Phosphonates as Unique Components of Plant Seeds—A Promising Approach to Use Phosphorus Profiles in Plant Chemotaxonomy
Abstract
:1. Introduction
2. Results
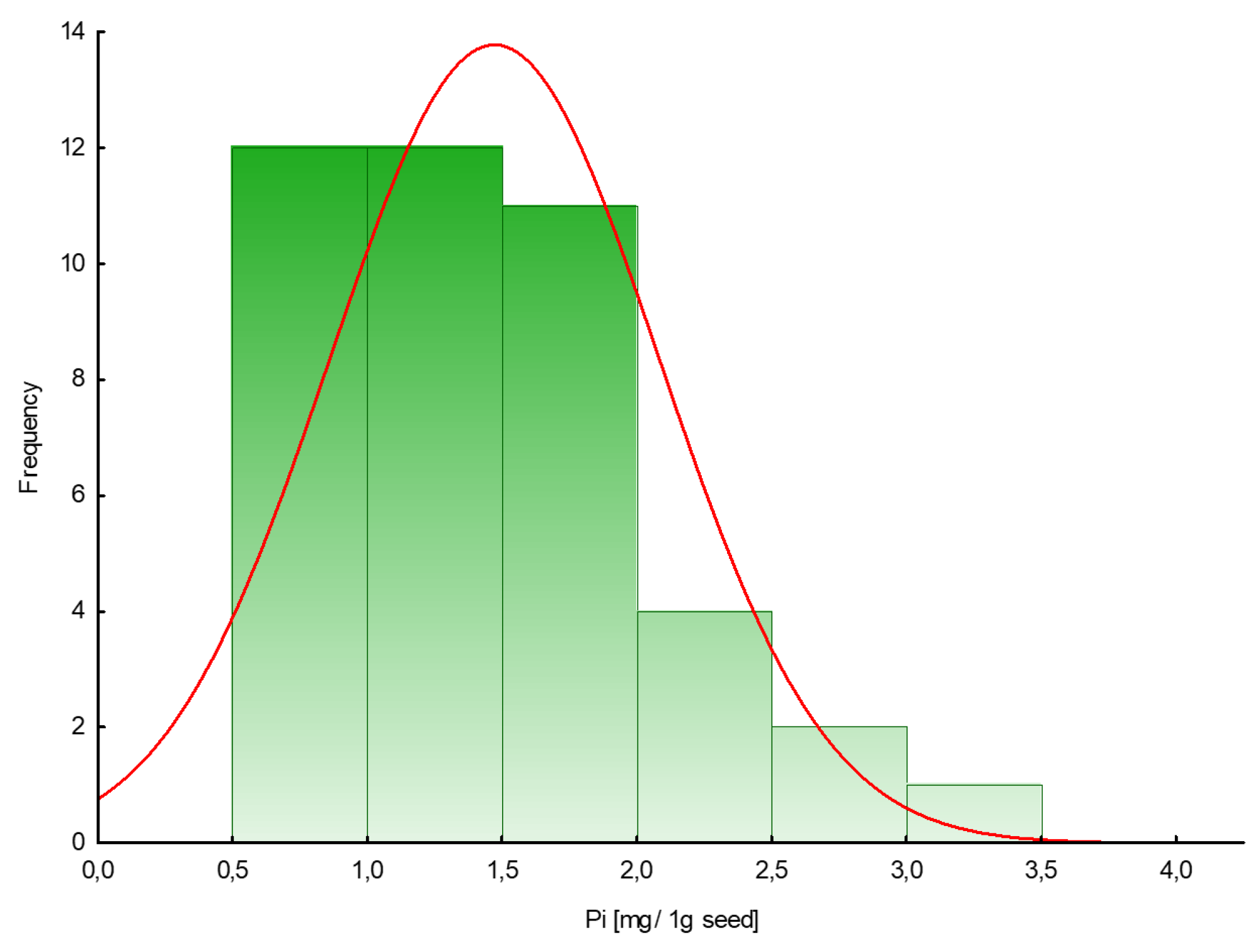
2.1. Inorganic Phosphates (Pi) in Plant Seeds
2.2. Phosphorus Speciation of Plant Seeds by 31P NMR
2.3. Natural Phosphonates in Plant Seeds
3. Discussion
4. Materials and Methods
4.1. Tested Plant Seeds
4.2. Isolation of Phosphorus Compounds from Seeds
4.3. Determination of Phosphate Content in Seeds
4.4. Determination of Phosphorus Profiles of the Seeds
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, M.S.; Oki, Y.; Adachi, T. Intraspecific Variations of Phosphorus Absorption and Remobilization, P Forms, and Their Internal Buffering in Brassica Cultivars Exposed to a P-Stressed Environment. J. Integr. Plant Biol. 2008, 50, 703–716. [Google Scholar] [CrossRef]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.P.; Kulakova, A.N.; Cooley, N.A.; McGrath, J.W. New ways to break an old bond: The bacterial carbon-phosphorus hydrolases and their role in biogeochemical phosphorus cycling. Environ. Microbiol. 2007, 9, 2392–2400. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kandatstu, M. Isolation of 2-Aminoethane Phosphonic Acid from Rumen Protozoa. Nature 1959, 184, 901–902. [Google Scholar] [CrossRef]
- Kafarski, P. Phosphonates: Their natural occurrence and physiological role. In Contemporary Topics about Phosphorus in Biology and Materials; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hendlin, D.; Stapley, E.O.; Jackson, M.; Wallick, H.; Miller, A.K.; Wolf, F.J.; Miller, T.W.; Chaiet, L.; Kahan, F.M.; Foltz, E.L.; et al. Phosphonomycin, a New Antibiotic Produced by Strains of Streptomyces. Science 1969, 166, 122–123. [Google Scholar] [CrossRef]
- Kahan, F.M.; Kahan, J.S.; Cassidy, P.J.; Kropp, H. The Mechanism of Action of Fosfomycin (Phosphonomycin). Ann. N. Y. Acad. Sci. 1974, 235, 364–386. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, Q.; Li, S.; Deng, Z.; Gao, J. The intriguing biology and chemistry of fosfomycin: The only marketed phosphonate antibiotic. RSC Adv. 2019, 9, 42204–42218. [Google Scholar] [CrossRef] [Green Version]
- Seto, H.; Kuzuyama, T. Bioactive natural products with carbon–phosphorus bonds and their biosynthesis. Nat. Prod. Rep. 1999, 16, 589–596. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Kim, A.; Kim, J.; Martin, B.M.; Dunaway-Mariano, D. Isolation and Characterization of the Carbon–Phosphorus Bond-forming Enzyme Phosphoenolpyruvate Mutase from the MolluskMytilus edulis. J. Biol. Chem. 1998, 273, 4443–4448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkarawi, H.H.; Zotz, G. Phytic acid in green leaves of herbaceous plants-temporal variation in situ and response to different nitrogen/phosphorus fertilizing regimes. AoB Plants 2014, 6, plu048. [Google Scholar] [CrossRef]
- Noack, S.R.; McLaughlin, M.J.; Smernik, R.J.; McBeath, T.M.; Armstrong, R.D. Phosphorus speciation in mature wheat and canola plants as affected by phosphorus supply. Plant Soil 2014, 378, 125–137. [Google Scholar] [CrossRef]
- Cai, H.; Chuang, W.-G.; Cui, X.; Cheng, R.-H.; Chiu, K.; Chen, Z.; Ding, S. High Resolution 31P NMR Spectroscopy Generates a Quantitative Evolution Profile of Phosphorous Translocation in Germinating Sesame Seed. Sci. Rep. 2018, 8, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschidis, M.C. Thin-layer chromatographic and infrared spectral evidence for the presence of phosphonolipids in ground apricot kernel. J. Chromatogr. A 1984, 294, 519–524. [Google Scholar] [CrossRef]
- Mukhamedova, K.S.; Tolibaev, I.; Glushenkova, A.I. Purification of total phospho- and phosphonolipids of cotton and kenaf seeds from accompanying substances. Chem. Nat. Compd. 1988, 24, 666–668. [Google Scholar] [CrossRef]
- McDowell, R.W.; Stewart, I. Peak assignments for phosphorus-31 nuclear magnetic resonance spectroscopy in pH range 5–13 and their application in environmental samples. Chem. Ecol. 2005, 21, 211–226. [Google Scholar] [CrossRef]
- Ebuele, V.O.; Santoro, A.; Thoss, V. Characterization of Plant Seeds by Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy. Anal. Lett. 2017, 50, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Bergman, B.; Zheng, W.-W.; Klint, J.; Ran, L. On the origin of plants and relations to contemporary cyanobacterial-plant symbioses. Plant Biotechnol. 2008, 25, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Koukol, O.; Novák, F.; Hrabal, R. Composition of the organic phosphorus fraction in basidiocarps of saprotrophic and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 2464–2467. [Google Scholar] [CrossRef]
- Karaismailoğlu, M.C. Comparative morphology and anatomy of seeds of some Aethionema W.T. Aiton (Brassicaceae) taxa from Turkey. Bangladesh J. Plant Taxon. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Forlani, G.; Pavan, M.; Gramek, M.; Kafarski, P.; Lipok, J. Biochemical Bases for a Widespread Tolerance of Cyanobacteria to the Phosphonate Herbicide Glyphosate. Plant Cell Physiol. 2008, 49, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Cade-Menun, B.J. Improved peak identification in 31P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 2015, 257–258, 102–114. [Google Scholar] [CrossRef]




| Symbol | Common Name | Variety of Seed | Pi Content (mg PO43−/g DW Seed) | P1 (%) | P2 (%) | P3 (%) | P4 (%) | P5 (%) |
|---|---|---|---|---|---|---|---|---|
| AMA1 | Beetroot | Opolski | 2.4 ± 0.1 | 0 | 10.7 | 83.3 | 5.9 | 0.1 |
| API1 | Carrot | Dolanka | 0.9 ± 0.0 | 0 | 38.6 | 19.6 | 32.1 | 9.7 |
| API2 | Celery | Talar | 1.1 ± 0.0 | 0 | 42.2 | 14 | 35.9 | 7.9 |
| API3 | Dill | Szmaragd | 0.6 ± 0.0 | 0 | 39.5 | 23.1 | 31.7 | 5.7 |
| API4 | Foliate celery | Verde Pascal | 0.9 ± 0.1 | 0.7 | 35.6 | 18 | 38.1 | 7.6 |
| API5 | Parsley | Alba | 0.6 ± 0.0 | 0.2 | 38.7 | 11.4 | 44.8 | 4.9 |
| API6 | Parsnip | Halflong White | 1.4 ± 0.0 | 0 | 26.6 | 39.9 | 27.3 | 6.2 |
| AST1 | Chicory | Palla Rossa 3 | 1.8 ± 0.1 | 0 | 68.9 | 20.9 | 8.9 | 1.3 |
| AST2 | Sunflower sprouting seeds | - | 1.2 ± 0.1 | 0 | 65.6 | 16.7 | 15.9 | 1.8 |
| BRA1 | Broccoli sprouting Seeds | - | 0.8 ± 0.0 | 0 | 34.8 | 51.8 | 8.3 | 5.1 |
| BRA2 | Brussels sprouts | Long Island | 1.5 ± 0.0 | 0 | 30.7 | 55.1 | 13 | 1.2 |
| BRA3 | Cauliflower | Beta | 1.5 ± 0.0 | 0 | 37.3 | 48.8 | 13.1 | 0.8 |
| BRA4 | Chinese cabbage | Bristol | 1.6 ± 0.0 | 0.8 | 23.8 | 57.3 | 17.4 | 0.7 |
| BRA5 | Cress sprouting seeds | - | 0.6 ± 0.1 | 0.1 | 33.7 | 52.1 | 9.6 | 4.5 |
| BRA6 | Kale sprouting Seeds, fringed cabbage | - | 1.2 ± 0.0 | 0 | 51 | 33.3 | 13.4 | 2.3 |
| BRA7 | Radish sprouting seeds | - | 1.0 ± 0.1 | 0.4 | 36.6 | 50.6 | 11.1 | 1.3 |
| BRA8 | Red cabbage sprouting seeds | - | 1.2 ± 0.1 | 0.1 | 43.8 | 44.7 | 9.9 | 1.5 |
| BRA9 | Savoy cabbage | Langedijska | 1.4 ± 0.0 | 0 | 46.2 | 42.1 | 9.8 | 1.9 |
| BRA10 | Swede (rutabaga) | Nadmorska | 0.7 ± 0.1 | 0.3 | 35.8 | 46.5 | 12.8 | 4.6 |
| BRA11 | Turnip | Di milano a collettoviola | 1.1 ± 0.0 | 0 | 56.8 | 22 | 18.3 | 2.9 |
| BRA12 | Turnip kale | Wener Witte | 0.9 ± 0.0 | 0.4 | 35.5 | 48.3 | 13.8 | 2 |
| BRA13 | White cabbage | Amager Polana | 1.3 ± 0.0 | 0.1 | 30 | 45.6 | 22.8 | 1.5 |
| BRA14 | White mustard | - | 0.9 ± 0.1 | 0 | 48 | 21.1 | 23.5 | 7.4 |
| CUC1 | Cucumber | Aladyn F1 | 0.8 ± 0.0 | 2 | 10.3 | 76.3 | 9.4 | 2 |
| CUC2 | Zucchini | Astra Polka | 1.6 ± 0.2 | 0 | 7.6 | 86.5 | 5.8 | 0.1 |
| CUC3 | Pumpkin | Bambino | 2.2 ± 0.1 | 0 | 4.4 | 92.3 | 3.1 | 0.2 |
| CUC4 | Pumpkin | Kabaczek Złoty Cepelin | 0.9 ± 0.0 | 0.6 | 8.2 | 90.7 | 0.2 | 0.3 |
| CUC5 | Summer squash | Patison Polo F1 | 1.9 ± 0.0 | 0 | 3.3 | 93 | 3.4 | 0.3 |
| FAB1 | Alfalfa sprouting seeds | - | 1.7 ± 0.1 | 0 | 3.3 | 11.3 | 85.4 | 0 |
| FAB2 | Beans for dry seeds | Borlotto lingua di fuoco nano | 2.6 ± 0.0 | 1.3 | 86.3 | 12.5 | 0 | 0 |
| FAB3 | Broad bean | - | 2.1 ± 0.1 | 1 | 7.5 | 85.5 | 6 | 0 |
| FAB3 | Dwarf bean | Esterka | 1.4 ± 0.1 | 2.1 | 23.8 | 54.8 | 19.3 | 0 |
| FAB5 | Green lentil sprouting seeds | - | 2.0 ± 0.1 | 0.8 | 8.8 | 79.4 | 10.7 | 0.3 |
| FAB6 | Mung bean sprouting seeds | - | 2.6 ± 0.1 | 0.8 | 39.9 | 41.1 | 17.7 | 0.5 |
| FAB7 | Pea sprouting seeds | - | 2.2 ± 0.1 | 1.8 | 14.8 | 73.6 | 9.2 | 0.6 |
| POA1 | Corn sprouting seeds | - | 1.2 ± 0.0 | 0 | 10.9 | 84 | 3.6 | 1.5 |
| SOL1 | Eggplant | Black Beauty | 1.6 ± 0.0 | 0 | 13.3 | 82.8 | 3.9 | 0 |
| SOL2 | Pepper | Kasia | 3.4 ± 0.0 | 0 | 3.9 | 88.9 | 6.2 | 1 |
| SOL3 | Pepper | Ożarowska | 1.7 ± 0.1 | 0 | 6.7 | 72.2 | 21.1 | 0 |
| SOL4 | Tomato | Betalux | 1.4 ± 0.2 | 0.3 | 9.2 | 85.2 | 4.9 | 0.4 |
| SOL5 | Tomato | Krakus | 1.6 ± 0.0 | 4 | 12.2 | 72.7 | 11.1 | 0 |
| SOL6 | Tomato | Malinowy Olbrzym | 1.9 ± 0.1 | 0.2 | 9 | 81.3 | 9.5 | 0.3 |
| Symbol | Common Name | Variety of Seed | Botanical Name | Family | Seeds per Gram (s/g) |
|---|---|---|---|---|---|
| AMA1 | Beetroot | Opolski | Beta vulgaris var. Conditiva | Amaranthaceae (AMA) | 40–60 |
| API1 | Carrot | Dolanka | Daucus carota | Apiaceae (API) | 600–700 |
| API2 | Celery | Talar | Apium graveolens | 2500–2700 | |
| API3 | Dill | Szmaragd | Anethum graveolens | 700 | |
| API4 | Foliate celery | Verde Pascal | Apium graveolens var. dulce | 2500–2700 | |
| API5 | Parsley | Alba | Petroselinum crispum convar. Radicosum | 500–600 | |
| API6 | Parsnip | Halflong White | Pastinaca sativa | 200–220 | |
| AST1 | Chicory | Palla Rossa 3 | Cichorium intybus var. foliosum | Asteraceae (AST) | 600–800 |
| AST2 | Sunflower sprouting seeds | - | Helianthus annuus | 10–20 | |
| BRA1 | Broccoli sprouting Seeds | - | Brassicaoleracea | Brassicaceae (BRA) | 315 |
| BRA2 | Brusselssprouts | Long Island | Brassica oleracea L. var. gemmifera | 350–400 | |
| BRA3 | Cauliflower | Beta | Brassica oleracea convar. Botrytis | 300–400 | |
| BRA4 | Chinese cabbage | Bristol | Brassica pekinensis | 350–400 | |
| BRA5 | Cress sprouting seeds | - | Lepidium sativum | 400–420 | |
| BRA6 | Kale sprouting seeds, fringed cabbage | - | Brassica oleracea L. | 250–300 | |
| BRA7 | Radish sprouting seeds | - | Raphanus sativus var. sativus | 55 | |
| BRA8 | Red cabbage sprouting seeds | - | Brassica oleracea var. capitata rubra | 350 | |
| BRA9 | Savoy cabbage | Langedijska | Brassica oleracea L. var. sabauda | 350–400 | |
| BRA10 | Swede (rutabaga) | Nadmorska | Brassica napus | 350 | |
| BRA11 | Turnip | Di milano a collettoviola | Brassica rapa | 500 | |
| BRA12 | Turnip kale | Wener Witte | Brassica oleracea var. gongylodes | 300–350 | |
| BRA13 | White cabbage | Amager Polana | Brassica oleracea var. capitata alba | 300–350 | |
| BRA14 | White mustard | - | Sinapis L. | 150 | |
| CUC1 | Cucumber | Aladyn F1 | Cucumis sativus | Cucurbitaceae (CUC) | 40–50 |
| CUC2 | Zucchini | Astra Polka | Cucurbita pepo | 3–5 | |
| CUC3 | Pumpkin | Bambino | Cucurbita maxima | 2–3 | |
| CUC4 | Pumpkin | Kabaczek Złoty Cepelin | Cucurbita pepo | 3–5 | |
| CUC5 | Summer squash | Patison Polo F1 | Cucurbita pepo | 6–8 | |
| FAB1 | Alfalfa Sprouting seeds | - | Medicago sativa L. | Fabaceae (FAB) | 470–500 |
| FAB2 | Beans for dry seeds | Borlotto lingua di fuoco nano | Phaseolus vulgaris | 2–6 | |
| FAB3 | Broad bean | - | Vicia faba L. | 1 | |
| FAB3 | Dwarf bean | Esterka | Phaseolus vulgaris | 2–6 | |
| FAB5 | Green lentil sprouting seeds | - | Lens culinaris | 25 | |
| FAB6 | Mung bean sprouting seeds | - | Vigna radiata | 13 | |
| FAB7 | Pea sprouting seeds | - | Pisum sativum L. | 4–5 | |
| POA1 | Corn sprouting seeds | - | Zea mays var. saccharata | Poaceae (POA) | 8–10 |
| SOL1 | Eggplant | Black Beauty | Solanum melongena | Solanaceae (SOL) | 200–250 |
| SOL2 | Pepper | Kasia | Capsicum annuum | 170–200 | |
| SOL3 | Pepper | Ożarowska | Capsicum annuum | 170–200 | |
| SOL4 | Tomato | Betalux | Solanum lycopersicum L. | 250–270 | |
| SOL5 | Tomato | Krakus | Solanum lycopersicum L. | 250–270 | |
| SOL6 | Tomato | Malinowy Olbrzym | Solanum lycopersicum L. | 250–270 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Phosphonates as Unique Components of Plant Seeds—A Promising Approach to Use Phosphorus Profiles in Plant Chemotaxonomy. Int. J. Mol. Sci. 2021, 22, 11501. https://doi.org/10.3390/ijms222111501
Wieczorek D, Żyszka-Haberecht B, Kafka A, Lipok J. Phosphonates as Unique Components of Plant Seeds—A Promising Approach to Use Phosphorus Profiles in Plant Chemotaxonomy. International Journal of Molecular Sciences. 2021; 22(21):11501. https://doi.org/10.3390/ijms222111501
Chicago/Turabian StyleWieczorek, Dorota, Beata Żyszka-Haberecht, Anna Kafka, and Jacek Lipok. 2021. "Phosphonates as Unique Components of Plant Seeds—A Promising Approach to Use Phosphorus Profiles in Plant Chemotaxonomy" International Journal of Molecular Sciences 22, no. 21: 11501. https://doi.org/10.3390/ijms222111501
APA StyleWieczorek, D., Żyszka-Haberecht, B., Kafka, A., & Lipok, J. (2021). Phosphonates as Unique Components of Plant Seeds—A Promising Approach to Use Phosphorus Profiles in Plant Chemotaxonomy. International Journal of Molecular Sciences, 22(21), 11501. https://doi.org/10.3390/ijms222111501






