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, B.; Xiong, X.; Li, F.; Ren, W.; Kong, X.; Qiu, W.; Hardwidge, P.R.; Yin, Y. Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids 2015, 10, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Ruckenstuhl, C.; Netzberger, C.; Entfellner, I.; Carmona-Gutierrez, D.; Kickenweiz, T.; Stekovic, S.; Gleixner, C.; Schmid, C.; Klug, L.; Sorgo, A.G.; et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 2014, 5, e1004347. [Google Scholar] [CrossRef] [Green Version]
- Pennington, S.M.; Klutho, P.R.; Xie, L.; Broadhurst, K.; Koval, O.M.; McCormick, M.L.; Spitz, D.R.; Grumbach, I.M. Defective protein repair under methionine sulfoxide A deletion drives autophagy and ARE-dependent gene transcription. Redox Biol. 2018, 401–413. [Google Scholar] [CrossRef]
- Li, R.; Wei, X.; Jiang, D.S. Protein methylation functions as the posttranslational modification switch to regulate autophagy. Cell Mol. Life Sci. 2019, 19, 3711–3722. [Google Scholar] [CrossRef]
- Wu, X.; Tu, B.P. Selective regulation of autophagy by the Iml1-Npr2-Npr3 complex in the absence of nitrogen starvation. Mol. Biol Cell 2011, 21, 4124–4133. [Google Scholar] [CrossRef] [PubMed]
- Neklesa, T.K.; Davis, R.W. A genome-wide screen for regulators of TORC1 in response to amino acid starvation reveals a conserved Npr2/3 complex. PLoS Genet. 2009, 6, e1000515. [Google Scholar] [CrossRef] [Green Version]
- Sutter, B.M.; Wu, X.; Laxman, S.; Tu, B.P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 2013, 2, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 10, 752–789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Qi, Y.; Wang, Y.C.; Chi, K.K.; Chung, Y.; Ouyang, C.; Chen, Y.R.; Oh, M.E.; Sheng, X.; Tang, Y.; et al. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun Biol. 2018, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strekalova, E.; Malin, D.; Good, D.M.; Cryns, V.L. Methionine Deprivation Induces a Targetable Vulnerability in Triple-Negative Breast Cancer Cells by Enhancing TRAIL Receptor-2 Expression. Clin. Cancer Res. 2015, 12, 2780–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, W.; Wang, K.; Wang, X.; Yin, F.; Li, C.; Wang, C.; Zhao, B.; Zhong, C.; Zhang, J.; et al. Methionine and cystine double deprivation stress suppresses glioma proliferation via inducing ROS/autophagy. Toxicol Lett. 2015, 2, 349–355. [Google Scholar] [CrossRef]
- Bernfeld, E.; Foster, D.A. Glutamine as an Essential Amino Acid for KRas-Driven Cancer Cells. Trends Endocrinol. Metab. 2019, 6, 357–368. [Google Scholar] [CrossRef]
- Cooke, J.P.; Singer, A.H.; Tsao, P.; Zera, P.; Rowan, R.A.; Billingham, M.E. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J. Clin. Investig. 1992, 3, 1168–1172. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 7, 1679. [Google Scholar] [CrossRef] [Green Version]
- van Loon, L.J.; Kruijshoop, M.; Menheere, P.P.; Wagenmakers, A.J.; Saris, W.H.; Keizer, H.A. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003, 3, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 6, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Thacker, P.A.; Zhang, G.; Qiao, S.; Ma, X. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J. Agric. Food Chem. 2014, 4, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Yu, B.; Yang, X.; Liu, Z.; Huang, Z.; Mao, X.; Tian, G.; He, J.; Han, G.; Chen, H.; et al. Inhibition of adipogenic differentiation by myostatin is alleviated by arginine supplementation in porcine-muscle-derived mesenchymal stem cells. Sci. China Life Sci. 2011, 10, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Luo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. The effect of arginine on the Wnt/β-catenin signaling pathway during porcine intramuscular preadipocyte differentiation. Food Funct. 2017, 1, 381–386. [Google Scholar] [CrossRef]
- Miller, R.A.; Shi, Y.; Lu, W.; Pirman, D.A.; Jatkar, A.; Blatnik, M.; Wu, H.; Cárdenas, C.; Wan, M.; Foskett, J.K.; et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat. Med. 2018, 4, 518–524. [Google Scholar] [CrossRef]
- Ravindran, R.; Loebbermann, J.; Nakaya, H.I.; Khan, N.; Ma, H.; Gama, L.; Machiah, D.K.; Lawson, B.; Hakimpour, P.; Wang, Y.C.; et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 2016, 7595, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.R.; Oh, Y.J.; Kang, S.W.; Lee, E.B.; Lee, W.W. Role of SLC7A5 in Metabolic Reprogramming of Human Monocyte/Macrophage Immune Responses. Front. Immunol. 2018, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 5, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 2, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Kono, M.; Yoshida, N.; Maeda, K.; Tsokos, G.C. Transcriptional factor ICER promotes glutaminolysis and the generation of Th17 cells. Proc. Natl. Acad. Sci. USA 2018, 10, 2478–2483. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Saegusa, J.; Sendo, S.; Okano, T.; Akashi, K.; Irino, Y.; Morinobu, A. Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res. Ther. 2017, 1, 76. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, S.G.; Kegelman, T.P.; Su, Z.Z.; Das, S.K.; Dash, R.; Dasgupta, S.; Barral, P.M.; Hedvat, M.; Diaz, P.; et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. J. Cell Physiol. 2011, 10, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernizzi, L.; Paiardi, C.; Licata, G.; Vitali, T.; Santarelli, S.; Raneli, M.; Manelli, V.; Rizzetto, M.; Gioria, M.; Pasini, M.E.; et al. Glutamine Synthetase 1 Increases Autophagy Lysosomal Degradation of Mutant Huntingtin Aggregates in Neurons, Ameliorating Motility in a Drosophila Model for Huntington’s Disease. Cells 2020, 1, 196. [Google Scholar] [CrossRef] [Green Version]
- Michaud, M.; Xie, X.; Bravo-San Pedro, J.M.; Zitvogel, L.; White, E.; Kroemer, G. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology 2014, 7, e944047. [Google Scholar] [CrossRef] [Green Version]
- Parodi, M.; Pedrazzi, M.; Cantoni, C.; Averna, M.; Patrone, M.; Cavaletto, M.; Spertino, S.; Pende, D.; Balsamo, M.; Pietra, G.; et al. Natural Killer (NK)/melanoma cell interaction induces NK-mediated release of chemotactic High Mobility Group Box-1 (HMGB1) capable of amplifying NK cell recruitment. Oncoimmunology 2015, 12, e1052353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeldt, M.T.; O’Prey, J.; Morton, J.P.; Nixon, C.; MacKay, G.; Mrowinska, A.; Au, A.; Rai, T.S.; Zheng, L.; Ridgway, R.; et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013, 7479, 296–300. [Google Scholar] [CrossRef]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.; Bold, R.J.; Kung, H.J. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009, 2, 700–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demontis, F.; Perrimon, N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 2010, 5, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, M.M.; Zheng, B.; Lu, T.; Yan, Z.; Py, B.F.; Ng, A.; Xavier, R.J.; Li, C.; Yankner, B.A.; Scherzer, C.R.; et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 32, 14164–14169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 12, 1338–1348. [Google Scholar] [CrossRef]
- Madeo, F.; Pietrocola, F.; Eisenberg, T.; Kroemer, G. Caloric restriction mimetics: Towards a molecular definition. Nat. Rev. Drug Discov. 2014, 10, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Sedej, S.; Carmona-Gutierrez, D.; Madeo, F.; Kroemer, G. Autophagy in Cardiovascular Aging. Circ. Res. 2018, 7, 803–824. [Google Scholar] [CrossRef]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 11, 1812–1824. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Regulation of protein synthesis by branched-chain amino acids. Curr. Opin. Clin. Nutr. Metab. Care. 2001, 1, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227–231. [Google Scholar] [CrossRef] [Green Version]
- van Loon, L.J. Leucine as a pharmaconutrient in health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2012, 1, 71–77. [Google Scholar] [CrossRef]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 12, 2625–2629. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, Y.; Gu, L.; Lan, M.; Liu, C.; Wang, M.; Su, Y.; Ge, M.; Wang, T.; Yu, Y.; et al. Islr regulates canonical Wnt signaling-mediated skeletal muscle regeneration by stabilizing Dishevelled-2 and preventing autophagy. Nat. Commun. 2018, 1, 5129. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Yao, J.; Guo, L.; Cao, Y.; Liang, Z.; Yang, X.; Cai, C. Leucine-induced promotion of post-absorptive EAA utilization and hepatic gluconeogenesis contributes to protein synthesis in skeletal muscle of dairy calves. J. Anim. Physiol. Anim. Nutr. 2019, 3, 705–712. [Google Scholar] [CrossRef]
- Xiong, Y.; Fru, M.F.; Yu, Y.; Montani, J.P.; Ming, X.F.; Yang, Z. Long term exposure to L-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling. Aging 2014, 5, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Bárcena, C.; Quirós, P.M.; Durand, S.; Mayoral, P.; Rodríguez, F.; Caravia, X.M.; Mariño, G.; Garabaya, C.; Fernández-García, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 9, 2392–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settembre, C.; De Cegli, R.; Mansueto, G.; Saha, P.K.; Vetrini, F.; Visvikis, O.; Huynh, T.; Carissimo, A.; Palmer, D.; Klisch, T.J.; et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 2013, 6, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 47, 19860–19865. [Google Scholar] [CrossRef] [Green Version]
- Soussi, H.; Reggio, S.; Alili, R.; Prado, C.; Mutel, S.; Pini, M.; Rouault, C.; Clément, K.; Dugail, I. DAPK2 downregulation associates with attenuated adipocyte autophagic clearance in human obesity. Diabetes 2015, 10, 3452–3463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 1, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Qu, G.; Choi, S.C.; Cornaby, C.; Titov, A.; Kanda, N.; Teng, X.; Wang, H.; Morel, L. Targeting T Cell Activation and Lupus Autoimmune Phenotypes by Inhibiting Glucose Transporters. Front. Immunol. 2019, 10, 833. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 2, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukishiro, T.; Shimizu, Y.; Higuchi, K.; Watanabe, A. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J. Gastroenterol. Hepatol. 2000, 8, 849–859. [Google Scholar] [CrossRef]
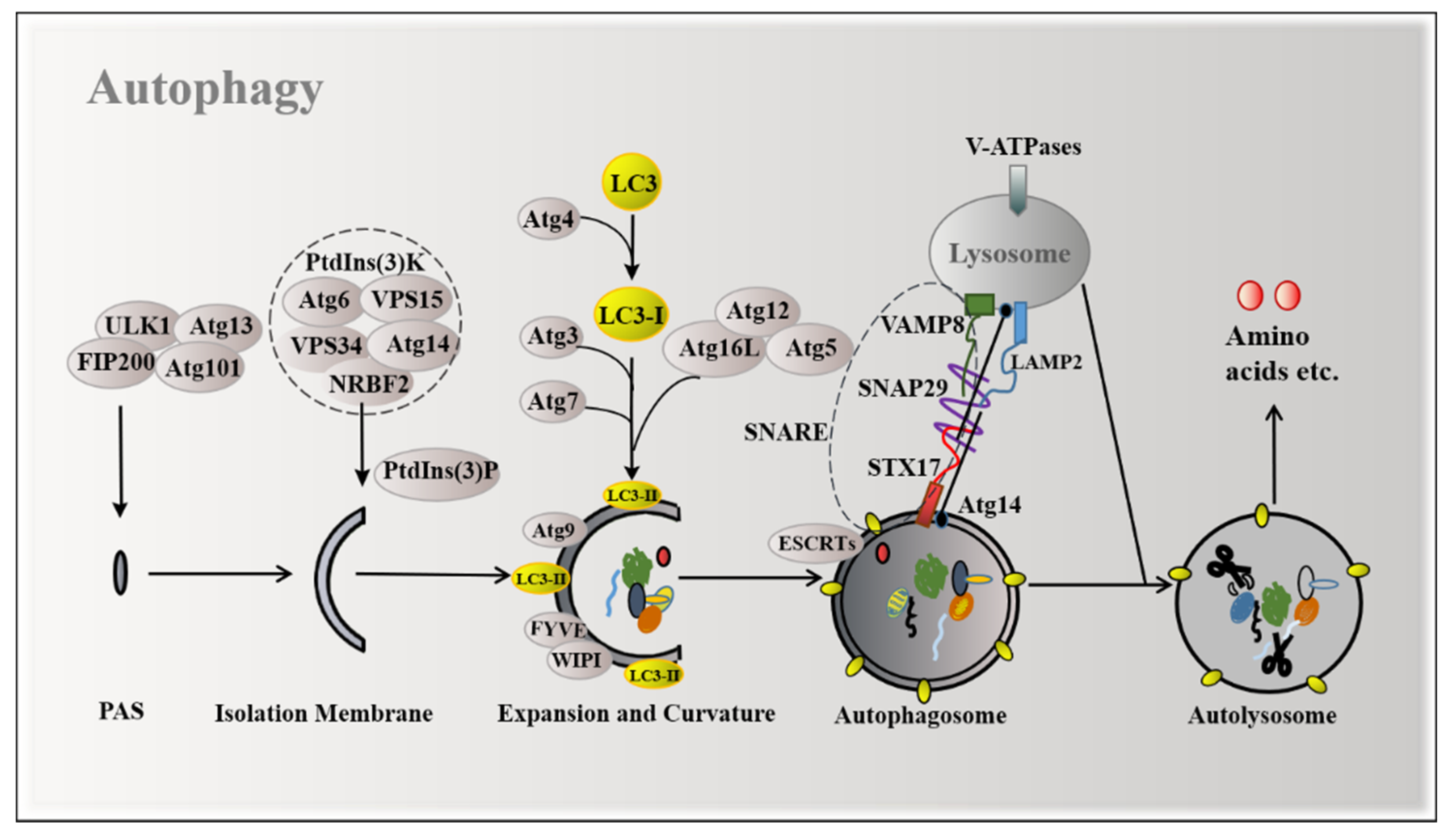
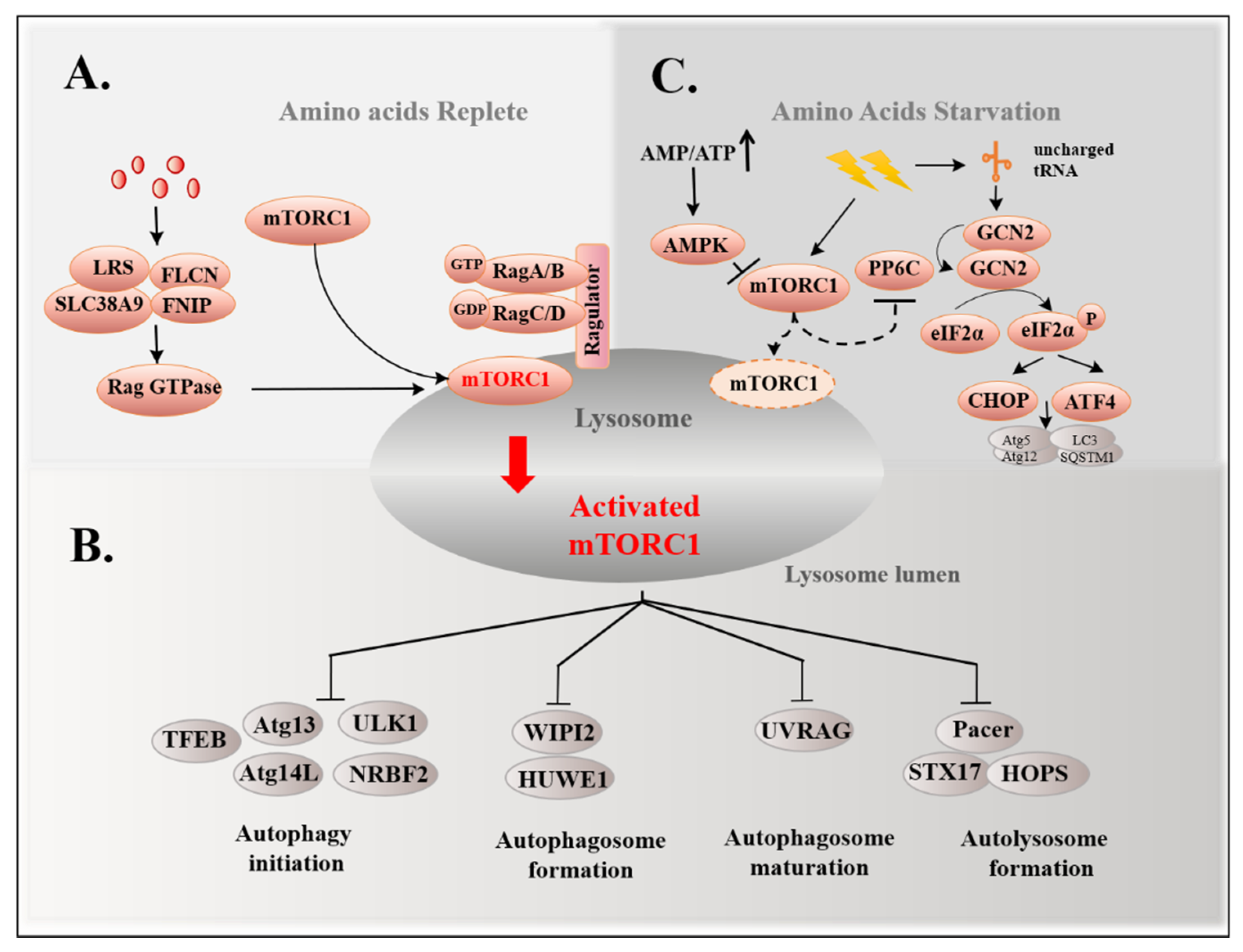
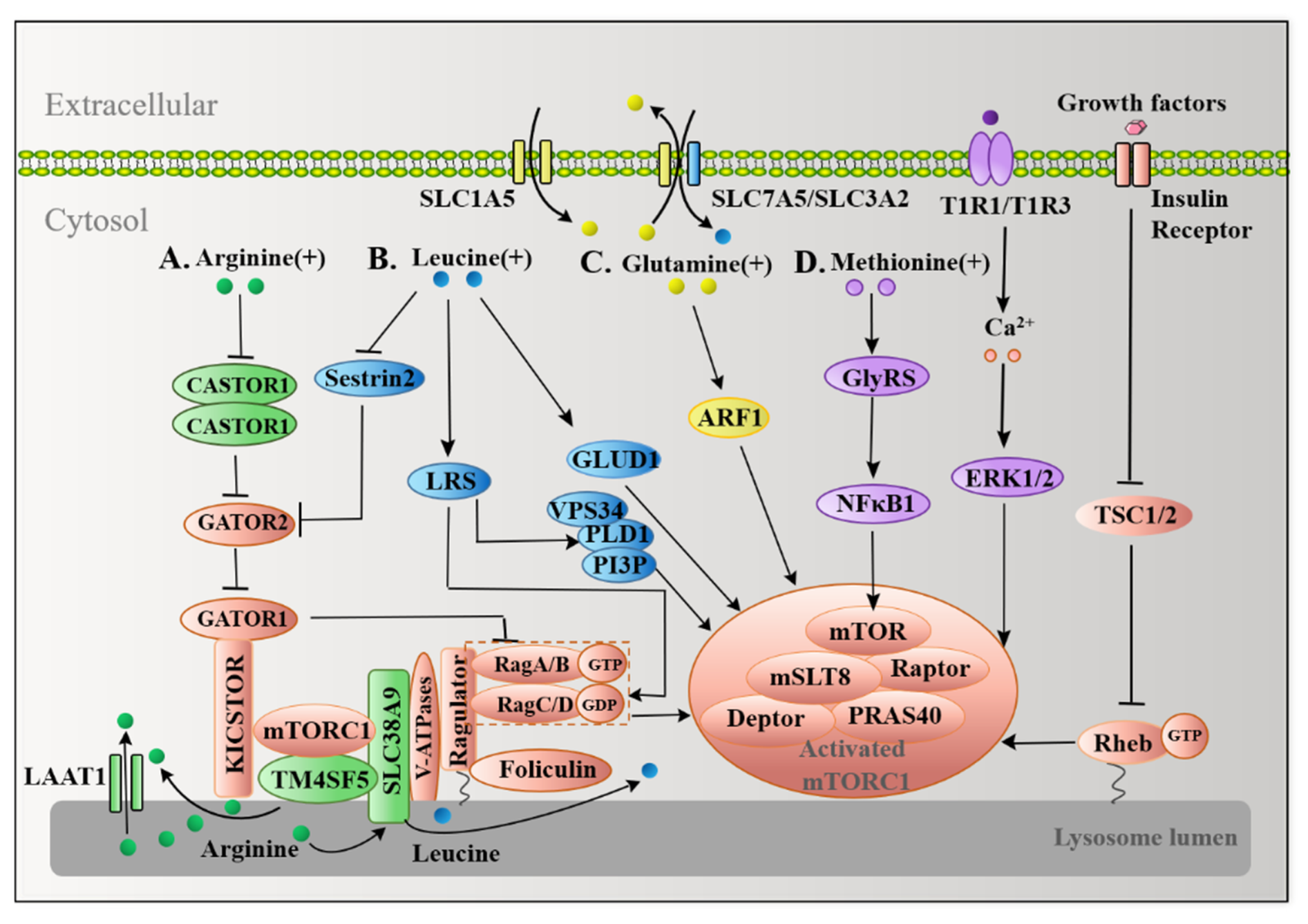

| Disease Types | Amino Acids Types | Key Findings | Reference |
|---|---|---|---|
| Cancer | Arginine | Restriction inhibits ASS1-deficient breast cancer cells | [101] |
| Methionine | Restriction inhibits breast cancer and glioma | [102,103] | |
| Glutamine | Restriction causes S phase stagnation in KRas-driven cancer cells | [104] | |
| Cardiovascular | Arginine | Supplements alleviate atherosclerosis and lowers blood pressure | [105,106] |
| Type 2 diabetes | Leucine | Increases the insulin response | [107] |
| Obesity | Arginine | Supplements and promotes lipolysis and reduces body/adipose tissue weight | [108,109,110,111] |
| Glutamine | Supplements and promotes the production of glucose | [112] | |
| Immune metabolism disorder | Leucine | Restriction reduces inflammation by inhibiting NLRP3t | [113,114] |
| Glutamine | Restriction activates T cells and produces IL-2 or IFN-γ | [115,116,117,118] | |
| Neurodegeneration | Glutamine | Glutamate is a neurotoxin | [119,120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ji, L.; Hu, J.; Zhao, Y.; Johnston, L.J.; Zhang, X.; Ma, X. Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application. Int. J. Mol. Sci. 2021, 22, 11427. https://doi.org/10.3390/ijms222111427
Liu C, Ji L, Hu J, Zhao Y, Johnston LJ, Zhang X, Ma X. Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application. International Journal of Molecular Sciences. 2021; 22(21):11427. https://doi.org/10.3390/ijms222111427
Chicago/Turabian StyleLiu, Chunchen, Linbao Ji, Jinhua Hu, Ying Zhao, Lee J. Johnston, Xiujun Zhang, and Xi Ma. 2021. "Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application" International Journal of Molecular Sciences 22, no. 21: 11427. https://doi.org/10.3390/ijms222111427
APA StyleLiu, C., Ji, L., Hu, J., Zhao, Y., Johnston, L. J., Zhang, X., & Ma, X. (2021). Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application. International Journal of Molecular Sciences, 22(21), 11427. https://doi.org/10.3390/ijms222111427






