Comparative Hessian Fly Larval Transcriptomics Provides Novel Insight into Host and Nonhost Resistance
Abstract
1. Introduction
2. Results and Discussion
2.1. Overview of Transcriptome of Larvae Feeding on Host Wheat and Nonhost Bd
2.2. Validation of RNA-Seq Expression
2.3. Larval Phenotypes on Host Wheat and Nonhost Bd
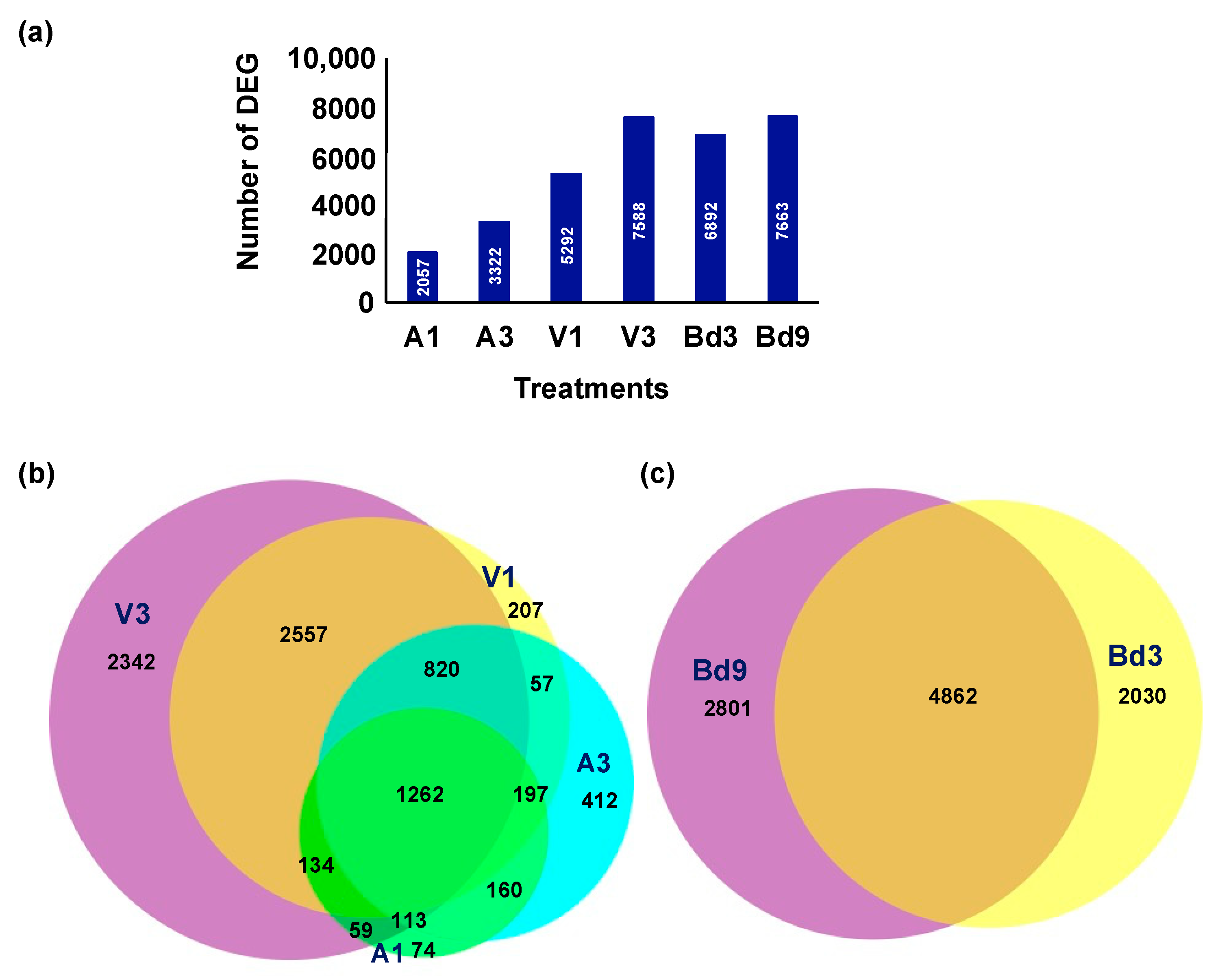
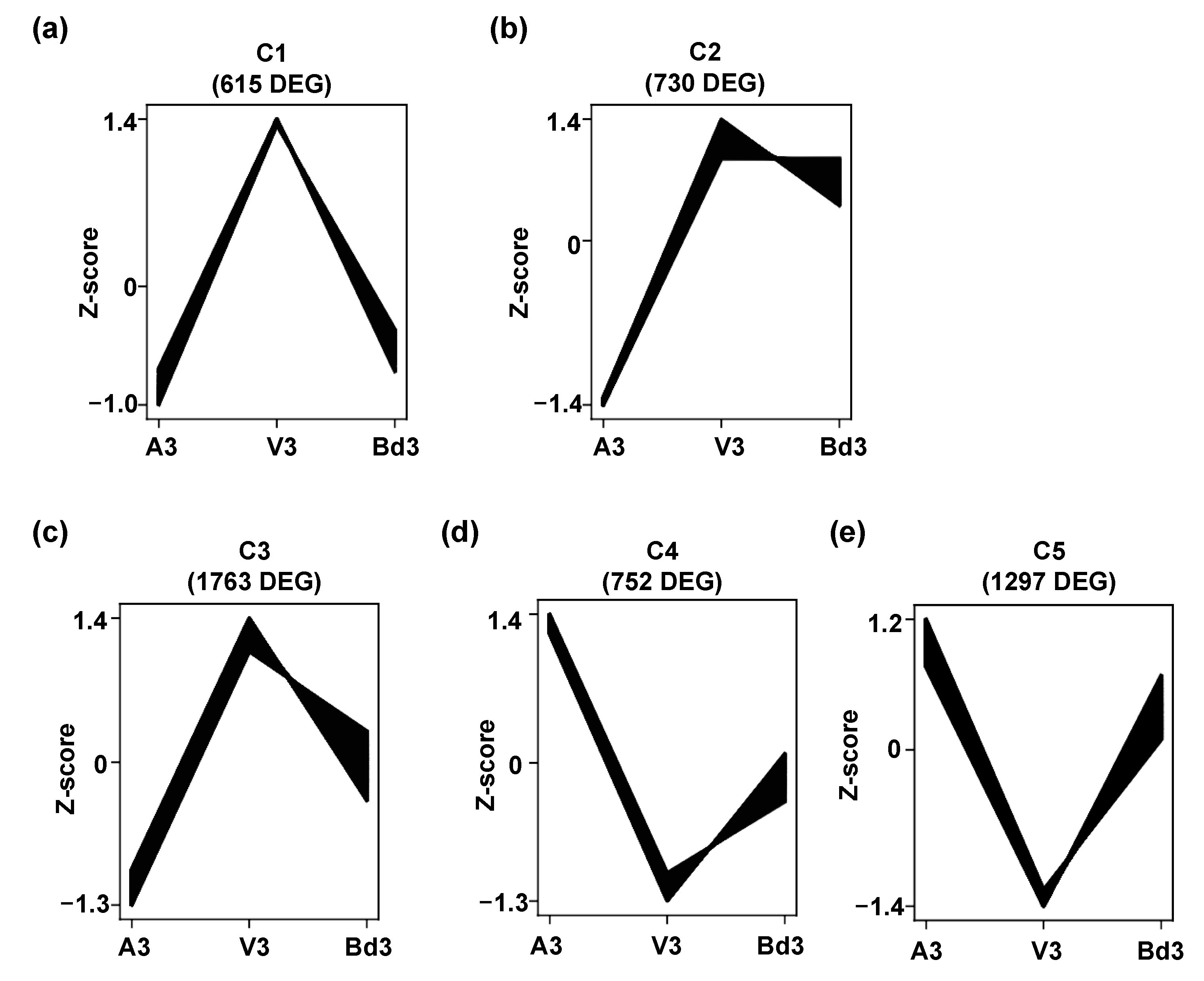
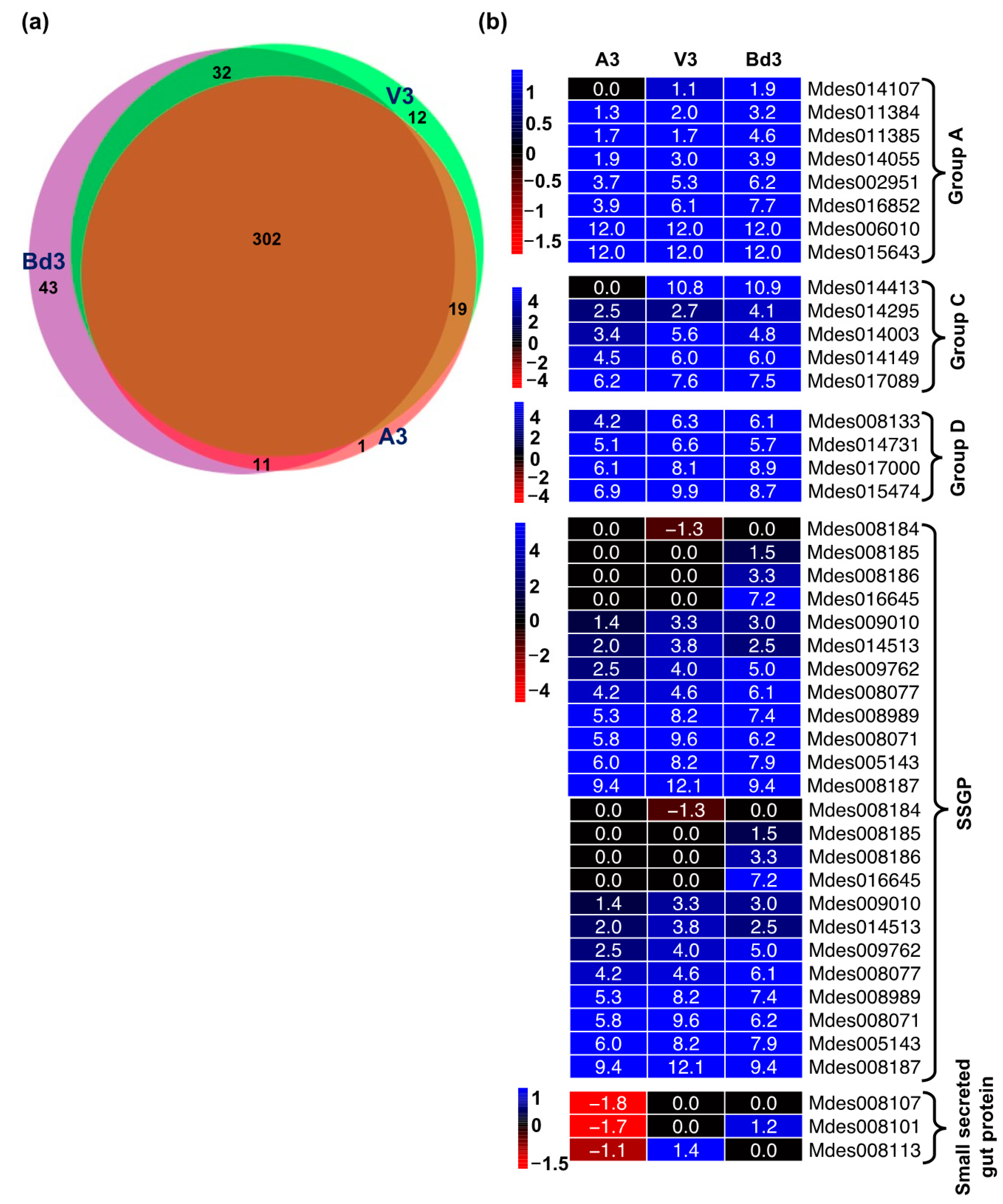
2.4. Identification and Clustering of Differentially Expressed Genes (DEGs)
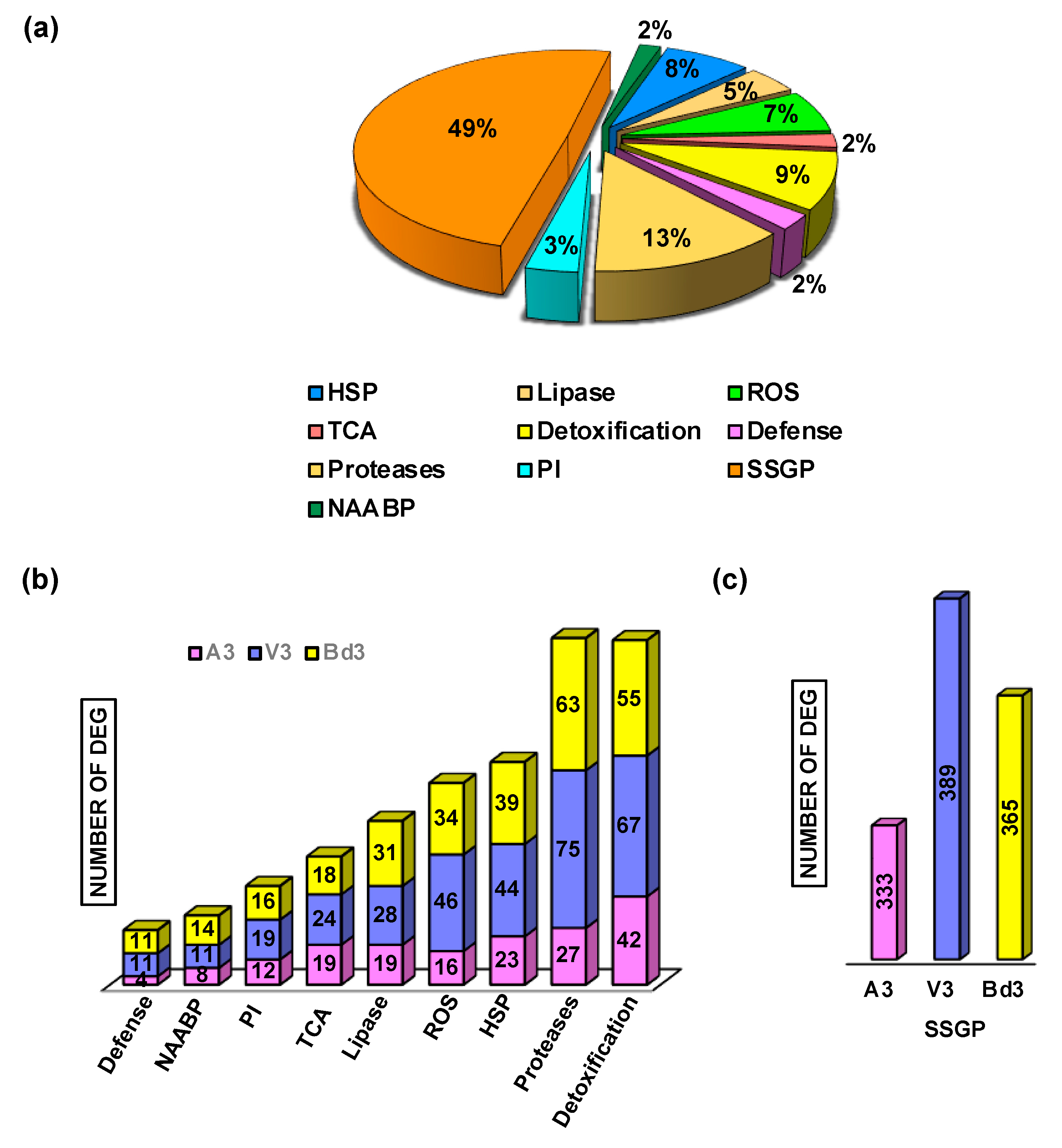
2.5. Differential Regulation of Specific Functional Categories
2.5.1. Regulation of Detoxification Enzymes
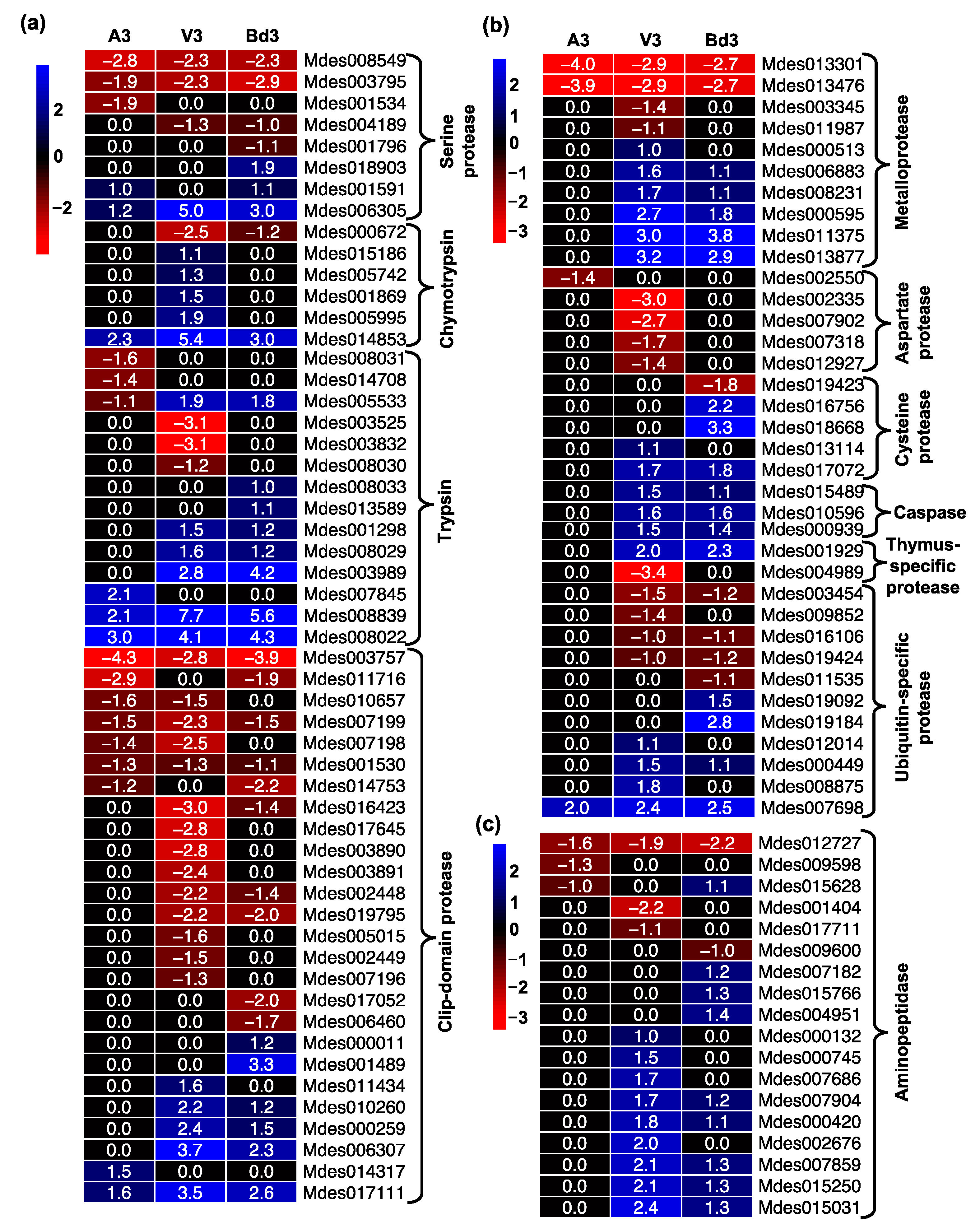
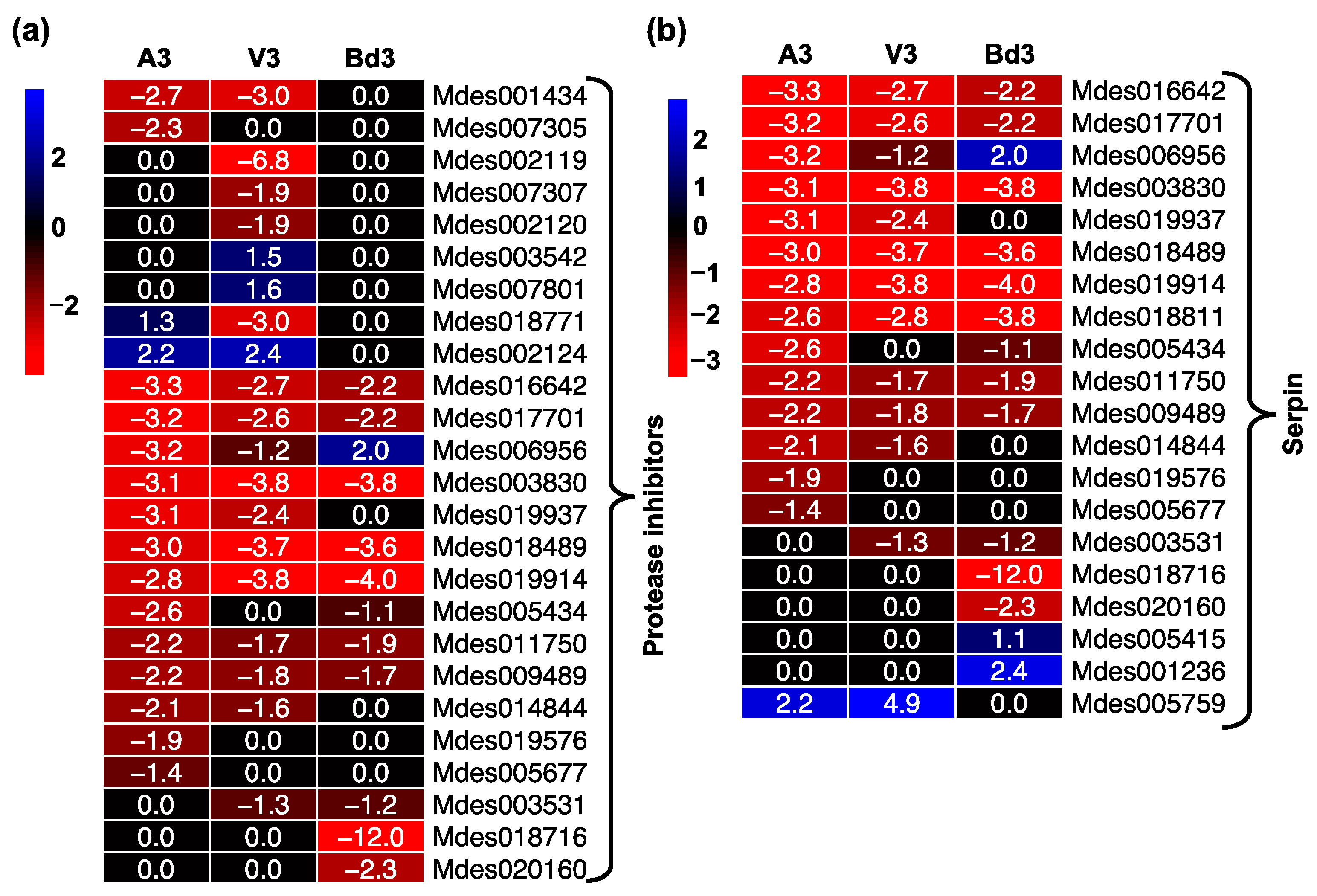
2.5.2. Gene Expression Profiles of Proteases and Protease Inhibitors
2.5.3. Regulation of Heat Shock Proteins
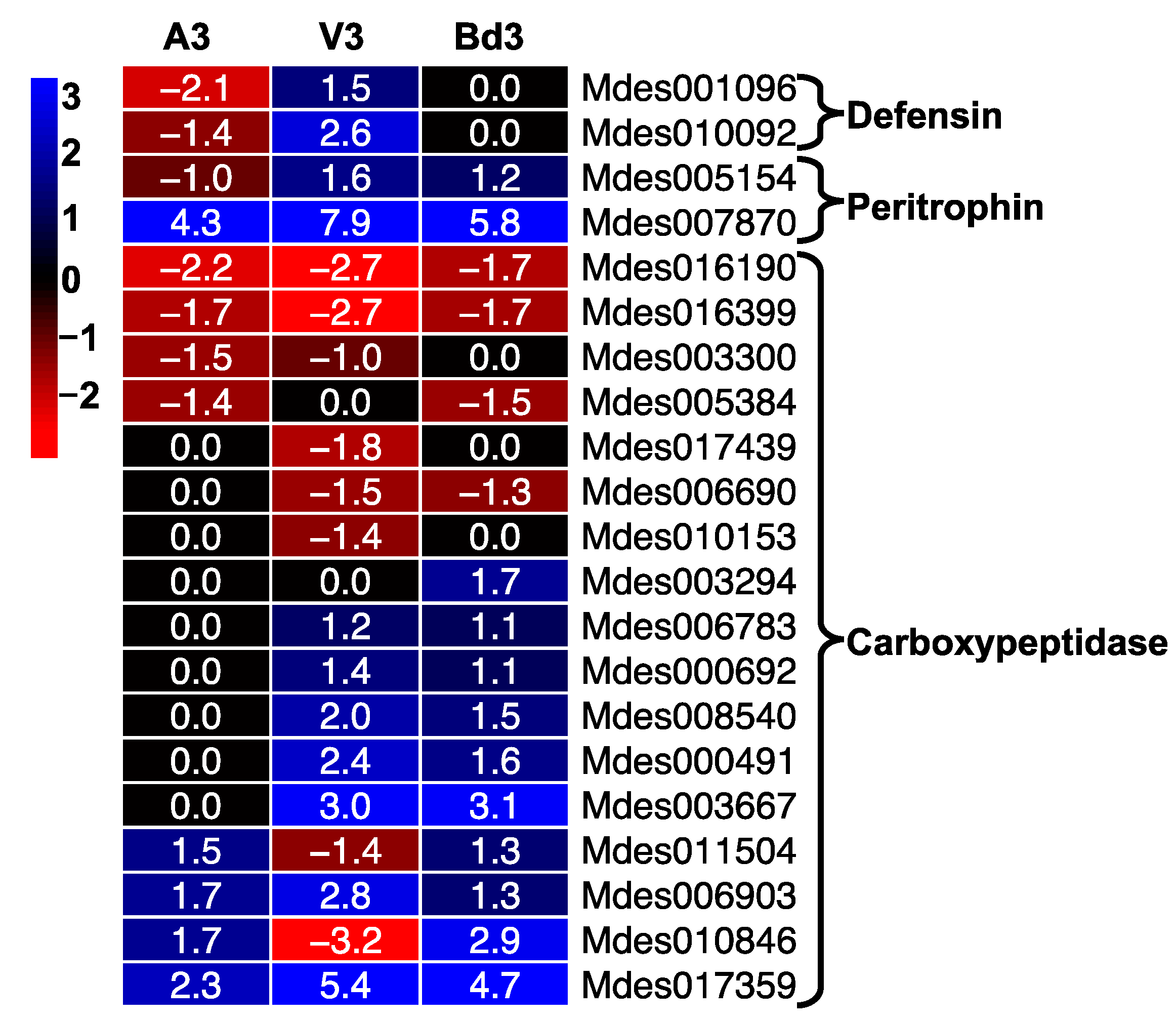
2.5.4. Expression of Defense Proteins
2.5.5. Lipases Differentially Expressed in Larvae Feeding on Host Wheat and Nonhost Bd Plants
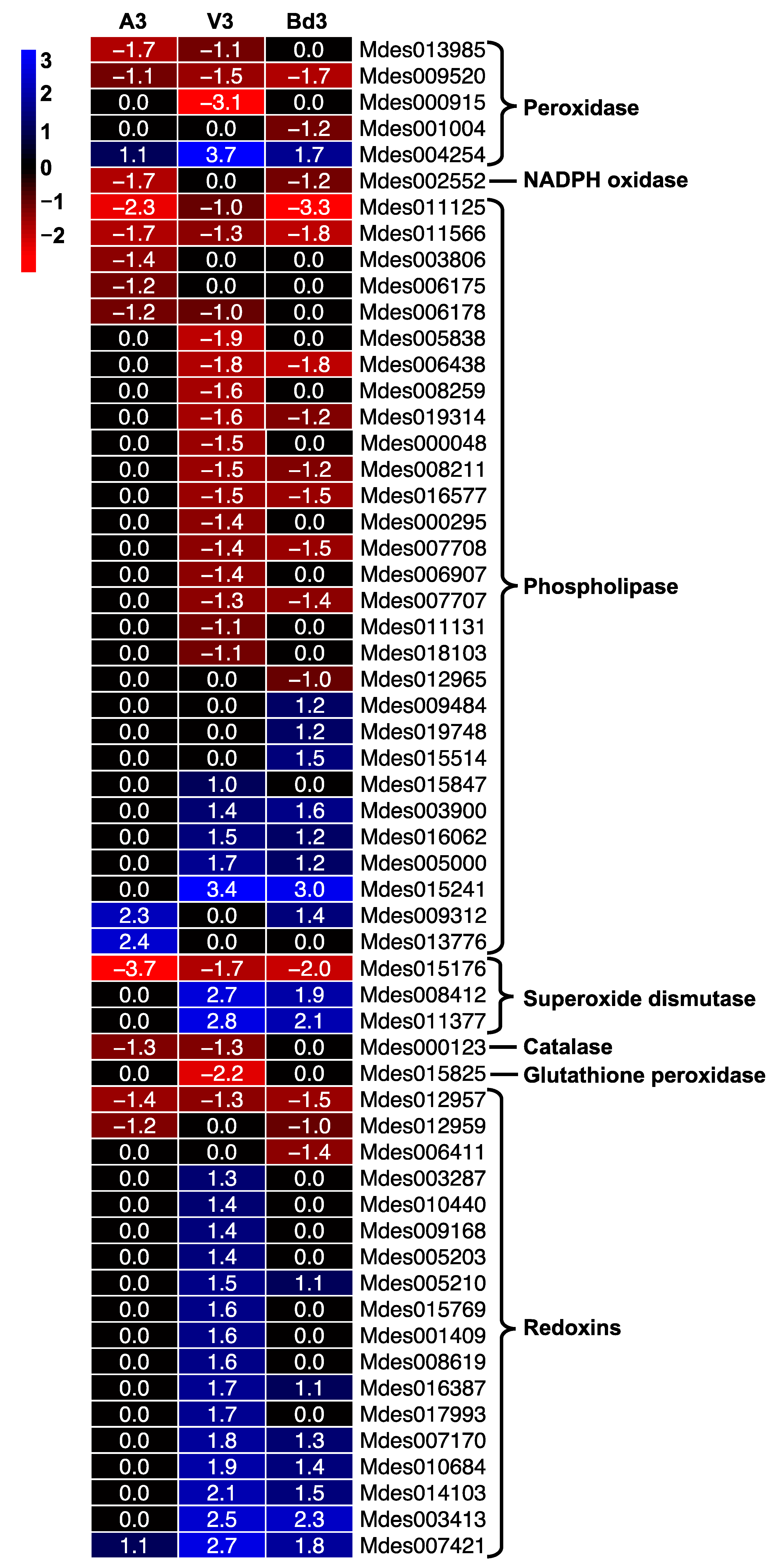
2.5.6. Genes Involved in Oxidative Stress
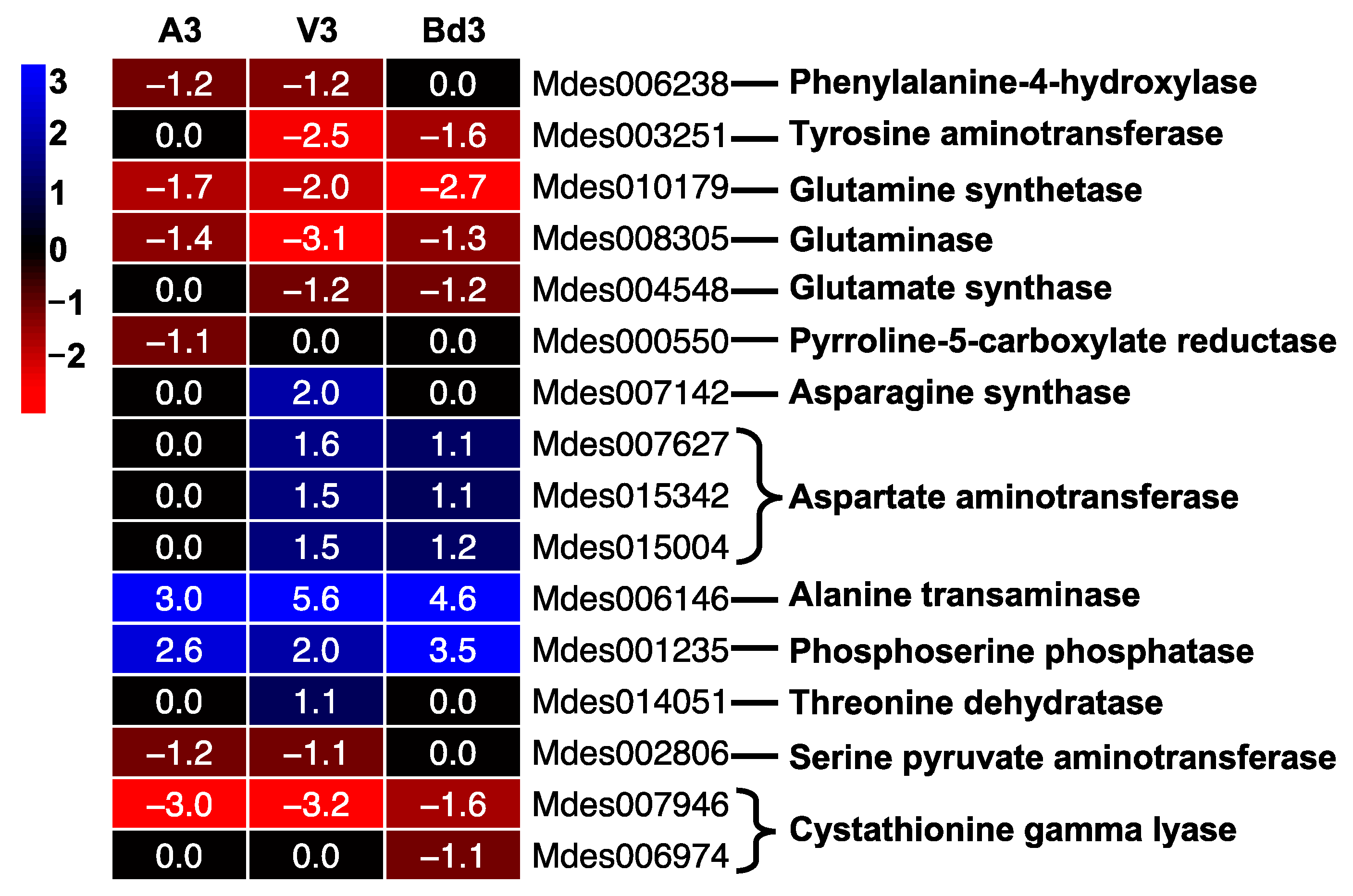
2.5.7. Modulation of Nonessential Amino Acid Biosynthetic Pathway (NAABP)
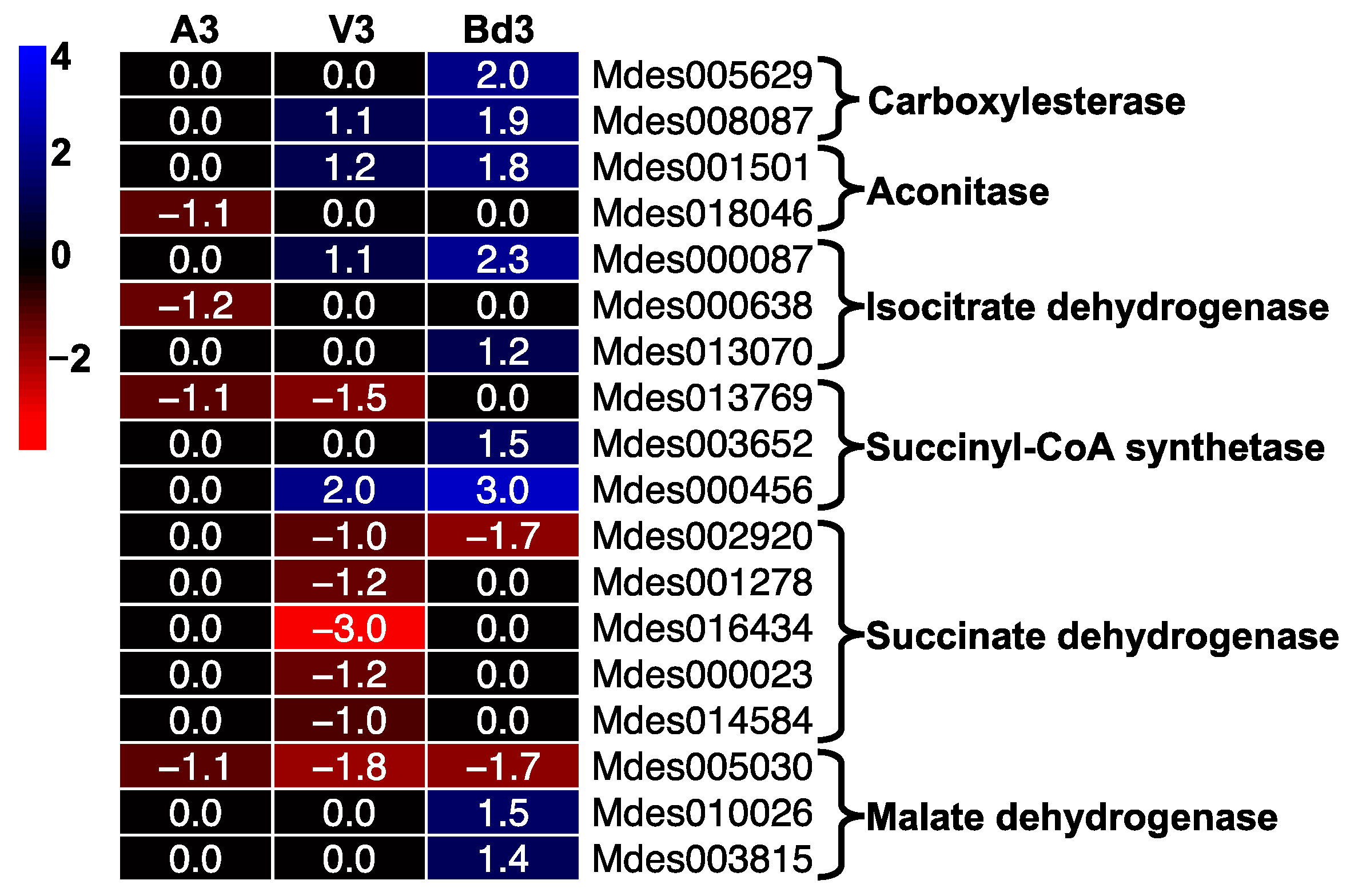
2.5.8. Regulation of Tricarboxylic Acid (TCA) Pathway
2.5.9. Differential Expression of Genes Encoding Secreted Salivary Gland Proteins
3. Materials and Methods
3.1. Insect and Plant Material
3.2. Plant Growth and Infestation
3.3. Insect Tissue Collections
3.4. RNA Isolation and Quantification
3.5. Illumina RNA-Sequencing and Data Processing
3.6. Identification and Analysis of Differentially Expressed Genes
3.7. Validation of RNA-Seq Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEG | Differentially expressed gene |
| SSGP | Secreted salivary gland protein |
| HSP | Heat shock protein |
| PI | Protease inhibitor |
| TCA | Tricarboxylic acid cycle |
| NAABP | Nonessential amino acid biosynthetic pathway |
| ROS | Reactive oxygen species |
| UGT | UDP-glycosyltransferase |
| GST | Glutathione-S-transferase |
| CYP450 | Cytochrome P450 |
| PM | Peritrophic matrix |
References
- Smiley, R.W.; Gourlie, J.A.; Whittaker, R.G.; Easley, S.A.; Kidwell, K.K. Economic impact of Hessian fly (Diptera: Cecidomyiidae) on spring wheat in Oregon and additive yield losses with Fusarium crown rot and lesion nematode. J. Econ. Entomol. 2004, 97, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, H.N.; Whitworth, J.R.; Chen, M.-S.; Cramer, G.; Maxwell, T. Impact of Hessian fly, Mayetiola destructor, on developmental aspects of hard red winter wheat in Kansas. Southwest. Entomol. 2016, 41, 321–330. [Google Scholar] [CrossRef]
- Hatchett, J.H.; Gallun, R.L. Genetics of ability of Hessian fly, Mayetiola destructor (Diptera: Cecidomyiidae), to survive on wheats having different genes for resistance. Ann. Entomol. Soc. Am. 1970, 63, 1400–1407. [Google Scholar] [CrossRef]
- Sardesai, N.; Subramanyam, S.; Nemacheck, J.A.; Williams, C.E. Modulation of defense-response gene expression in wheat during Hessian fly larval feeding. J. Plant Interact. 2005, 1, 39–50. [Google Scholar] [CrossRef]
- Subramanyam, S.; Sardesai, N.; Puthoff, D.P.; Meyer, J.M.; Nemacheck, J.A.; Gonzalo, M.; Williams, C.E. Expression of two wheat defense-response genes, Hfr-1 and Wci-1, under biotic and abiotic stresses. Plant Sci. 2006, 170, 90–103. [Google Scholar] [CrossRef]
- Subramanyam, S.; Smith, D.F.; Clemens, J.C.; Webb, M.A.; Sardesai, N.; Williams, C.E. Functional characterization of HFR1, a high-mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol. 2008, 147, 1412–1426. [Google Scholar] [CrossRef]
- Subramanyam, S.; Zheng, C.; Shukle, J.T.; Williams, C.E. Hessian fly larval attack triggers elevated expression of disease resistance dirigent-like protein-encoding gene, HfrDrd, in resistant wheat. Arthropod-Plant Interact. 2013, 7, 389–402. [Google Scholar] [CrossRef]
- Shukle, R.H.; Subramanyam, S.; Saltzmann, K.A.; Williams, C.E. Ultrastructural changes in the midguts of Hessian fly larvae feeding on resistant wheat. J. Insect Physiol. 2010, 56, 754–760. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Liu, X.; Jeannotte, R.; Reese, J.C.; Harris, M.; Stuart, J.J.; Chen, M.-S. Hessian fly (Mayetiola destructor) attack causes a dramatic shift in carbon and nitrogen metabolism in wheat. Mol. Plant Microbe Interact. 2008, 21, 70–78. [Google Scholar] [CrossRef]
- Puthoff, D.P.; Sardesai, N.; Subramanyam, S.; Nemacheck, J.A.; Williams, C.E. Hfr-2, a wheat cytolytic toxin-like gene, is up-regulated by virulent Hessian fly larval feeding. Mol. Plant Pathol. 2005, 6, 411–423. [Google Scholar] [CrossRef]
- Liu, X.; Khajuria, C.; Li, J.; Trick, H.N.; Huang, L.; Gill, B.S.; Reeck, G.R.; Antony, G.; White, F.F.; Chen, M.-S. Wheat Mds-1 encodes a heat-shock protein and governs susceptibility towards the Hessian fly gall midge. Nat. Commun. 2013, 4, 2070. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, S.; Sardesai, N.; Minocha, S.C.; Zheng, C.; Shukle, R.H.; Williams, C.E. Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat. BMC Plant Biol. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.O.; Freeman, T.P.; Rohfristch, O.; Anderson, K.G.; Payne, S.A.; Moore, J.A. Virulent Hessian fly (Diptera: Cecidomyiidae) larvae induce a nutritive tissue during compatible interactions with wheat. Ann. Entomol. Soc. Am. 2006, 99, 305–316. [Google Scholar] [CrossRef]
- Saltzmann, K.D.; Giovanini, M.P.; Zheng, C.; Williams, C.E. Virulent Hessian fly larvae manipulate the free amino acid content of host wheat plants. J. Chem. Ecol. 2008, 34, 1401–1410. [Google Scholar] [CrossRef]
- Subramanyam, S.; Shreve, J.T.; Nemacheck, J.A.; Johnson, A.J.; Schemerhorn, B.J.; Shukle, R.H.; Williams, C.E. Modulation of nonessential amino acid biosynthetic pathways in virulent Hessian fly larvae (Mayetiola destructor), feeding on susceptible host wheat (Triticum aestivum). J. Insect Physiol. 2018, 105, 54–63. [Google Scholar] [CrossRef]
- Subramanyam, S.; Nemacheck, J.A.; Bernal-Crespo, V.; Sardesai, N. Insect derived extra oral GH32 plays a role in susceptibility of wheat to Hessian fly. Sci. Rep. 2021, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.G.; Harris, M.O. Does R gene resistance allow wheat to prevent plant growth effects associated with Hessian fly (Diptera: Cecidomyiidae) attack? J. Econ. Entomol. 2006, 99, 1842–1853. [Google Scholar] [CrossRef]
- Schmid, R.S.; Knutson, A.; Giles, K.L.; McCornack, B.P. Hessian fly (Diptera: Cecidomyiidae) biology and management in wheat. J. Integr. Pest Manag. 2018, 9, 14. [Google Scholar] [CrossRef]
- Berzonsky, W.A.; Ding, H.; Haley, S.D.; Harris, M.O.; Lamb, R.J.; McKenzie, R.I.H.; Ohm, H.W.; Patterson, F.L.; Peairs, F.B.; Porter, D.R.; et al. Breeding wheat for resistance to insects. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; Volume 22, pp. 221–296. [Google Scholar]
- Sardesai, N.; Nemacheck, J.A.; Subramanyam, S.; Williams, C.E. Identification and mapping of H32, a new wheat gene conferring resistance to Hessian fly. Theor. Appl Genet. 2005, 111, 1167–1173. [Google Scholar] [CrossRef]
- Bassi, F.M.; Brahmi, H.; Sabraoui, A.; Amri, A.; Nsarellah, N.; Nachit, M.M.; Al-Abdallat, A.; Chen, M.S.; Lazraq, A.; El Bouhssini, M. Genetic identification of loci for Hessian fly resistance in durum wheat. Mol. Breed. 2019, 39, 24. [Google Scholar] [CrossRef]
- Zhao, L.; Abdelsalam, N.R.; Xu, Y.; Chen, M.-S.; Feng, Y.; Kong, L.; Bai, G. Identification of two novel Hessian fly resistance genes H35 and H36 in a hard winter wheat line SD06165, Theor. Appl. Genet. 2020, 133, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.E.; Ohm, H.W.; Patterson, F.L.; Taylor, P.L. Effectiveness of deploying single gene resistances in wheat for controlling damage by the Hessian fly (Diptera: Cecidomyiidae). Environ. Entomol. 1991, 20, 964–969. [Google Scholar] [CrossRef]
- Ratcliffe, R.H.; Ohm, H.W.; Patterson, F.L.; Cambron, S.E.; Safranski, G.G. Response of resistance genes H9-H19 in wheat to Hessian fly (Dioptera: Cecidomyiidae) laboratory biotypes and field populations from the Eastern United States. J. Econ. Entomol. 1996, 89, 1309–1317. [Google Scholar] [CrossRef]
- Subramanyam, S.; Nemacheck, J.A.; Hargarten, A.M.; Sardesai, N.; Schemerhorn, B.J.; Williams, C.E. Multiple molecular defense strategies in Brachypodium distachyon surmount Hessian fly (Mayetiola destructor) larvae-induced susceptibility for plant survival. Sci. Rep. 2019, 9, 2596. [Google Scholar] [CrossRef] [PubMed]
- Nemacheck, J.A.; Schemerhorn, B.J.; Scofield, S.R.; Subramanyam, S. Phenotypic and molecular characterization of Hessian fly resistance in diploid wheat, Aegilops tauschii. BMC Plant Biol. 2019, 19, 439. [Google Scholar] [CrossRef]
- Subramanyam, S.; Nemacheck, J.A. Nonhost Kitaake rice displays phenotypic characteristics of host resistant wheat and molecular biomarkers of both resistant and susceptible wheat in response to feeding by Hessian fly larvae. J. Plant Interact. 2021, 16, 156–165. [Google Scholar] [CrossRef]
- Brkljacic, J.; Grotewold, E.; Scholl, R.; Mockler, T.; Garvin, D.F.; Vain, P.; Brutnell, T.; Sibout, R.; Bevan, M.; Budak, H.; et al. Brachypodium as a model for the grasses: Today and the future. Plant Physiol. 2011, 157, 3–13. [Google Scholar] [CrossRef]
- Bragg, J.N.; Wu, J.; Gordon, S.P.; Guttman, M.E.; Thilmony, R.; Lazo, G.R.; Gu, Y.Q.; Vogel, J.P. Generation and characterization of the Western Regional Research Center Brachypodium T-DNA insertional mutant collection. PLoS ONE 2012, 7, e41916. [Google Scholar]
- Gill, U.S.; Uppalapati, S.R.; Nakashima, J.; Mysore, K.S. Characterization of Brachypodium distachyon as a nonhost model against switchgrass rust pathogen Puccinia emaculata. BMC Plant Biol. 2015, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Sandoya, G.V.; de Oliveira Buanafina, M.M. Differential responses of Brachypodium distachyon genotypes to insect and fungal pathogens. Physiol. Mol. Plant Pathol. 2014, 85, 53–64. [Google Scholar] [CrossRef]
- Hargarten, A.M.; Nemacheck, J.A.; Subramanyam, S.; Xiao, X.; Schemerhorn, B.J.; Williams, C.E. Physical and metabolic consequences of Hessian fly infestation are more severe on nonhost Brachypodium distachyon than on host-plant resistant wheat. Arthropod-Plant Interact. 2017, 11, 767–783. [Google Scholar] [CrossRef]
- Chen, M.-S.; Liu, S.; Wang, H.; Cheng, X.; El Bouhssini, M.; Whitworth, J. Genes expressed differentially in Hessian fly larvae feeding in resistant and susceptible plants. Int. J. Mol. Sci. 2016, 17, 1324. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Nemacheck, J.A.; Shukle, J.T.; Subramanyam, S.; Saltzmann, K.D.; Shukle, R.H. Induced permeability modulates resistance and susceptibility of wheat seedlings to herbivory by Hessian fly larvae. J. Exp. Bot. 2011, 62, 4521–4531. [Google Scholar] [CrossRef]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, 93–102. [Google Scholar] [CrossRef]
- Leicach, S.R.; Chludil, H.D. Plant secondary metabolites: Structure-activity relationships in human health prevention and treatment of common diseases. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 42, pp. 267–304. [Google Scholar]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Yu, Q.; Lu, C.; Li, B.; Fang, S.; Zuo, W.; Dai, F.; Zhang, Z.; Xiang, Z. Identification, genomic organization and expression pattern of glutathionse S-transferase in the silkworm, Bombyx mori. Insect Biochm. Mol. Biol. 2008, 38, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Hussain, B.; Buhroo, A.A.; Ignacimuthu, S.; Sharma, H.C. Effect of plant secondary metabolites on legume pod borer, Helicoverpa armigera. J. Pest Sci. 2013, 86, 399–408. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Shukle, R.H. Molecular cloning and characterization of a glutathione S-transferase gene from Hessian fly (Diptera: Cecidomyiidae). Ann. Entomol. Soc. Am. 2004, 97, 1285–1293. [Google Scholar] [CrossRef][Green Version]
- Mittapalli, O.; Neal, J.J.; Shukle, R.H. Tissue and life stage specificity of glutathione S-transferase expression in the Hessian fly, Mayetiola destructor: Implications for resistance to host allelochemicals. J. Insect Sci. 2007, 7, 20. [Google Scholar] [CrossRef]
- Subramanyam, S.; Nemacheck, J.A. Initiation of compatible wheat-Hessian fly interactions triggers the expression of a novel UDP-glycosyltransferase, MdesUGT1, in virulent Hessian fly larvae. Arthropod-Plant Interact. 2021, 15, 363–374. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Clem, R.J. Insect proteases. In Insect Molecular Biology and Biochemistry, 1st ed.; Gilbert, L., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 346–364. [Google Scholar]
- Li, H.; Oppert, B.; Higgins, R.A.; Huang, F.; Zhu, K.Y.; Buschman, L.L. Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and -suseptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 2004, 34, 753–762. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Chen, C.-X.; Yan, Y.; Xu, K.-K.; Li, C. Clip-domain serine proteinase gene (LsCLIP3) is essential for larval-pupal molting and immunity in Lasioderma serricorne. Front. Physiol. 2020, 10, 1631. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Liu, X.; Maddur, A.A.; Oppert, B.; Chen, M.-S. Cloning and characterization of chymotrypsin- and trypsin-like cDNA from the gut of the Hessian fly [Mayetiola destructor (Say)]. Insect Biochem. Mol. Biol. 2005, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Stuart, J.J.; Shukle, R.H. Molecular cloning and characterization of two digestive serine proteases from the Hessian fly, Mayetiola destructor. Insect Mol. Biol. 2005, 14, 209–318. [Google Scholar] [CrossRef]
- Jang, I.H.; Nam, H.-J.; Lee, W.-J. CLIP-domain serine proteases in Drosophila innate immunity. BMB Rep. 2008, 41, 102–107. [Google Scholar] [CrossRef]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar] [CrossRef]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The insect caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Biochemistry and Molecular Biology of Digestion. In Insect Molecular Biology and Biochemistry, 1st ed.; Gilbert, L., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 365–418. [Google Scholar]
- Meekins, D.A.; Kanost, M.R.; Michel, K. Serpins in arthropod biology. Semin Cell Dev. Biol. 2017, 62, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Xiaoxia, X.; Mandal, S.D.; Jin, F. Role of serine protease inhibitors in insect-host-pathogen interactions. Arch. Insect Biochem. Physiol. 2019, 102, e21556. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.-D.; Li, D.-T.; Jiang, M.-X.; Zhang, C.-X. HSP70/DNAJ family of genes in the brown planthopper, Nilaparvata lugens: Diversity and function. Genes 2021, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Porto, W.F.; Fensterseifer, G.M.; Fanco, O.L. In silico identification, structural characterization and phylogenetic analysis of MdesDEF-2: A novel defensin from the Hessian fly, Mayetiola destructor. J. Mol. Model. 2014, 20, 2339. [Google Scholar] [CrossRef] [PubMed]
- Boosalis, G.M. Hessian fly in relation to the development of crown and basal stem rot of wheat. Phytopathology 1954, 44, 224–229. [Google Scholar]
- Mittapalli, O.; Shukle, R.H.; Sardesai, N.; Giovanini, M.P.; Williams, C.E. Expression patterns of antibacterial genes in the Hessian Fly. J. Insect Physiol. 2006, 52, 1143–1152. [Google Scholar] [CrossRef]
- Bansal, R.; Hulbert, S.; Schemerhorn, B.; Reese, J.C.; Whitworth, J.; Stuart, J.J.; Chen, M.-S. Hessian fly-associated bacteria: Transmission, essentiality and composition. PLoS ONE 2011, 6, e23170. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.D.; Toprak, U.; Erlandson, M. Peritrophic matrix formation. J. Insect Physiol. 2019, 117, 103898. [Google Scholar] [CrossRef]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef] [PubMed]
- Tellam, R.L.; Wijffels, G.; Willadsen, P. Peritrophic matrix proteins. Insect Biochem. Mol. Biol. 1999, 29, 87–101. [Google Scholar]
- Zha, X.-L.; Yu, X.-B.; Zhang, H.-Y.; Wang, H.; Huang, X.-Z.; Shen, Y.-H.; Lu, C. Identification of peritrophins and antiviral effect of Bm01504 again BmNPV in the silkworm, Bombyx mori. J. Mol. Sci. 2020, 21, 7973. [Google Scholar] [CrossRef]
- Mittapalli, O.; Sardesai, N.; Shukle, R.H. cDNA cloning and transcriptional expression of a peritrophin-like gene in the Hessian fly, Mayetiola destructor [Say]. Arch. Insect. Biochem. Physiol. 2007, 64, 19–29. [Google Scholar]
- Brown, D.P.; Gatehouse, J.A. Characterization of a digestive carboxypeptidase from the insect pest corn earworm (Helicoverpa armigera) with novel specificity towards C-terminal glutamate residues. Eur. J. Biochem. 2004, 271, 2000–2011. [Google Scholar]
- Vendrell, J.; Aviles, F.X.; Bayes, A. Insect gut carboxypeptidase 3. In Handbook of Proteolytic Enzymes, Rawlings, N.D., 3rd ed.; Salvesen, G., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 1, pp. 1370–1375. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Shukle, R.H.; Mittapalli, O.; Morton, P.K.; Chen, M.-S. Characterization and expression analysis of a gene encoding a secreted lipase-like protein expressed in the salivary glands of the larval Hessian fly, Mayetiola destructor (Say). J. Insect Physiol. 2009, 55, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007, 68, 2946–2959. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Mittapalli, O.; Neal, J.J.; Shukle, R.H. Antioxidant defense response in a galling insect. Proc. Natl. Acad. Sci. USA 2007, 104, 1889–1894. [Google Scholar] [CrossRef]
- Hu, D.; Luo, W.; Fan, L.F.; Liu, F.L.; Gu, J.; Deng, H.M.; Zhang, C.; Huang, L.H.; Feng, Q.L. Dynamics and regulation of glycoslysis-tricarboxylic acid metabolism in the midgut of Spodoptera litura during metamorphosis. Insect Mol. Biol. 2016, 25, 153–162. [Google Scholar] [CrossRef]
- Bos, J.I.B.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Chen, M.-S.; Fellers, J.P.; Stuart, J.J.; Reese, J.C.; Liu, X. A group of related cDNAs encoding secreted proteins from Hessian fly [Mayetiola destructor (Say)] salivary glands. Insect Mol. Biol. 2004, 13, 101–108. [Google Scholar] [CrossRef]
- Chen, M.-S.; Fellers, J.P.; Zhu, Y.C.; Stuart, J.J.; Hulbert, S.; El-Bouhssini, M.; Liu, X. A super-family of genes coding for secreted salivary gland proteins from the Hessian fly, Mayetiola destructor. J. Insect Sci. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Zhao, H.-X.; Zhu, Y.C.; Scheffler, B.; Liu, X.; Liu, X.; Hulbert, S.; Stuart, J.J. Analysis of transcripts and proteins expressed in the salivary glands of Hessian fly (Mayetiola destructor) larvae. J. Insect Physiol. 2008, 54, 1–16. [Google Scholar] [CrossRef]
- Chen, M.-S.; Liu, X.; Yang, Z.; Zhao, H.; Shukle, R.H.; Stuart, J.J.; Hulbert, S. Unusual conservation among genes encoding small secreted salivary gland proteins from a gall midge. BMC Evol. Biol. 2010, 10, 296. [Google Scholar] [CrossRef]
- Zhao, C.; Escalante, L.N.; Chen, H.; Benatti, T.R.; Qu, J.; Chellapilla, S.; Waterhouse, R.M.; Wheeler, D.; Andersson, M.N.; Bao, R.; et al. A massive expansion of effector genes underlies gall-formation in the wheat pest Mayetiola destructor. Curr. Biol. 2015, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Al-Jbory, Z.; El-Bouhssini, M.; Chen, M.-S. Conserved and unique putative effectors expressed in the salivary glands of three related gall midge species. J. Insect Sci. 2018, 18, 15. [Google Scholar] [CrossRef]
- Sosa, O.; Gallun, R.L. Purification of races B and C of the Hessian Fly by genetic manipulation. Ann. Entomol. Soc. Am. 1973, 66, 1065–1070. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hulsen, T.; De Vlieg, J.; Alkerma, W. BioVenn—A web application for the comparison and visualization of biological lists using area -proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Abu-Jamous, B.; Kelly, S. Clust: Automatic extraction of optimal co-expressed gene clusters from gene expression data. Genome Biol. 2018, 19, 172. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanyam, S.; Nemacheck, J.A.; Xie, S.; Bhide, K.; Thimmapuram, J.; Scofield, S.R.; Sardesai, N. Comparative Hessian Fly Larval Transcriptomics Provides Novel Insight into Host and Nonhost Resistance. Int. J. Mol. Sci. 2021, 22, 11498. https://doi.org/10.3390/ijms222111498
Subramanyam S, Nemacheck JA, Xie S, Bhide K, Thimmapuram J, Scofield SR, Sardesai N. Comparative Hessian Fly Larval Transcriptomics Provides Novel Insight into Host and Nonhost Resistance. International Journal of Molecular Sciences. 2021; 22(21):11498. https://doi.org/10.3390/ijms222111498
Chicago/Turabian StyleSubramanyam, Subhashree, Jill A. Nemacheck, Shaojun Xie, Ketaki Bhide, Jyothi Thimmapuram, Steven R. Scofield, and Nagesh Sardesai. 2021. "Comparative Hessian Fly Larval Transcriptomics Provides Novel Insight into Host and Nonhost Resistance" International Journal of Molecular Sciences 22, no. 21: 11498. https://doi.org/10.3390/ijms222111498
APA StyleSubramanyam, S., Nemacheck, J. A., Xie, S., Bhide, K., Thimmapuram, J., Scofield, S. R., & Sardesai, N. (2021). Comparative Hessian Fly Larval Transcriptomics Provides Novel Insight into Host and Nonhost Resistance. International Journal of Molecular Sciences, 22(21), 11498. https://doi.org/10.3390/ijms222111498







