Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice
Abstract
:1. Introduction
2. Results
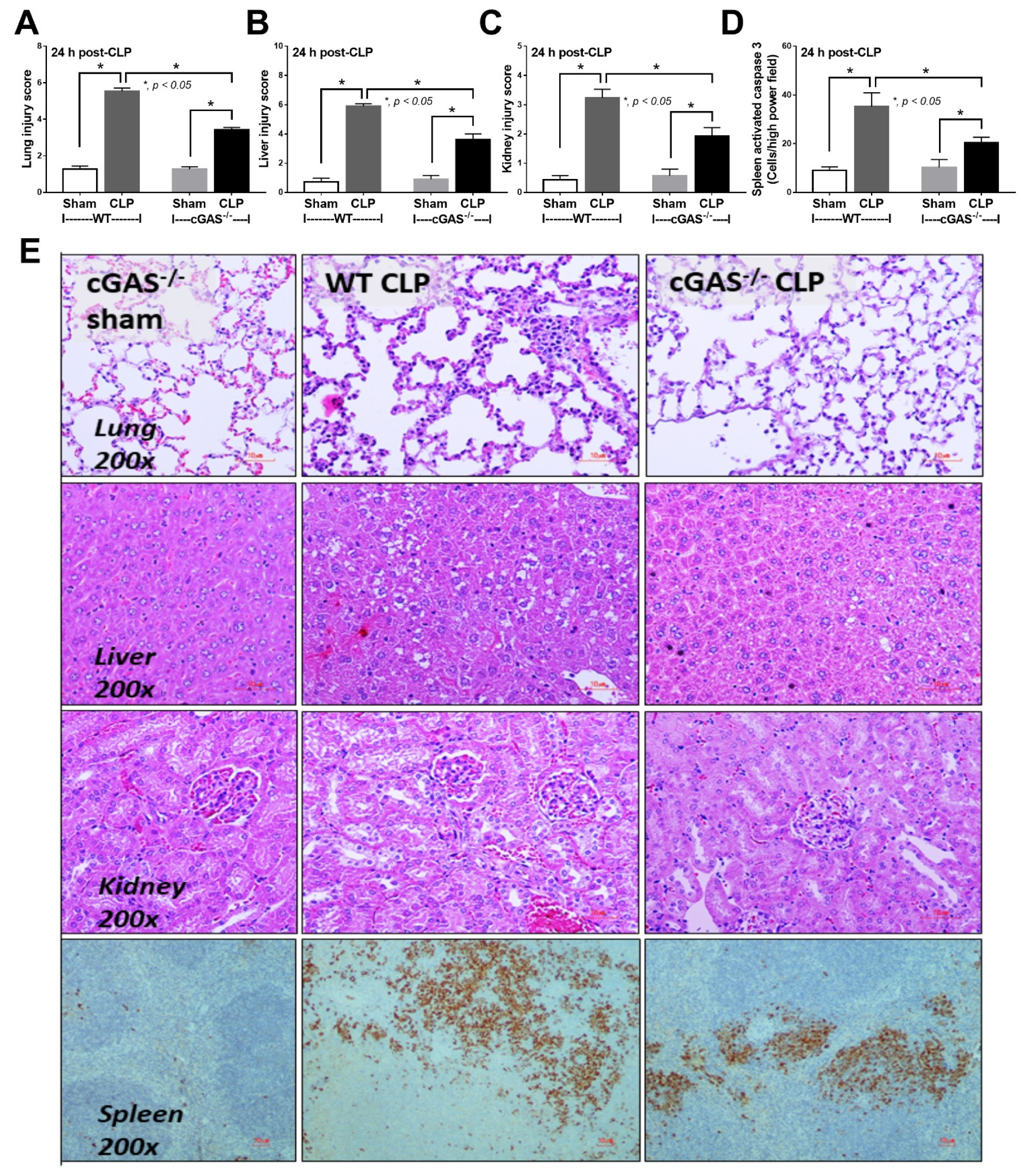
2.1. Less Severe Sepsis Was Observed in the Cecal Ligation and Puncture Model and the LPS Injection Model with cGAS Deficiency (cGAS-/-) Compared with Wildtype Mice
2.2. Less Prominent Inflammatory Responses against LPS in cGAS Deficient (cGAS-/-) Macrophages Compared with Wildtype Cells
2.3. Less Prominent LPS-Induced Mitochondrial Injury in cGAS Deficient (cGAS-/-) Macrophages Compared with Wildtype Cells
3. Discussion
3.1. Less Severe Sepsis in cGAS Deficient Mice Due to Reduced Pro-inflammatory Responses
3.2. The Co-Presentation of LPS with Bacterial DNA Enhanced Wildtype Macrophage Responses through M1 Polarization and cGAS Upregulation
3.3. Less Mitochondrial Damage in cGAS Deficient Macrophages after LPS Stimulation a Possible Effect of Mitochondrial DNA in Sepsis
4. Materials and Methods
4.1. Animal
4.2. Animal Model
4.3. Blood Sample Analysis
4.4. Histology
4.5. Macrophage Preparation
4.6. Cytokines, 2’3’-cGAMP and Gene Expression
4.7. Mitochondrial Evaluation and Extracellular Flux Analysis
4.8. RNA Sequencing
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.C.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rajaee, A.; Barnett, R.; Cheadle, W.G. Pathogen- and Danger-Associated Molecular Patterns and the Cytokine Response in Sepsis. Surg. Infect. 2018, 19, 107–116. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 2020, 18, 579–587. [Google Scholar] [CrossRef]
- Kotas, M.E.; Matthay, M.A. Mesenchymal stromal cells and macrophages in sepsis: New insights. Eur. Respir. J. 2018, 51, 1800510. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.P.; Nizet, V. Cell death during sepsis: Integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis 2009, 14, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, A.; Wort, S.J.; Thomas, H.; Collinson, P.; Bennett, E.D. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 2006, 10, R60. [Google Scholar] [CrossRef] [Green Version]
- Martins, G.A.; Kawamura, M.T.; Carvalho, M.D.G.D.C. Detection of DNA in the Plasma of Septic Patients. Ann. New York Acad. Sci. 2006, 906, 134–140. [Google Scholar] [CrossRef]
- Miyake, K.; Onji, M. Endocytosis-free DNA sensing by cell surface TLR9 in neutrophils: Rapid defense with autoimmune risks. Eur. J. Immunol. 2013, 43, 2006–2009. [Google Scholar] [CrossRef]
- Behnen, M.; Leschczyk, C.; Moller, S.; Batel, T.; Klinger, M.; Solbach, W.; Laskay, T. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J. Immunol. 2014, 193, 1954–1965. [Google Scholar] [CrossRef]
- Margolis, S.R.; Wilson, S.C.; Vance, R.E. Evolutionary Origins of cGAS-STING Signaling. Trends Immunol. 2017, 38, 733–743. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Pandol, S.J.; Li, L.; Habtezion, A. STING Signaling Promotes Inflammation in Experimental Acute Pancreatitis. Gastroenterology 2018, 154, 1822–1835.e2. [Google Scholar] [CrossRef] [Green Version]
- Torralba, D.; Baixauli, F.; Villarroya Beltri, C.; Fernández Delgado, I.; Latorre Pellicer, A. Priming of dendritic cells by DNA containing extracellular vesicles from activated T cells through antigen driven con tacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef] [Green Version]
- Ablasser, A.; Gulen, M.F. The role of cGAS in innate immunity and beyond. J. Mol. Med. 2016, 94, 1085–1093. [Google Scholar] [CrossRef]
- Zhou, C.M.; Wang, B.; Wu, Q.; Lin, P.; Qin, S.G.; Pu, Q.Q.; Yu, X.-J.; Wu, M. Identification of cGAS as an innate immune sensor of extracellular bacterium Pseudomonas aeruginosa. Iscience 2021, 24, 101928. [Google Scholar] [CrossRef]
- Huang, L.S.; Hong, Z.; Wu, W.; Xiong, S.; Gao, X.; Rehman, J.; Malik, A.B. mtDNA Activates cGAS Signaling and Suppresses the YAP-Mediated Endothelial Cell Proliferation Program to Promote Inflammatory Injury. Immunity 2020, 52, 475–486.e5. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Dai, T.; He, X.L.; Zhang, Z.K.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The interactions between cGAS-STING pathway and pathogens. Signal Transduct. Target. Ther. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.S.; Hamann, L.; Shah, J.A.; Verbon, A.; Mockenhaupt, F.P.; Puzianowska-Kuznicka, M.; Naujoks, J.; Sander, L.E.; Witzenrath, M.; Cambier, J.C.; et al. The common HAQ STING variant impairs cGAS-dependent antibacterial responses and is associated with susceptibility to Legionnaires’ disease in humans. PLoS Pathog. 2018, 14, e1006829. [Google Scholar] [CrossRef]
- Ahn, J.; Barber, G.N. STING signaling and host defense against microbial infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Watson, R.O.; Bell, S.; MacDuff, D.A.; Kimmey, J.M.; Diner, E.J.; Olivas, J.; Vance, R.E.; Stallings, C.L.; Virgin, H.; Cox, J.S. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 2015, 17, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Wang, S.; Gao, P.; Gao, G.; Fan, Z. DNA sensor cGAS-mediated immune recognition. Protein Cell 2016, 7, 777–791. [Google Scholar] [CrossRef] [Green Version]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 1–22. [Google Scholar] [CrossRef]
- Heipertz, E.L.; Harper, J.; Walker, W.E. STING and TRIF Contribute to Mouse Sepsis, Depending on Severity of the Disease Model. Shock 2017, 47, 621–631. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, H.; Li, G.; Wang, D.; Zhou, Q.; Wu, J.; Zheng, J.; Huang, J.; Slade, D.A.; Wu, X.; et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine 2019, 41, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Abrams, S.T.; Austin, J.; Toh, J.; Wang, S.S.; Wang, Z.; Yu, Q.; Yu, W.; Toh, C.H.; Wang, G. The Central Role and Possible Mechanisms of Bacterial DNAs in Sepsis Development. Mediat. Inflamm. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Gosiewski, T.; Ludwig-Galezowska, A.H.; Huminska-Lisowska, K.; Sroka-Oleksiak, A.; Radkowski, P.; Salamon, D.; Wojciechowicz, J.; Kus-Slowinska, M.; Bulanda, M.; Wolkow, P.P. Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method—The observation of DNAemia. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 36, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, S.; Akeda, Y.; Yamamoto, N.; Seki, M.; Yamamoto, K.; Oishi, K.; Tomono, K. Origin of Circulating Free DNA in Sepsis: Analysis of the CLP Mouse Model. Mediat. Inflamm. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cornélie, S.; Wiel, E.; Lund, N.; Lebuffe, G.; Vendeville, C.; Riveau, G.; Vallet, B.; Ban, E. Cytosine-phosphate-guanine (CpG) motifs are sensitizing agents for lipopolysaccharide in toxic shock model. Intensiv. Care Med. 2002, 28, 1340–1347. [Google Scholar] [CrossRef]
- Yi, A.-K.; Yoon, J.-G.; Hong, S.-C.; Redford, T.W.; Krieg, A.M. Lipopolysaccharide and CpG DNA synergize for tumor necrosis factor-α production through activation of NF-κB. Int. Immunol. 2001, 13, 1391–1404. [Google Scholar] [CrossRef] [Green Version]
- Sparwasser, T.; Miethke, T.; Lipford, G.; Borschert, K.; Häcker, H.; Heeg, K.; Wagner, H. Bacterial DNA causes septic shock. Nature 1997, 386, 336–337. [Google Scholar] [CrossRef]
- Liao, W.; Zuo, X.; Lin, G.; Zhou, Y.; Fu, Y.; Cai, S.; Wei, P.-P.; Liu, Y.-X.; Liu, Y.; Ma, G.; et al. Microbial cell-free DNA in plasma of patients with sepsis: A potential diagnostic methodology. Discov. Med. 2020, 29, 129–137. [Google Scholar]
- Chen, P.; Li, S.; Li, W.; Ren, J.; Sun, F.; Liu, R.; Zhou, X.J. Rapid diagnosis and comprehensive bacteria profiling of sepsis based on cell-free DNA. J. Transl. Med. 2020, 18, 5–10. [Google Scholar] [CrossRef]
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med. Exp. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.M.; van Deventer, S.J.; Kuijper, E.J.; Speelman, P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Chemother. 1994, 38, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimmele, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilleri, D.; Gerna, G.; Furione, M.; Bernardo, M.E.; Giorgiani, G.; Telli, S.; Baldanti, F.; Locatelli, F. Use of a DNAemia cut-off for monitoring human cytomegalovirus infection reduces the number of preemptively treated children and young adults receiving hematopoietic stem-cell transplantation compared with qualitative pp65 antigenemia. Blood 2007, 110, 2757–2760. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 2012, 339, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Van der Slikke, E.C.; Star, B.S.; van Meurs, M.; Henning, R.H.; Moser, J.; Bouma, H.R. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Timmermans, K.; Kox, M.; Scheffer, G.J.; Pickkers, P. Plasma Nuclear and Mitochondrial DNA Levels, and Markers of Inflammation, Shock, and Organ Damage in Patients with Septic Shock. Shock 2016, 45, 607–612. [Google Scholar] [CrossRef]
- Di Caro, V.; Walko, T.D.; Bola, R.A., 3rd; Hong, J.D.; Pang, D.; Hsue, V.; Au, A.K.; Halstead, E.S.; Carcillo, J.A.; Clark, R.S.B.; et al. Plasma Mitochondrial DNA—A Novel DAMP in Pediatric Sepsis. Shock 2016, 45, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Xue, Q.; Papasian, C.J.; Morrison, D.C. Bacterial DNA and Lipopolysaccharide Induce Synergistic Production of TNF-α Through a Post-Transcriptional Mechanism. J. Immunol. 2001, 166, 6855–6860. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Zanoni, I.; Cullen, T.W.; Goodman, A.L.; Kagan, J.C. Mechanisms of Toll-like Receptor 4 Endocytosis Reveal a Common Immune-Evasion Strategy Used by Pathogenic and Commensal Bacteria. Immunity 2015, 43, 909–922. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.-L.; Yang, X.-L.; Zhou, H.-J.; Long, J.; Liu, B.; Zhang, L.-M.; Lu, D. cGAS knockdown promotes microglial M2 polarization to alleviate neuroinflammation by inhibiting cGAS-STING signaling pathway in cerebral ischemic stroke. Brain Res. Bull. 2021, 171, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwitchawan, T.; Visitchanakun, P.; Dang, P.C.; Ritprajak, P.; Palaga, T.; Leelahavanichkul, A. Dysregulation of lipid metabolism in macrophages is responsible for severe endotoxin tolerance in FcgRIIB-deficient lupus mice. Front. Immunol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.P.; Issara-Amphorn, J.; Charoensappakit, A.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Saisorn, W.; Sae-Khow, K.; Leelahavanichkul, A. BAM15, a Mitochondrial Uncoupling Agent, Attenuates Inflammation in the LPS Injection Mouse Model: An Adjunctive Anti-Inflammation on Macrophages and Hepatocytes. J. Innate Immun. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Issara-Amphorn, J.; Dang, C.; Saisorn, W.; Limbutara, K.; Leelahavanichkul, A. Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1⟶3)-β-D-glucan That Enhances Systemic Inflammation through Energy Augmentation in Macrophages. Int. J. Mol. Sci. 2021, 22, 5031. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Schulze-Osthoff, K. Cell death in sepsis: A matter of how, when, and where. Crit. Care 2009, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Worasilchai, N.; Wannalerdsakun, S.; Jutivorakool, K.; Somparn, P.; Issara-Amphorn, J. Gastrointestinal Leakage Detected by Serum (1-->3)-beta-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock 2016, 46, 506–518. [Google Scholar] [CrossRef]
- Amornphimoltham, P.; Yuen, P.S.T.; Star, R.A.; Leelahavanichkul, A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci. 2019, 64, 2416–2428. [Google Scholar] [CrossRef]
- Udompornpitak, K.; Bhunyakarnjanarat, T.; Charoensappakit, A.; Dang, C.P.; Saisorn, W.; Leelahavanichkul, A. Lipopolysaccharide-Enhanced Responses against Aryl Hydrocarbon Receptor in FcgRIIb-Deficient Macrophages, a Profound Impact of an Environmental Toxin on a Lupus-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 4199. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.-X.; Kang, R.; Tang, D.-L. STING1 in sepsis: Mechanisms, functions, and implications. Chin. J. Traumatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Busani, S.; De Biasi, S.; Nasi, M.; Paolini, A.; Venturelli, S.; Tosi, M.; Girardis, M.; Cossarizza, A. Increased Plasma Levels of Mitochondrial DNA and Normal Inflammasome Gene Expression in Monocytes Characterize Patients With Septic Shock Due to Multidrug Resistant Bacteria. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Soto-Heredero, G.; Heras, M.M.G.D.L.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Liu, F. The cGAS-cGAMP-STING pathway: A molecular link between immunity and metabolism. Diabetes 2019, 68, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.; Lin, L.; Ai, Q.; Wan, J.; Dai, J.; Liu, G.; Tang, L.; Yang, Y.; Ge, P.; Jiang, R.; et al. Lipopolysaccharide-Induced Dephosphorylation of AMPK-Activated Protein Kinase Potentiates Inflammatory Injury via Repression of ULK1-Dependent Autophagy. Front. Immunol. 2018, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Li, C.; Li, Y.; Yu, Q.; Yu, H.; Li, C. Perillaldehyde Inhibition of cGAS Reduces dsDNA-Induced Interferon Response. Front. Immunol. 2021, 12, 655637. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Tangtanatakul, P.; Trithiphen, O.; Soonthornchai, W.; Wongphoom, J.; Tachaboon, S.; Srisawat, N.; Leelahavanichkul, A. Plasma miR-370-3P as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays From Mouse Brains. Shock 2019, 54, 347–357. [Google Scholar] [CrossRef]
- Dang, C.P.; Leelahavanichkul, A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS ONE 2020, 15, e0236038. [Google Scholar] [CrossRef]
- Yang, J.; Wu, R.; Qiang, X.; Zhou, M.; Dong, W.; Ji, Y.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Human Adrenomedullin and Its Binding Protein Attenuate Organ Injury and Reduce Mortality after Hepatic Ischemia-Reperfusion. Ann. Surg. 2009, 249, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Chatthanathon, P.; Somboonna, N.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Gut leakage enhances sepsis susceptibility in iron-overloaded β-thalassemia mice through macrophage hyperinflammatory responses. Am. J. Physiol. Liver Physiol. 2020, 318, G966–G979. [Google Scholar] [CrossRef]
- Taratummarat, S.; Sangphech, N.; Vu, C.T.B.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Panpetch, W.; Saisorn, W.; Chatthanathon, P.; Wannigama, D.L.; Thim-Uam, A. Increased susceptibility to dextran sulfate-induced mucositis of iron-overload β-thalassemia mice, another endogenous cause of septicemia in thalassemia. Clin. Sci. 2021, 135, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Bhunyakarnjanarat, T.; Udompornpitak, K.; Saisorn, W.; Chantraprapawat, B.; Visitchanakun, P.; Dang, C.; Issara-Amphorn, J.; Leelahavanichkul, A. Prominent Indomethacin-Induced Enteropathy in Fcgriib Defi-cient lupus Mice: An Impact of Macrophage Responses and Immune Deposition in Gut. Int. J. Mol. Sci. 2021, 22, 1377. [Google Scholar] [CrossRef]







| Primers | Forward | Reverse |
|---|---|---|
| AMPK (PRKAA1) | 5’ -AGAGGGCCGCAATAAAAGAT- 3’ | 5’ -TGTTGTACAGGCAGCTGAGG- 3’ |
| AMPK (PRKAA2) | 5’ -TGGCTGCCTTCTTATGCTTT- 3’ | 5’ -GCTTTGAAACGGCTTCTCAC- 3’ |
| Arginase-1 (Arg-1) | 5’ -CTTGGCTTGCTTCGGAACTC- 3’ | 5’ -GGAGAAGGCGTTTGCTTAGTTC- 3’ |
| cyclic GMP–AMP syntase pair 9 (cGAS pair 9) | 5’ -ATGTGAAGATTTCYGCTCCTAATGA- 3’ | 5’ -GAAATGACTCAGCGGATTTCCT- 3’ |
| Inducible nitric oxide synthase (iNOS) | 5’ -ACCCACATCTGGCAGAATGAG- 3’ | 5’ -AGCCATGACCTTTCGCATTAG- 3’ |
| Interferon gamma (IFN-γ) | 5’ -ACTGACTTGAATGTCCAACGCA- 3’ | 5’ -ATCTGACTCCTTTTTCGCTTCC- 3’ |
| Interleukin-10 (IL-10) | 5’ -GCTCTTACTGACTGGCATGAG- 3’ | 5’ -CGCAGCTCTAGGAGCATGTG- 3’ |
| Interleukin-1β (IL-1β | 5’ -GAAATGCCACCTTTTGACAGTG- 3’ | 5’ -TGGATGCTCTCATCAGGACAG- 3’ |
| Interleukin-6 (IL-6) | 5’ -TACCACTTCACAAGTCGGAGGC- 3’ | 5’ -CTGCAAGTGCATCATCGTTGTTC- 3’ |
| Mitochrondia DNA (mtDNA) | 5’ -CGTACACCCTCTAACCTAGAGAAGG- 3’ | 5’ -GGTTTTAAGTCTTACGCAATTTCC- 3’ |
| Nuclear factor-κB (NF-κB) | 5’ -CTTCCTCAGCCATGGTACCTCT- 3’ | 5’ -CAAGTCTTCATCAGCATCAAACTG- 3’ |
| Resistin-like molecule-α (FIZZ-1) | 5’ -GCCAGGTCCTGGAACCTTTC- 3’ | 5’ -GGAGCAGGGAGATGCAGATGA- 3’ |
| Toll like receptor 4 (TLR-4) | 5’ -GGCAGCAGGTGGAATTGTAT- 3’ | 5’ -AGGCCCCAGAGTTTTGTTCT- 3’ |
| Transforming Growth Factor-β (TGF-β) | 5’ -CAGAGCTGCGCTTGCAGAG- 3’ | 5’ -GTCAGCAGCCGGTTACCAAG- 3’ |
| Tumor necrosis factor α (TNF-α) | 5’ -CCTCACACTCAGATCATCTTCTC- 3’ | 5’ -AGATCCATGCCGTTGGCCAG- 3’ |
| β2-microglobulin (β2M) | 5′ -TTCTGGTGCTTGTCTCACTGA- 3′ | 5′ -CAGTATGTTCGGCTTCCCATTC- 3’ |
| β-actin | 5’ -CGGTTCCGATGCCCTGAGGCTCTT- 3’ | 5’ -CGTCACACTTCATGATGGAATTGA- 3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visitchanakun, P.; Kaewduangduen, W.; Chareonsappakit, A.; Susantitaphong, P.; Pisitkun, P.; Ritprajak, P.; Townamchai, N.; Leelahavanichkul, A. Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11450. https://doi.org/10.3390/ijms222111450
Visitchanakun P, Kaewduangduen W, Chareonsappakit A, Susantitaphong P, Pisitkun P, Ritprajak P, Townamchai N, Leelahavanichkul A. Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice. International Journal of Molecular Sciences. 2021; 22(21):11450. https://doi.org/10.3390/ijms222111450
Chicago/Turabian StyleVisitchanakun, Peerapat, Warerat Kaewduangduen, Awirut Chareonsappakit, Paweena Susantitaphong, Prapaporn Pisitkun, Patcharee Ritprajak, Natavudh Townamchai, and Asada Leelahavanichkul. 2021. "Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice" International Journal of Molecular Sciences 22, no. 21: 11450. https://doi.org/10.3390/ijms222111450
APA StyleVisitchanakun, P., Kaewduangduen, W., Chareonsappakit, A., Susantitaphong, P., Pisitkun, P., Ritprajak, P., Townamchai, N., & Leelahavanichkul, A. (2021). Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice. International Journal of Molecular Sciences, 22(21), 11450. https://doi.org/10.3390/ijms222111450







