Developing Wound Dressings Using 2-deoxy-D-Ribose to Induce Angiogenesis as a Backdoor Route for Stimulating the Production of Vascular Endothelial Growth Factor
Abstract
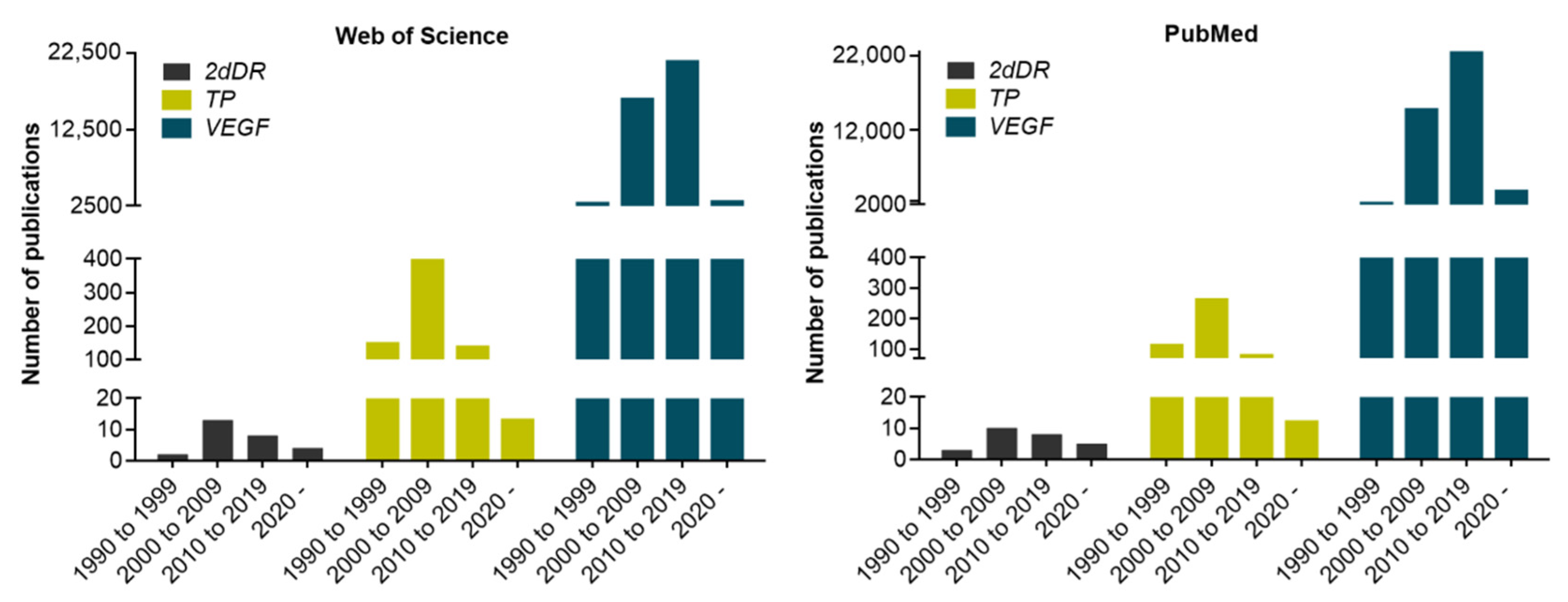
1. Background to Identifying 2dDR as a Pro-Angiogenic Sugar
2. Exploration of the Dose-Dependent Biological Activity of 2dDR
3. What Do We Understand of the Mechanism of Action of 2dDR? How Does Sugar Structure Affect Function?
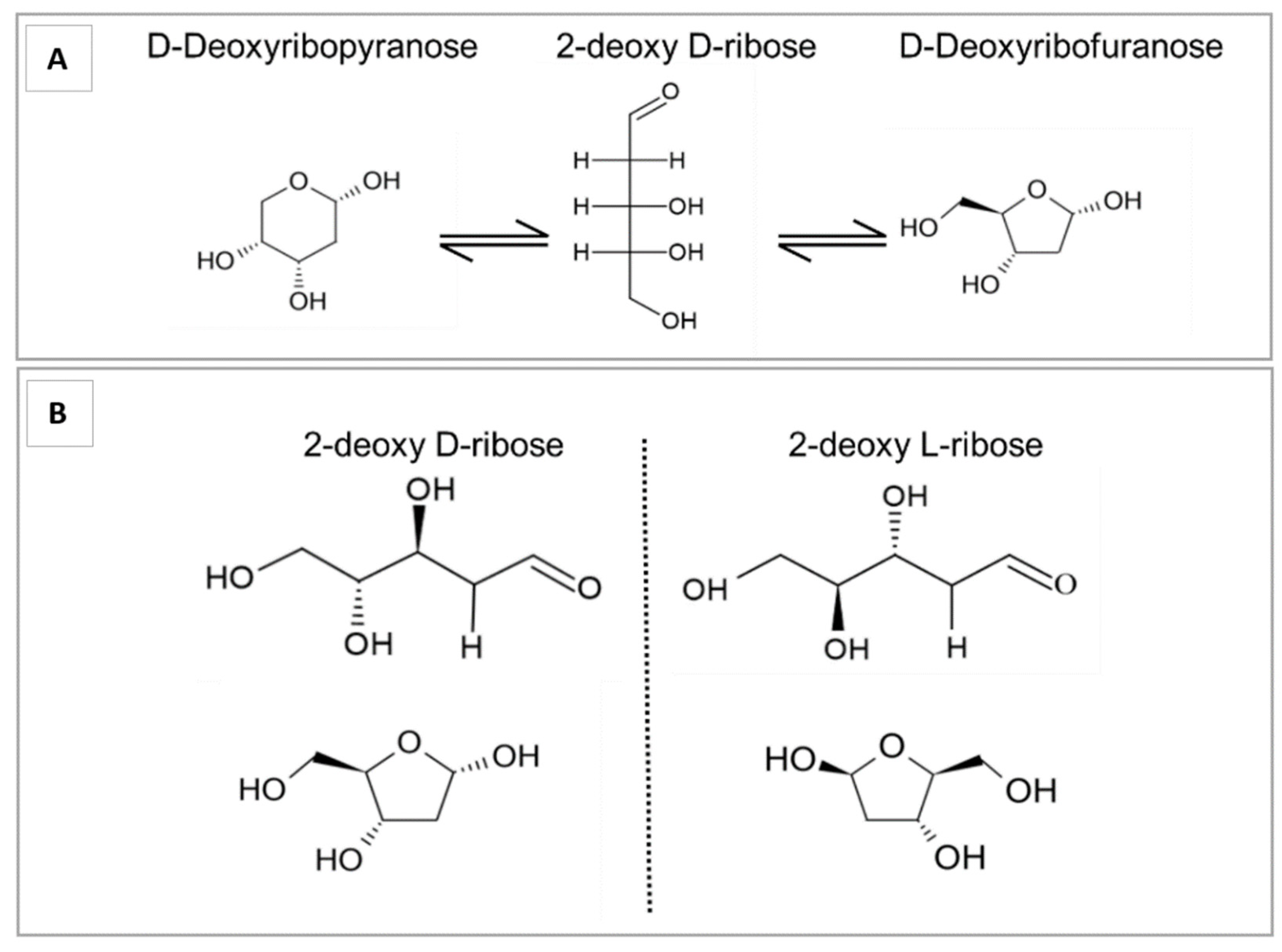
Isomers and Conformational Structures of Deoxyribose
4. Pro- and Anti-Angiogenic Activity of Small Sugars
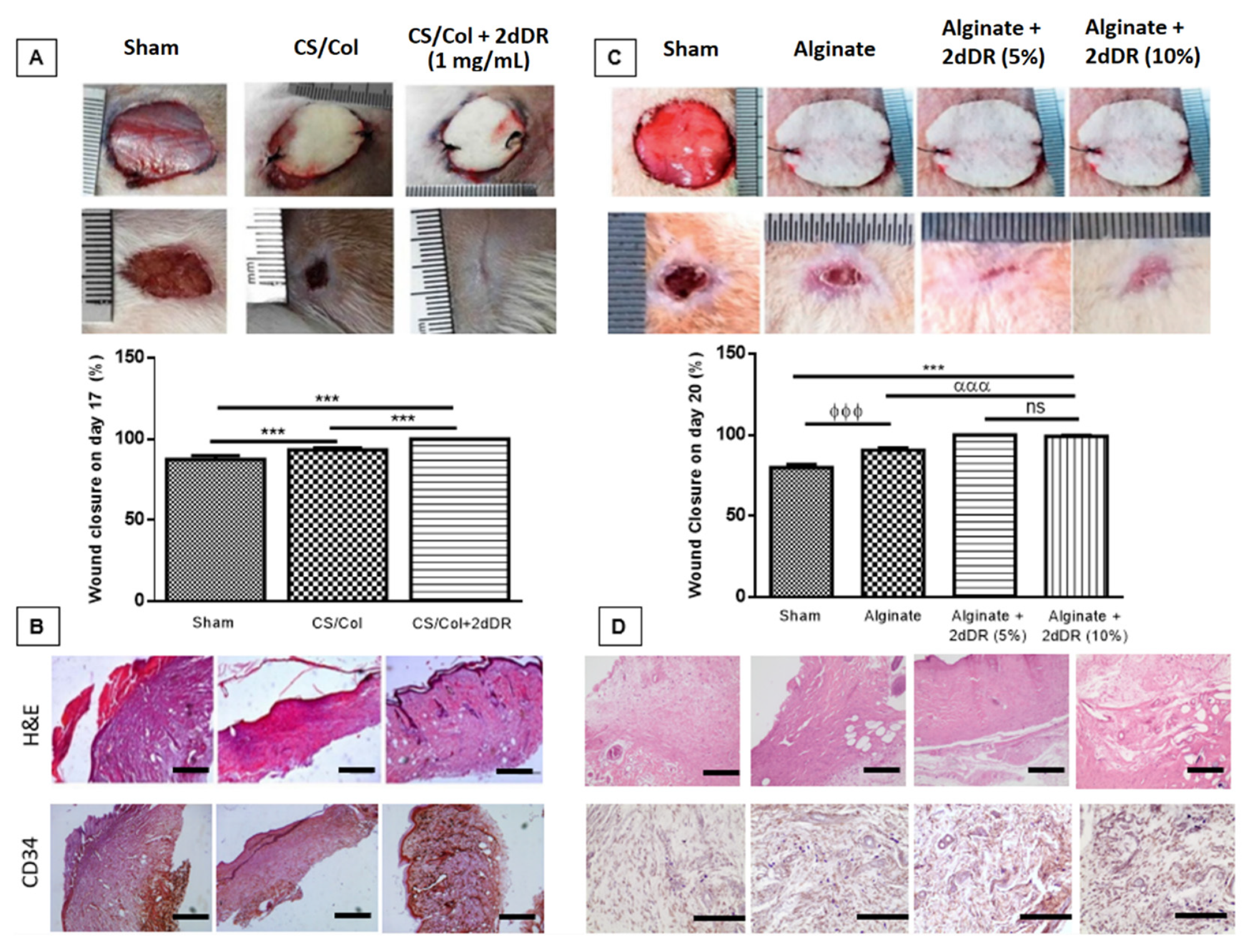
5. Developing Dressings Containing 2dDR to Stimulate Angiogenesis
6. Understanding the Mechanism of Action of 2dDR
7. The Stability of 2dDR
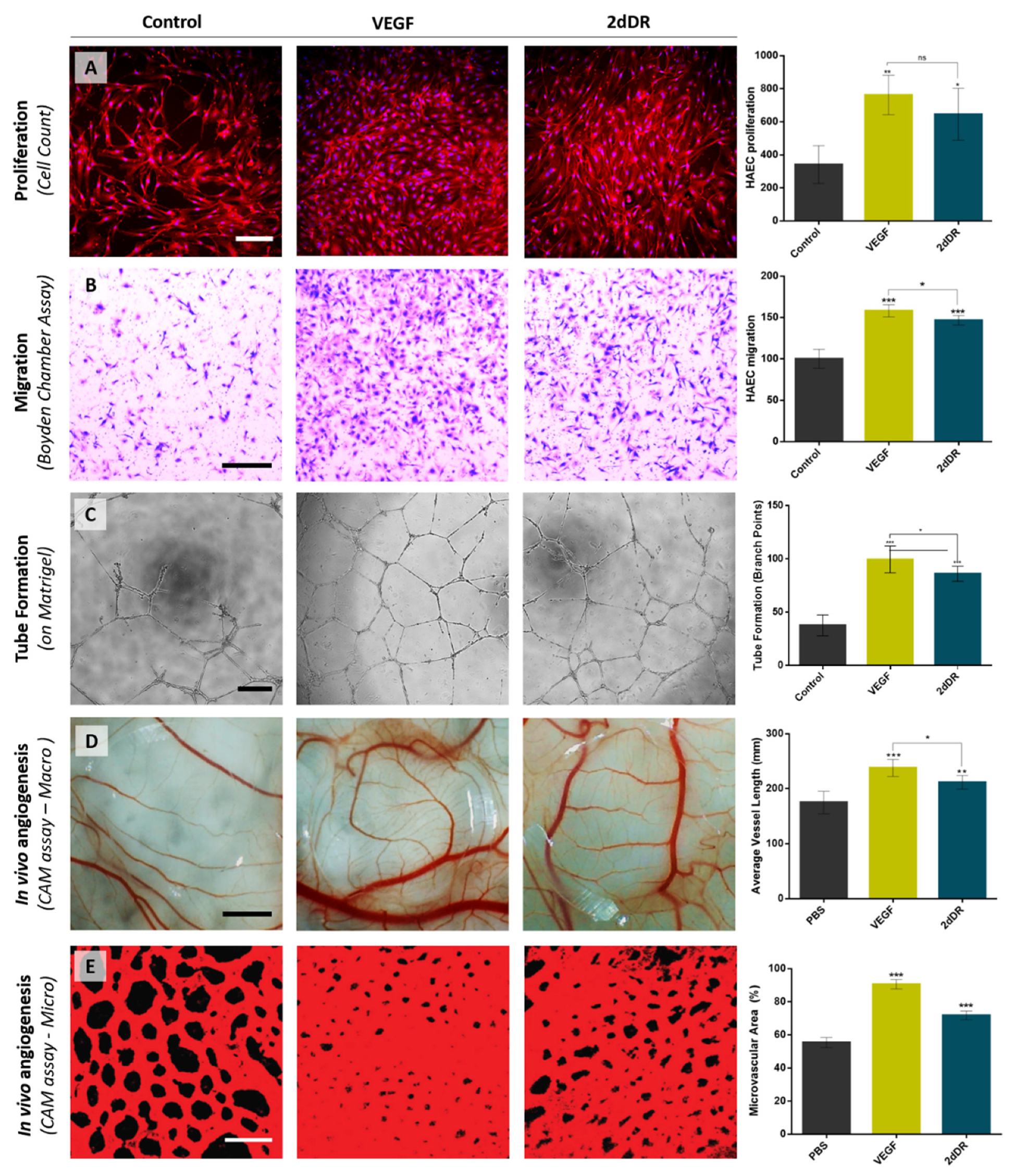
8. The Pro-Angiogenic Potential of 2dDR Compared to VEGF
9. Ongoing Work
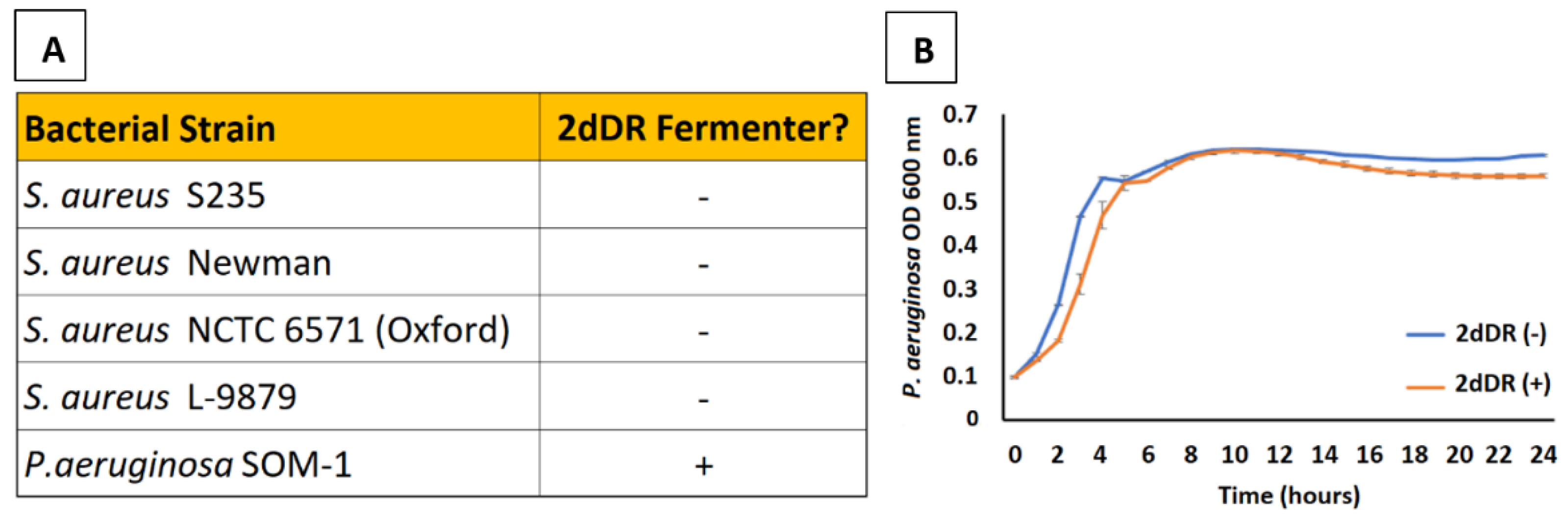
9.1. 2dDR and Skin Microbiology
9.2. Stability and Sterilisation
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levene, P.A.; Mikeska, L.A.; Mori, T. On the carbohydrate of thymonucleic acid. J. Biol. Chem. 1930, 85, 785–787. [Google Scholar] [CrossRef]
- Frixione, E.; Ruiz-Zamarripa, L. The “scientific catastrophe” in nucleic acids research that boosted molecular biology. J. Biol. Chem. 2019, 294, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Priestle, J.P.; Paris, C.G. Experimental Techniques and Data Banks. In Guidebook on Molecular Modeling in Drug Design; Academic Press: Cambridge, MA, USA, 1996; pp. 139–217. [Google Scholar]
- Desgranges, C.; Razaka, G.; Rabaud, M.; Bricaud, H. Catabolism of thymidine in human blood platelets purification and properties of thymidine phosphorylase. BBA Sect. Nucleic Acids Protein Synth. 1981, 654, 211–218. [Google Scholar] [CrossRef]
- Brown, N.S.; Bicknell, R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem. J. 1998, 334, 1–8. [Google Scholar] [CrossRef]
- Barton, G.J.; Ponting, C.P.; Spraggon, G.; Finnis, C.; Sleep, D. Human platelet-derived endothelial cell growth factor is homologous to Escherichia coli thymidine phosphorylase. Protein Sci. 1992, 1, 688–690. [Google Scholar] [CrossRef]
- Usuki, K.; Saras, J.; Waltenberger, J.; Miyazono, K.; Pierce, G.; Thomason, A.; Heldin, C.H. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem. Biophys. Res. Commun. 1992, 184, 1311–1316. [Google Scholar] [CrossRef]
- Friedkin, M.; Roberts, D. The enzymatic synthesis of nucleosides. I. Thymidine phosphorylase in mammalian tissue. J. Biol. Chem. 1954, 207, 245–256. [Google Scholar] [CrossRef]
- Furukawa, T.; Yoshimura, A.; Sumizawa, T.; Haraguchi, M.; Akiyama, S.I.; Fukui, K.; Ishizawa, M.; Yamada, Y. Angiogenic factor. Nature 1992, 356, 668. [Google Scholar] [CrossRef]
- Pauly, J.L.; Schuller, M.G.; Zelcer, A.A.; Kirss, T.A.; Gore, S.S.; Germain, M.J. Identification and comparative analysis of thymidine phosphorylase in the plasma of healthy subjects and cancer patients: Brief communication. J. Natl. Cancer Inst. 1977, 58, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Moghaddam, A.; Zhang, H.T.; Fan, T.P.; Hu, D.E.; Lees, V.C.; Turley, H.; Fox, S.B.; Gatter, K.C.; Harris, A.L.; Bicknell, R. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc. Natl. Acad. Sci. USA 1995, 92, 998–1002. [Google Scholar] [CrossRef]
- Ishikawa, F.; Miyazono, K.; Hellman, U.; Drexler, H.; Wernstedt, C.; Hagiwara, K.; Usuki, K.; Takaku, F.; Risau, W.; Heldin, C.H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature 1989, 338, 557–562. [Google Scholar] [CrossRef]
- Miyadera, K.; Sumizawa, T.; Haraguchi, M.; Yoshida, H.; Konstanty, W.; Yamada, Y.; Akiyama, S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995, 55, 1687–1690. [Google Scholar] [PubMed]
- Moghaddam, A.; Choudhuri, R.; Bicknell, R. Thymidine phosphorylase/platelet-derived endothelial cell growth factor: An angiogenic enzyme Tumour Angiogenesis. In Tumour Angiogenesis; Oxford University Press: Oxford, UK, 1997; pp. 251–260. [Google Scholar]
- Finnis, C.; Dodsworth, N.; Pollitt, C.E.; Carr, G.; Sleep, D. Thymidine phosphorylase activity of platelet-derived endothelial cell growth factor is responsible for endothelial cell mitogenicity. Eur. J. Biochem. 1993, 212, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Sellers, L.A.; Matheson, H.B.; Fan, T.P.D. Thymidine phosphorylase induces angiogenesis in vivo and in vitro: An evaluation of possible mechanisms. Br. J. Pharmacol. 2003, 139, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, H.; Furukawa, T.; Okamoto, M.; Nakajima, Y.; Matsushita, S.; Ikeda, R.; Gotanda, T.; Haraguchi, M.; Sumizawa, T.; Ono, M.; et al. Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose. Cancer Res. 2002, 62, 2834–2839. [Google Scholar]
- Nakajima, Y.; Madhyastha, R.; Maruyama, M. 2-Deoxy-D-Ribose, a Downstream Mediator of Thymidine Phosphorylase, Regulates Tumor Angiogenesis and Progression. Anticancer. Agents Med. Chem. 2009, 9, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Che, X.F.; Ushiyama, M.; Yamaguchi, T.; Okumura, H.; Nakajima, Y.; Takeda, Y.; Shibayama, Y.; Furukawa, T.; Yamamoto, M.; et al. 2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. Biochem. Biophys. Res. Commun. 2006, 342, 280–285. [Google Scholar] [CrossRef]
- Nakajima, Y.; Gotanda, T.; Uchimiya, H.; Furukawa, T.; Haraguchi, M.; Ikeda, R.; Sumizawa, T.; Yoshida, H.; Akiyama, S.I. Inhibition of Metastasis of Tumor Cells Overexpressing Thymidine Phosphorylase by 2-Deoxy-L-Ribose. Cancer Res. 2004, 64, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using ex Ovo Chick Chorioallantoic Membrane (CAM) Assay to Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Pre-Seeding of Simple Electrospun Scaffolds with a Combination of Endothelial Cells and Fibroblasts Strongly Promotes Angiogenesis. Tissue Eng. Regen. Med. 2020, 17, 445–458. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Decellularised baby spinach leaves and their potential use in tissue engineering applications: Studying and promoting neovascularisation. J. Biomater. Appl. 2019, 34, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S.; Aldemir Dikici, B.; MacNeil, S.; Claeyssens, F. Decellularised extracellular matrix decorated PCL PolyHIPE scaffolds for enhanced cellular activity, integration and angiogenesis. Biomater. Sci. 2021. Advance Article. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Reilly, G.C.; Claeyssens, F. Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by in Vitro Generated Extracellular Matrix Decoration. ACS Appl. Mater. Interfaces 2020, 12, 12510–12524. [Google Scholar] [CrossRef]
- Yar, M.; Shahzadi, L.; Mehmood, A.; Raheem, M.I.; Román, S.; Chaudhry, A.A.; ur Rehman, I.; Ian Douglas, C.W.; MacNeil, S. Deoxy-sugar releasing biodegradable hydrogels promote angiogenesis and stimulate wound healing. Mater. Today Commun. 2017, 13, 295–305. [Google Scholar] [CrossRef]
- Dikici, S.; Mangir, N.; Claeyssens, F.; Yar, M.; MacNeil, S. Exploration of 2-deoxy-D-ribose and 17β-Estradiol as alternatives to exogenous VEGF to promote angiogenesis in tissue-engineered constructs. Regen. Med. 2019, 14, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S.; Aldemir Dikici, B.; Bhaloo, S.I.; Balcells, M.; Edelman, E.R.; MacNeil, S.; Reilly, G.C.; Sherborne, C.; Claeyssens, F. Assessment of the angiogenic potential of 2-deoxy-D-ribose using a novel in vitro 3D dynamic model in comparison with established in vitro assays. Front. Bioeng. Biotechnol. 2019, 7, 451. [Google Scholar] [CrossRef]
- Dikici, S.; Bullock, A.J.; Yar, M.; Claeyssens, F.; MacNeil, S. 2-deoxy-D-ribose (2dDR) upregulates vascular endothelial growth factor (VEGF) and stimulates angiogenesis. Microvasc. Res. 2020, 131, 104035. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Dikici, S.; Roman, S.; Mehmood, A.; Chaudhry, A.A.; U Rehman, I.; MacNeil, S.; Yar, M. Addition of 2-deoxy-d-ribose to clinically used alginate dressings stimulates angiogenesis and accelerates wound healing in diabetic rats. J. Biomater. Appl. 2019, 34, 463–475. [Google Scholar] [CrossRef]
- Andleeb, A.; Dikici, S.; Waris, T.S.; Bashir, M.M.; Akhter, S.; Chaudhry, A.A.; MacNeil, S.; Yar, M. Developing affordable and accessible pro-angiogenic wound dressings; incorporation of 2 deoxy D-ribose (2dDR) into cotton fibres and wax-coated cotton fibres. J. Tissue Eng. Regen. Med. 2020, 14, 973–988. [Google Scholar] [CrossRef]
- Haraguchi, M.; Miyadera, K.; Uemura, K.; Sumizawa, T.; Furukawa, T.; Yamada, K.; Akiyama, S.I.; Yamada, Y. Angiogenic activity of enzymes. Nature 1994, 368, 198. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Choudhuri, R.; Scott, P.A.; Zhang, L.; Ziche, M.; Morbidelli, L.; Donnini, S.; Jagger, R.T.; Chan, H.-Y.; Smith, K.; et al. Angiogenic Polypeptides in Breast Cancer: Expression of Mrna’s in Primary Human Tumours, MCF-7 Cell Transfection and Xenograft Models. In Angiogenesis; Springer: Boston, MA, USA, 1998; pp. 213–221. [Google Scholar]
- Nakajima, Y.; Haraguchi, M.; Furukawa, T.; Yamamoto, M.; Nakanishi, H.; Tatematsu, M.; Akiyama, S.I. 2-Deoxy-L-ribose inhibits the invasion of thymidine phosphorylase- overexpressing tumors by suppressing matrix metalloproteinase-9. Int. J. Cancer 2006, 119, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Vara, D.; Watt, J.M.; Fortunato, T.M.; Mellor, H.; Burgess, M.; Wicks, K.; Mace, K.; Reeksting, S.; Lubben, A.; Wheeler-Jones, C.P.D.; et al. Direct Activation of NADPH Oxidase 2 by 2-Deoxyribose-1-Phosphate Triggers Nuclear Factor Kappa B-Dependent Angiogenesis. Antioxid. Redox Signal. 2018, 28, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Bioengineering Vascular Networks to Study Angiogenesis and Vascularization of Physiologically Relevant Tissue Models in Vitro. ACS Biomater. Sci. Eng. 2020, 6, 3513–3528. [Google Scholar] [CrossRef] [PubMed]
- Ramos-rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Asencio, I.O. Delivery of bioactive compounds to improve skin cell responses on microfabricated electrospun microenvironments. Bioengineering 2021, 8, 105. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Anderson, L.; Conner, A.H. The mutarotation of 2-deoxy-β-d-erythro-pentose (“2-deoxy-β-d-ribose”). Conformations, kinetics, and equilibria. Carbohydr. Res. 1971, 20, 59–72. [Google Scholar] [CrossRef]
- Fan, C.; Deng, Q.; Zhu, T.F. Bioorthogonal information storage in l-DNA with a high-fidelity mirror-image Pfu DNA polymerase. Nat. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Vogel, T.; Blake, D.A.; Whikehart, D.R.; Guo, N.-H.; Zabrenetzky, V.S.; Roberts, D.D. Specific simple sugars promote chemotaxis and chemokinesis of corneal endothelial cells. J. Cell. Physiol. 1993, 157, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, S.; Yamauchi, K.; Nakajima, K.; Iijima, S.; Aizawa, T.; Hashizume, K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr. J. 1999, 46, 59–62. [Google Scholar] [CrossRef]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.J.; De Caterina, R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 2016, 15, 18. [Google Scholar] [CrossRef]
- Teixeira, A.S.; Andrade, S.P. Glucose-induced inhibition of angiogenesis in the rat sponge granuloma is prevented by aminoguanidine. Life Sci. 1999, 64, 655–662. [Google Scholar] [CrossRef]
- Jiraritthamrong, C.; Kheolamai, P.; U-Pratya, Y.; Chayosumrit, M.; Supokawej, A.; Manochantr, S.; Tantrawatpan, C.; Sritanaudomchai, H.; Issaragrisil, S. In vitro vessel-forming capacity of endothelial progenitor cells in high glucose conditions. Ann. Hematol. 2012, 91, 311–320. [Google Scholar] [CrossRef]
- Ikeda, R.; Furukawa, T.; Kitazono, M.; Ishitsuka, K.; Okumura, H.; Tani, A.; Sumizawa, T.; Haraguchi, M.; Komatsu, M.; Uchimiya, H.; et al. Molecular basis for the inhibition of hypoxia-induced apoptosis by 2-deoxy-D-ribose. Biochem. Biophys. Res. Commun. 2002, 291, 806–812. [Google Scholar] [CrossRef]
- Tagg, S.L.C.; Foster, P.A.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A.; Newman, S.P. 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: A potential treatment for breast and prostate cancer. Br. J. Cancer 2008, 99, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Merchan, J.R.; Kovács, K.; Railsback, J.W.; Kurtoglu, M.; Jing, Y.; Piña, Y.; Gao, N.; Murray, T.G.; Lehrman, M.A.; Lampidis, T.J. Antiangiogenic activity of 2-deoxy-D-glucose. PLoS ONE 2010, 5, e13699. [Google Scholar] [CrossRef]
- Chuang, I.C.; Yang, C.M.; Song, T.Y.; Yang, N.C.; Hu, M.L. The anti-angiogenic action of 2-deoxyglucose involves attenuation of VEGFR2 signaling and MMP-2 expression in HUVECs. Life Sci. 2015, 139, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Decatur, C.; Toro, M.; Pham, D.G.; Liu, H.; Jing, Y.; Murray, T.G.; Lampidis, T.J.; Merchan, J.R. 2-deoxy-glucose downregulates endothelial AKT and ERK via interference with N-linked glycosylation, induction of endoplasmic reticulum stress, and GSK3β activation. Mol. Cancer Ther. 2016, 15, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fei, Q.; Li, J.; Zhang, C.; Sun, Y.; Zhu, C.; Wang, F.; Sun, Y. 2-Deoxyglucose reverses the promoting effect of insulin on colorectal cancer cells in vitro. PLoS ONE 2016, 11, e0151115. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, S.; Chawla, A.S.; Bhatt, A.N.; Roy, B.G.; Saluja, D.; Dwarakanath, B.S. Dietary 2-deoxy-D-glucose impairs tumour growth and metastasis by inhibiting angiogenesis. Eur. J. Cancer 2019, 123, 11–24. [Google Scholar] [CrossRef]
- Yar, M.; Gigliobianco, G.; Shahzadi, L.; Dew, L.; Siddiqi, S.A.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; MacNeil, S. Production of chitosan PVA PCL hydrogels to bind heparin and induce angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 466–476. [Google Scholar] [CrossRef]
- Gigliobianco, G.; Chong, C.K.; MacNeil, S. Simple surface coating of electrospun poly-L-lactic acid scaffolds to induce angiogenesis. J. Biomater. Appl. 2015, 30, 50–60. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Nagane, M.; Huang, H.S.; Cavenee, W.K. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc. Natl. Acad. Sci. USA 1997, 94, 12081–12087. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Eriksson, A.; Kubo, H.; Alitalo, K.; Cao, Y.; Thyberg, J. Comparative Evaluation of FGF-2-, VEGF-A-, and VEGF-C-Induced Angiogenesis Lymphangiogenesis, Vascular Fenestrations, and Permeability. Circ. Res. 2004, 94, 664–670. [Google Scholar] [CrossRef]
- Oka, N.; Soeda, A.; Inagaki, A.; Onodera, M.; Maruyama, H.; Hara, A.; Kunisada, T.; Mori, H.; Iwama, T. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2007, 360, 553–559. [Google Scholar] [CrossRef]
- Mizumachi, H.; Ijima, H. Measuring Stability of Vascular Endothelial Growth Factor using an Immobilization Technique. Adv. Biomed. Eng. 2013, 2, 130–136. [Google Scholar] [CrossRef]
- Hoeben, A.N.N.; Landuyt, B.; Highley, M.S.M.; Wildiers, H.; Oosterom, A.T.V.A.N.; Bruijn, E.A.D.E.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Parra, M.; Verdin, E.; Bassel-Duby, R.; Olson, E.N. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc. Natl. Acad. Sci. USA 2008, 105, 7738–7743. [Google Scholar] [CrossRef]
- Domagk, G.F.; Horecker, B.L. Pentose fermentation by Lactobacillus plantarum. V. Fermentation of 2-deoxy-D-ribose. J. Biol. Chem. 1958, 233, 283–286. [Google Scholar] [CrossRef]
- Rasmussen, M.A. Isolation and characterization of Selenomonas ruminantium strains capable of 2-deoxyribose utilization. Appl. Environ. Microbiol. 1993, 59, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Borza, T.; Dandanell, G.; Gilles, A.M.; Barzu, O.; Kelln, R.A.; Neuhard, J. Regulation of expression of the 2-deoxy-D-ribose utilization regulon, deoQKPX, from Salmonella enterica serovar Typhimurium. J. Bacteriol. 2003, 185, 6042–6050. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikici, S.; Yar, M.; Bullock, A.J.; Shepherd, J.; Roman, S.; MacNeil, S. Developing Wound Dressings Using 2-deoxy-D-Ribose to Induce Angiogenesis as a Backdoor Route for Stimulating the Production of Vascular Endothelial Growth Factor. Int. J. Mol. Sci. 2021, 22, 11437. https://doi.org/10.3390/ijms222111437
Dikici S, Yar M, Bullock AJ, Shepherd J, Roman S, MacNeil S. Developing Wound Dressings Using 2-deoxy-D-Ribose to Induce Angiogenesis as a Backdoor Route for Stimulating the Production of Vascular Endothelial Growth Factor. International Journal of Molecular Sciences. 2021; 22(21):11437. https://doi.org/10.3390/ijms222111437
Chicago/Turabian StyleDikici, Serkan, Muhammad Yar, Anthony J. Bullock, Joanna Shepherd, Sabiniano Roman, and Sheila MacNeil. 2021. "Developing Wound Dressings Using 2-deoxy-D-Ribose to Induce Angiogenesis as a Backdoor Route for Stimulating the Production of Vascular Endothelial Growth Factor" International Journal of Molecular Sciences 22, no. 21: 11437. https://doi.org/10.3390/ijms222111437
APA StyleDikici, S., Yar, M., Bullock, A. J., Shepherd, J., Roman, S., & MacNeil, S. (2021). Developing Wound Dressings Using 2-deoxy-D-Ribose to Induce Angiogenesis as a Backdoor Route for Stimulating the Production of Vascular Endothelial Growth Factor. International Journal of Molecular Sciences, 22(21), 11437. https://doi.org/10.3390/ijms222111437







