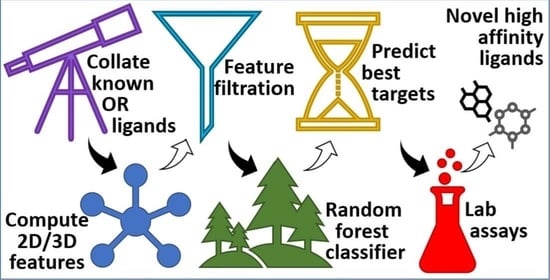
Machine Learning Assisted Approach for Finding Novel High Activity Agonists of Human Ectopic Olfactory Receptors
Abstract
:1. Introduction
2. Methods
2.1. Ligand Dataset Collection for the Receptors and Chemical Descriptors Calculation
2.2. Data Pre-Processing, Feature Selection and Class Balancing
2.3. Classifiers
2.3.1. Random Forest
2.3.2. Support Vector Machine
2.3.3. Naïve Bayes
2.4. Model Validation
2.5. Filtration of Compounds for Virtual Screening on the Basis of Chemical Similarity
2.6. OR1A1 and OR2W1 Homology Modelling
2.7. Molecular Docking of Highly Probable Predicted Compounds
2.8. Cell Culture
2.9. Dual-Glo Luciferase Reporter Gene Assay
2.10. Cell Surface Expression Evaluation by Flow Cytometry
3. Results
3.1. Chemical Diversity Analysis
3.2. Feature Selection and Performance of the Classifiers
3.3. Putative Ligand Screening through Machine Learning Based Classification
3.4. Homology Model and Molecular Docking Analysis
3.5. In Vitro Testing of Predicted Agonists Using Luciferase Assay
3.6. Binding Mode of the Tested Ligands
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharm. 2018, 93, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Buck, L.; Axel, R. A Novel Multigene Family may Encode Odorant Receptors: A Molecular Basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Baker, M.S.; Ahn, S.B.; Mohamedali, A.; Islam, M.T.; Cantor, D.; Verhaert, P.; Fanayan, S.; Sharma, S.; Nice, E.C.; Connor, M.; et al. Accelerating the Search for the Missing Proteins in the Human Proteome. Nat. Commun. 2017, 8, 14271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, M.; Briand, L.; de March, C.A.; Matsunami, H.; Yamashita, A.; Meyerhof, W.; Weyand, S. Structure–Function Relationships of Olfactory and Taste Receptors. Chem. Senses 2018, 43, 81–87. [Google Scholar] [CrossRef]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J.; et al. Expression of Members of the Putative Olfactory Receptor Gene Family in Mammalian Germ Cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression Profile of Ectopic Olfactory Receptors Determined by Deep Sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef] [Green Version]
- Massberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Lee, S.-J.; Depoortere, I.; Hatt, H. Therapeutic Potential of Ectopic Olfactory and Taste Receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138. [Google Scholar] [CrossRef]
- Del Mármol, J.; Yedlin, M.A.; Ruta, V. The Structural Basis of Odorant Recognition in Insect Olfactory Receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, A.; Mohamedali, A.; Ranganathan, S. Looking for Missing Proteins, in Reference Module in Life Sciences; Elsevier: Amsterdam, The Nederland, 2019. [Google Scholar] [CrossRef]
- Bush, C.F.; Hall, R.A. Olfactory Receptor Trafficking to the Plasma Membrane. Experientia 2008, 65, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- De March, C.A.; Ryu, S.; Sicard, G.; Moon, C.; Golebiowski, J. Structure–Odour Relationships Reviewed in the Postgenomic Era. Flavour Fragr. J. 2015, 30, 342–361. [Google Scholar] [CrossRef]
- Abaffy, T.; Bain, J.R.; Muehlbauer, M.J.; Spasojevic, I.; Lodha, S.; Bruguera, E.; O’Neal, S.; Kim, S.Y.; Matsunami, H. A Testosterone Metabolite 19-Hydroxyandrostenedione Induces Neuroendocrine Trans-Differentiation of Prostate Cancer Cells via an Ectopic Olfactory Receptor. Front. Oncol. 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushdid, C.; de March, C.A.; Fiorucci, S.; Matsunami, H.; Golebiowski, J. Agonists of G-Protein-Coupled Odorant Receptors Are Predicted from Chemical Features. J. Phys. Chem. Lett. 2018, 9, 2235–2240. [Google Scholar] [CrossRef]
- Yang, K.K.; Wu, Z.; Arnold, F.H. Machine-Learning-Guided Directed Evolution for Protein Engineering. Nat. Methods 2019, 16, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Le, N.Q.K.; Kha, Q.H.; Nguyen, V.H.; Chen, Y.-C.; Cheng, S.-J.; Chen, C.-Y. Machine Learning-Based Radiomics Signatures for EGFR and KRAS Mutations Prediction in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9254. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.H.T.; Le, N.; Van Tuan, L.; Ban, H.T.; Hung, T.N.K.; Nguyen, N.; Dang, L.H.; Le, N. Machine Learning Model for Identifying Antioxidant Proteins Using Features Calculated from Primary Sequences. Biology 2020, 9, 325. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Wu, W.; Pang, T.; Hu, H.; Chan, W.K.B.; Ke, X.; Zhang, Y. WDL-RF: Predicting Bioactivities of Ligand Molecules Acting with G Protein-Coupled Receptors by Combining Weighted Deep Learning and Random Forest. Bioinformatics 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- He, S.-B.; Hu, B.; Kuang, Z.-K.; Wang, D.; Kong, D.-X. Predicting Subtype Selectivity for Adenosine Receptor Ligands with Three-Dimensional Biologically Relevant Spectrum (BRS-3D). Sci. Rep. 2016, 6, 36595. [Google Scholar] [CrossRef] [Green Version]
- Schmiedeberg, K.; Shirokova, E.; Weber, H.-P.; Schilling, B.; Meyerhof, W.; Krautwurst, D. Structural Determinants of Odorant Recognition by the Human Olfactory Receptors OR1A1 and OR1A2. J. Struct. Biol. 2007, 159, 400–412. [Google Scholar] [CrossRef]
- Geithe, C.; Protze, J.; Kreuchwig, F.; Krause, G.; Krautwurst, D. Structural Determinants of a Conserved Enantiomer-Selective Carvone Binding Pocket in the Human Odorant Receptor OR1A1. Cell. Mol. Life Sci. 2017, 74, 4209–4229. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, Y.; Block, E.; Buehl, M.; Corr, M.J.; Cormanich, R.; Gundala, S.; Matsunami, H.; O’Hagan, D.; Özbil, M.; et al. Molecular Mechanism of Activation of Human Musk Receptors OR5AN1 and OR1A1 by (R)-Muscone and Diverse Other Musk-Smelling Compounds. Proc. Natl. Acad. Sci. USA 2018, 115, E3950–E3958. [Google Scholar] [CrossRef] [Green Version]
- Launay, G.; Téletchéa, S.; Wade, F.; Pajot-Augy, E.; Gibrat, J.-F.; Sanz, G. Automatic Modeling of Mammalian Olfactory Receptors and Docking of Odorants. Protein Eng. Des. Sel. 2012, 25, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, S.; Jovancevic, N.; Gelis, L.; Pietsch, S.; Hatt, H.; Gerwert, K. Dynamical Binding Modes Determine Agonistic and Antagonistic Ligand Effects in the Prostate-Specific G-Protein Coupled Receptor (PSGR). Sci. Rep. 2017, 7, 16007. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, R.C.; Casajuana-Martin, N.; Recio, A.G.; Alcántara, L.; Pardo, L.; Campillo, M.; Gonzalez, A. The Mutational Landscape of Human Olfactory G Protein-Coupled Receptors. BMC Biol. 2021, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.J.Y.; Du, W.; Chen, Y.Z.; Fan, H. A Benchmarking Study on Virtual Ligand Screening Against Homology Models of Human GPCRs. Proteins Struct. Funct. Bioinform. 2018, 86, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phytotherapy Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, A.; Ranganathan, S. Applications of Machine Learning in GPCR Bioactive Ligand Discovery. Curr. Opin. Struct. Biol. 2019, 55, 66–76. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly Accurate Protein Structure Prediction for the Human Proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, A.; Vijayram, R.; Ranganathan, S. BIO-GATS: A Tool for Automated GPCR Template Selection Through a Biophysical Approach for Homology Modeling. Front. Mol. Biosci. 2021, 8, 617176. [Google Scholar] [CrossRef]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin Cells of the Human Gut: Sensors for Spices and Odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef]
- Tan, Q.; Cui, J.; Huang, J.; Ding, Z.; Lin, H.; Niu, X.; Li, Z.; Wang, G.; Luo, Q.; Lu, S. Genomic Alteration During Metastasis of Lung Adenocarcinoma. Cell. Physiol. Biochem. 2016, 38, 469–486. [Google Scholar] [CrossRef]
- Wu, C.; Jia, Y.; Hae, J.L.; Kim, Y.; Sekharan, S.; Batista, V.S.; Lee, S.-J. Act. OR1A1 Suppresses PPAR-Gamma Expr. By Inducing HES-1 Cult. Hepatocytes. Int. J. Biochem. Cell Biol. 2015, 64, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Mimaki, S.; Makinoshima, H.; Tada, S.; Ishii, G.; Ohmatsu, H.; Niho, S.; Yoh, K.; Matsumoto, S.; Takahashi, A.; et al. Therapeutic Priority of the PI3K/AKT/mTOR Pathway in Small Cell Lung Cancers as Revealed by a Comprehensive Genomic Analysis. J. Thorac. Oncol. 2014, 9, 1324–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, N.; Yamano, E.; Ishii, A.; Tanaka, M.; Nakamura, J.; Watanabe, Y. Involvement of the Olfactory System in the Induction of Anti-Fatigue Effects by Odorants. PLoS ONE 2018, 13, e019526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, H.; Tian, Y.-S.; Kawashita, N.; Takagi, T. Mordred: A Molecular Descriptor Calculator. J. Cheminform. 2018, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Vidal, G.; Bouysset, C.; Grunig, H.; Fiorucci, S.; Montagné, N.; Golebiowski, J.; Jacquin-Joly, E. Machine Learning Decodes Chemical Features to Identify Novel Agonists of a Moth Odorant Receptor. Sci. Rep. 2020, 10, 1655. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 21 October 2021).
- Chawla, N.V.; Bowyer, K.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T.; Ohl, P.; Thiel, K.; Wiswedel, B. KNIME-the Konstanz Information Miner: Version 2.0 and beyond. AcM SIGKDD Explor. Newsl. 2009, 11, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Fawagreh, K.; Gaber, M.; Elyan, E. Random Forests: From Early Developments to Recent Advancements. Syst. Sci. Control. Eng. 2014, 2, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Shi, Y.; Liu, X. Recent Advances on Support Vector Machines Research. Technol. Econ. Dev. Econ. 2012, 18, 5–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kumar, S.; Lee, S.-Y.; Park, S.J.; Kim, M.-H. Development of Predictive Models for Identifying Potential S100A9 Inhibitors Based on Machine Learning Methods. Front. Chem. 2019, 7, 779. [Google Scholar] [CrossRef]
- Irwin, J.; Shoichet, B.K. ZINC–A Free Database of Commercially Available Compounds for Virtual Screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Allison, P.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Degtyarenko, K.; De Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcántara, R.; Darsow, M.; Guedj, M.; Ashburner, M. ChEBI: A Database and Ontology for Chemical Entities of Biological Interest. Nucleic Acids Res. 2008, 36, D344–D350. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Nojavani, M.B.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Cancer Odor Database (COD): A Critical Databank for Cancer Diagnosis Research. Database 2017, 2017, bax055. [Google Scholar] [CrossRef]
- Marenco, L.; Wang, R.; McDougal, R.; Olender, T.; Twik, M.; Bruford, E.; Liu, X.; Zhang, J.; Lancet, D.; Shepherd, G.; et al. ORDB, HORDE, ODORactor and other On-Line Knowledge Resources of Olfactory Receptor-Odorant Interactions. Database 2016, 2016, baw132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, A.; Mohamedali, A.; Ranganathan, S. Protocol for Protein Structure Modelling. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Nakai, K., Schonbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 252–272. [Google Scholar] [CrossRef]
- Okada, T.; Sugihara, M.; Bondar, A.-N.; Elstner, M.; Entel, P.; Buss, V. The Retinal Conformation and its Environment in Rhodopsin in Light of a New 2.2Å Crystal Structure. J. Mol. Biol. 2004, 342, 571–583. [Google Scholar] [CrossRef]
- Cvicek, V.; Goddard, W.A., 3rd; Abrol, R. Structure-Based Sequence Alignment of the Transmembrane Domains of All Human GPCRs: Phylogenetic, Structural and Functional Implications. PLoS Comput. Biol. 2016, 12, e1004805. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. Methods Mol. Biol. 2017, 1654, 39–54. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Wang, Q.; Canutescu, A.A.; Dunbrack, R.L., Jr. SCWRL and MolIDE: Computer Programs for Side-Chain Conformation Prediction and Homology Modeling. Nat. Protoc. 2008, 3, 1832–1847. [Google Scholar] [CrossRef] [Green Version]
- Abagyan, R.; Totrov, M.; Kuznetsov, D. ICM: A New Method for Protein Modeling and Design: Applications to Docking and Structure Prediction from the Distorted Native Conformation. J. Comput. Chem. 1994, 15, 488–506. [Google Scholar] [CrossRef]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP Family Members Induce Functional Expression of Mammalian Odorant Receptors. Cell 2004, 119, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahoun, T.; Grasso, L.; Vogel, H.; Pick, H. Recombinant Expression and Functional Characterization of Mouse Olfactory Receptor mOR256-17 in Mammalian Cells. Biochemistry 2011, 50, 7228–7235. [Google Scholar] [CrossRef]
- Gelis, L.; Wolf, S.; Hatt, H.; Neuhaus, E.M.; Gerwert, K. Prediction of a Ligand-Binding Niche Within a Human Olfactory Receptor by Combining Site-Directed Mutagenesis with Dynamic Homology Modeling. Angew. Chem. Int. Ed. Engl. 2012, 51, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Busse, D.; Kudella, P.; Grüning, N.-M.; Gisselmann, G.; Ständer, S.; Luger, T.; Jacobsen, F.; Steinsträßer, L.; Paus, R.; Gkogkolou, P.; et al. A Synthetic Sandalwood Odorant Induces Wound-Healing Processes in Human Keratinocytes via the Olfactory Receptor OR2AT4. J. Investig. Dermatol. 2014, 134, 2823–2832. [Google Scholar] [CrossRef] [Green Version]
- Maßberg, D.; Simon, A.; Häussinger, D.; Keitel, V.; Gisselmann, G.; Conrad, H.; Hatt, H. Monoterpene (−)-Citronellal Affects Hepatocarcinoma Cell Signaling Via an Olfactory Receptor. Arch. Biochem. Biophys. 2015, 566, 100–109. [Google Scholar] [CrossRef]
- Thach, T.T.; Hong, Y.-J.; Lee, S.; Lee, S.-J. Molecular Determinants of the Olfactory Receptor Olfr544 Activation by Azelaic Acid. Biochem. Biophys. Res. Commun. 2017, 485, 241–248. [Google Scholar] [CrossRef]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory Receptor 10J5 Responding to A-Cedrene Regulates Hepatic Steatosis via the cAMP-PKA Pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef] [Green Version]
- Weber, L.; Schulz, W.; Philippou, S.; Eckardt, J.; Ubrig, B.; Hoffmann, M.J.; Tannapfel, A.; Kalbe, B.; Gisselmann, G.; Hatt, H. Characterization of the Olfactory Receptor OR10H1 in Human Urinary Bladder Cancer. Front. Physiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Liu, M.T.; Ho, J.; Liu, J.K.; Purakait, R.; Morzan, U.N.; Ahmed, L.; Batista, V.S.; Matsunami, H.; Ryan, K. Carbon Chain Shape Selectivity by the Mouse Olfactory Receptor OR-I7. Org. Biomol. Chem. 2018, 16, 2541–2548. [Google Scholar] [CrossRef]
- Choi, Y.; Shim, J.; Park, J.-H.; Kim, Y.-S.; Kim, M. Discovery of Orphan Olfactory Receptor 6M1 as a New Anticancer Target in MCF-7 Cells by a Combination of Surface Plasmon Resonance-Based and Cell-Based Systems. Sensors 2021, 21, 3468. [Google Scholar] [CrossRef] [PubMed]
- Gat, U.; Nekrasova, E.; Lancet, D.; Natochin, M. Olfactory Receptor Proteins. Expression, Characterization and Partial Purification. Eur. J. Biochem. 1994, 225, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Katada, S.; Nakagawa, T.; Kataoka, H.; Touhara, K. Odorant Response Assays for a Heterologously Expressed Olfactory Receptor. Biochem. Biophys. Res. Commun. 2003, 305, 964–969. [Google Scholar] [CrossRef]
- Bushdid, C.; de March, C.A.; Matsunami, H.; Golebiowski, J. Numerical Models and In vitro Assays to Study Odorant. Receptors 2018, 1820, 77–93. [Google Scholar] [CrossRef]
- Zhuang, H.; Matsunami, H. Evaluating Cell-Surface Expression and Measuring Activation of Mammalian Odorant Receptors in Heterologous Cells. Nat. Protoc. 2008, 3, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Krautwurst, D.; Yau, K.-W.; Reed, R.R. Identification of Ligands for Olfactory Receptors by Functional Expression of a Receptor Library. Cell 1998, 95, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Matsunami, H. Synergism of Accessory Factors in Functional Expression of Mammalian Odorant Receptors. J. Biol. Chem. 2007, 282, 15284–15293. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.R.; Matsunami, H. Activation State of the M3 Muscarinic Acetylcholine Receptor Modulates Mammalian Odorant Receptor Signaling. Sci. Signal. 2011, 4, ra1. [Google Scholar] [CrossRef] [Green Version]
- Ikegami, K.; de March, C.A.; Nagai, M.H.; Ghosh, S.; Do, M.; Sharma, R.; Bruguera, E.S.; Lu, Y.E.; Fukutani, Y.; Vaidehi, N.; et al. Structural Instability and Divergence from Conserved Residues Underlie Intracellular Retention of Mammalian Odorant Receptors. Proc. Natl. Acad. Sci. USA 2020, 117, 2957–2967. [Google Scholar] [CrossRef]
- Laird, D.W.; Molday, R.S. Evidence Against the Role of Rhodopsin in Rod Outer Segment Binding to RPE Cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 419–428. [Google Scholar]
- Dey, S.; Matsunami, H. Calreticulin Chaperones Regulate Functional Expression of Vomeronasal Type 2 Pheromone Receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 16651–16656. [Google Scholar] [CrossRef] [Green Version]
- Jović, A.; Brkić, K.; Bogunović, N. A Review of Feature Selection Methods with Applications. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 25–29 May 2015. [Google Scholar] [CrossRef]
- Aboudi, N.E.; Benhlima, L. Review on Wrapper Feature Selection Approaches. In Proceedings of the 2016 International Conference on Engineering & MIS (ICEMIS), Agadir, Morocco, 22–24 September 2016. [Google Scholar] [CrossRef]
- Li, M.; Ling, C.; Xu, Q.; Gao, J. Classification of G-Protein Coupled Receptors Based on a Rich Generation of Convolutional Neural Network, N-Gram Transformation and Multiple Sequence Alignments. Amino Acids 2018, 50, 255–266. [Google Scholar] [CrossRef]
- Yang, M.; Tao, B.; Chen, C.; Jia, W.; Sun, S.; Zhang, T.; Wang, X. Machine Learning Models Based on Molecular Fingerprints and an Extreme Gradient Boosting Method Lead to the Discovery of JAK2 Inhibitors. J. Chem. Inf. Model. 2019, 59, 5002–5012. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, A.B.; Thiessen, A.P.; Yu, B.; et al. PubChem 2019 update: Improved Access to Chemical Data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar] [CrossRef]
- Saito, H.; Chi, Q.; Zhuang, H.; Matsunami, H.; Mainland, J.D. Odor Coding by a Mammalian Receptor Repertoire. Sci. Signal. 2009, 2, ra9. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, H.H.; Tran, N.K.; Betts, E.V.; Howell, L.P.; Green, R. Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods. Acad. Pathol. 2019, 6, 2374289519873088. [Google Scholar] [CrossRef]
- de March, C.A.; Yu, Y.; Ni, M.J.; Adipietro, K.A.; Matsunami, H.; Ma, M.; Golebiowski, J. Conserved Residues Control Activation of Mammalian G Protein-Coupled Odorant Receptors. J. Am. Chem. Soc. 2015, 137, 8611–8616. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Zhuang, H.; Chi, Q.; Vosshall, L.B.; Matsunami, H. Genetic Variation in a Human Odorant Receptor Alters Odour Perception. Nature 2007, 449, 468–472. [Google Scholar] [CrossRef]
- Belloir, C.; Miller-Leseigneur, M.L.; Neiers, F.; Briand, L.; Le Bon, A.M. Biophysical and functional characterization of the human olfactory receptor OR1A1 expressed in a mammalian inducible cell line. Protein Expr. Purif. 2017, 129, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Moberly, A.H.; Adipietro, K.A.; Liu, W.L.; Zhuang, H.; et al. The missense of smell: Functional variability in the human odorant receptor repertoire. Nat. Neurosci. 2014, 17, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Sato-Akuhara, N.; Horio, N.; Kato-Namba, A.; Yoshikawa, K.; Niimura, Y.; Ihara, S.; Shirasu, M.; Touhara, K. Ligand Specificity and Evolution of Mammalian Musk Odor Receptors: Effect of Single Receptor Deletion on Odor Detection. J. Neurosci. 2016, 36, 4482–4491. [Google Scholar] [CrossRef] [Green Version]
- Adipietro, K.A.; Mainland, J.D.; Matsunami, H. Functional evolution of mammalian odorant receptors. PLoS Genet. 2012, 8, e1002821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ahmed, L.; Zhang, R.; Pan, Y.; Matsunami, H.; Burger, J.L.; Block, E.; Batista, V.S.; Zhuang, H. Smelling Sulfur: Copper and Silver Regulate the Response of Human Odorant Receptor OR2T11 to Low-Molecular-Weight Thiols. J. Am. Chem. Soc. 2016, 138, 13281–13288. [Google Scholar] [CrossRef]
- 92. Audouze, K.; Tromelin, A.; Le Bon, A.M.; Belloir, C.; Petersen, R.K.; Kristiansen, K.; Brunak, S.; Taboureau, O. Identification of odorant-receptor interactions by global mapping of the human odorome. PLoS ONE 2014, 9, e93037. [Google Scholar] [CrossRef] [Green Version]
- McRae, J.F.; Mainland, J.D.; Jaeger, S.R.; Adipietro, K.A.; Matsunami, H.; Newcomb, R.D. Genetic variation in the odorant receptor OR2J3 is associated with the ability to detect the “grassy” smelling odor, cis-3-hexen-1-ol. Chem. Senses 2012, 37, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| PubChem_CID | Compound Name | Database | Chemical Nature | ICM Docking Score |
|---|---|---|---|---|
| 3102 | Benzophenone | Control | Ketone | −12.8345 |
| 10465547 | [(Z)-Pent-3-enyl] 2-aminobenzoate | ZINC | Heterocyclic compound | −24.058033 |
| 70545042 | Prop-2-enyl 3-iodobenzoate | ZINC | Heterocyclic compound | −20.113147 |
| 56806459 | 2-(4-Methylphenoxy)pentan-3-one | ZINC | Ketone | −19.297316 |
| 84603836 | (3-Fluorophenyl)methyl 4-methylpentanoate | ZINC | Ester | −18.084389 |
| 101977 | D-citronellol | ChEBI, HMDB | Terpene | −18.009 |
| 56828593 | 4-(3-Fluorophenyl)-3-methyl-4-oxobutanenitrile | ZINC | Heterocyclic compound | −17.436507 |
| 17973047 | 4-(1-Methylcyclopropyl)phenol | ZINC | Heterocyclic compound | −17.286264 |
| 30842889 | Prop-2-enyl 2-(2,4-difluorophenyl)acetate | ZINC | Ester | −17.136729 |
| 22048986 | 6-Chloro-1-(3-fluorophenyl)hexan-1-one | ZINC | Ketone | −17.053948 |
| 11470552 | Ethyl 2-(3-bromophenyl)acetate | ZINC | Ester | −16.812695 |
| 45085600 | (5S)-5,6-dimethylhept-6-en-2-one | ZINC | Ketone | −16.733556 |
| 5352782 | 3-[(E)-But-1-enyl]pyridine | ZINC | Heterocyclic compound | −16.708306 |
| 7021479 | Methyl 2-(2-methylphenyl)acetate | ZINC | Ester | −16.649985 |
| 59382573 | (3-Methoxyphenyl)methyl butanoate | ZINC | Ester | −16.572913 |
| 84177 | Ethyl 2-(4-chlorophenyl)acetate | ZINC | Ester | −16.278326 |
| 6368521 | 1-[(E)-2-Chloroethenyl]-4-methoxybenzene | ZINC | Heterocyclic compound | −16.266073 |
| 78901972 | (3-Fluorophenyl)methyl 2-propylsulfanylacetate | ZINC | Ester | −16.165344 |
| 8842 | Citronellol | OdorDB, ChEBI | Terpene | −14.6017 |
| 22311 | Dipentene | OdorDB, COD | Terpene | −13.9064 |
| 1318 | 1,10-Phenanthroline | ChEBI | Heterocyclic compound | −13.7334 |
| 24473 | Dihydrocarvone | OdorDB | Ketone | −11.3054 |
| 131752167 | 2,10-Bisaboladiene-1,4-diol | HMDB | Alcohol | −10.6224 |
| 78236 | 4-Nonanone | HMDB | Ketone | −10.5291 |
| PubChem_CID | Compound Name | Database | Chemical Nature | ICM Docking Score |
|---|---|---|---|---|
| 3102 | Benzophenone | Control | Ketone | −11.8875 |
| 13433021 | 4-Methyl-2-m-tolylpyridine | ZINC | Heterocyclic compound | −14.515694 |
| 2733871 | 2,4-Dimethyl-1-phenylpyrrole | ZINC | Heterocyclic compound | −14.150921 |
| 249799 | 1-Butoxy-4-phenylbenzene | ZINC | Heterocyclic compound | −13.419691 |
| 22562335 | Methyl 3-(4-ethoxyphenyl)prop-2-ynoate | ZINC | Heterocyclic compound | −12.639271 |
| 16530415 | (2,3,4,5,6-Pentafluorophenyl)methyl 2-hydroxy-3-methylbenzoate | ZINC | Heterocyclic compound | −11.02916 |
| 3847415 | 1-Ethenyl-4-[4-(4-ethenylphenoxy)butoxy]benzene | ZINC | Heterocyclic compound | −8.767672 |
| 12252872 | Ethyl 4-hydroxy-3-prop-2-enylbenzoate | ZINC | Heterocyclic compound | −8.68684 |
| 231770 | 1,3-bis(4-Bromophenyl)prop-2-en-1-one | ZINC | Heterocyclic compound | −8.668044 |
| 7129 | 2-Ethoxynaphthalene | ZINC | Ether | −8.208685 |
| 60008260 | Ethyl 2-amino-5-cyanobenzoate | ZINC | Heterocyclic compound | −8.011839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, A.; de March, C.A.; Matsunami, H.; Ranganathan, S. Machine Learning Assisted Approach for Finding Novel High Activity Agonists of Human Ectopic Olfactory Receptors. Int. J. Mol. Sci. 2021, 22, 11546. https://doi.org/10.3390/ijms222111546
Jabeen A, de March CA, Matsunami H, Ranganathan S. Machine Learning Assisted Approach for Finding Novel High Activity Agonists of Human Ectopic Olfactory Receptors. International Journal of Molecular Sciences. 2021; 22(21):11546. https://doi.org/10.3390/ijms222111546
Chicago/Turabian StyleJabeen, Amara, Claire A. de March, Hiroaki Matsunami, and Shoba Ranganathan. 2021. "Machine Learning Assisted Approach for Finding Novel High Activity Agonists of Human Ectopic Olfactory Receptors" International Journal of Molecular Sciences 22, no. 21: 11546. https://doi.org/10.3390/ijms222111546
APA StyleJabeen, A., de March, C. A., Matsunami, H., & Ranganathan, S. (2021). Machine Learning Assisted Approach for Finding Novel High Activity Agonists of Human Ectopic Olfactory Receptors. International Journal of Molecular Sciences, 22(21), 11546. https://doi.org/10.3390/ijms222111546