Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages
Abstract
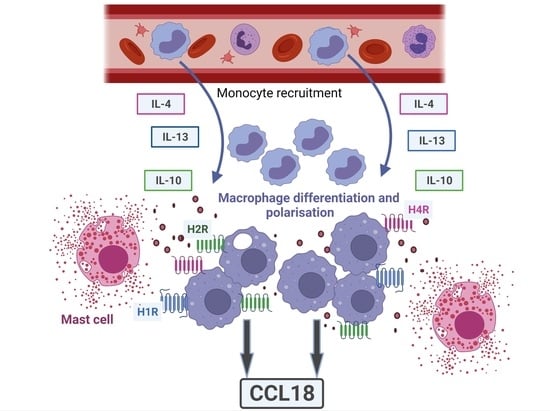
:1. Introduction
2. Results
2.1. Histamine Increases the IL-4- and the IL-13-Induced Upregulation of CCL18 mRNA Expresssion by Stimulating the H2R in Human M2 Macrophages
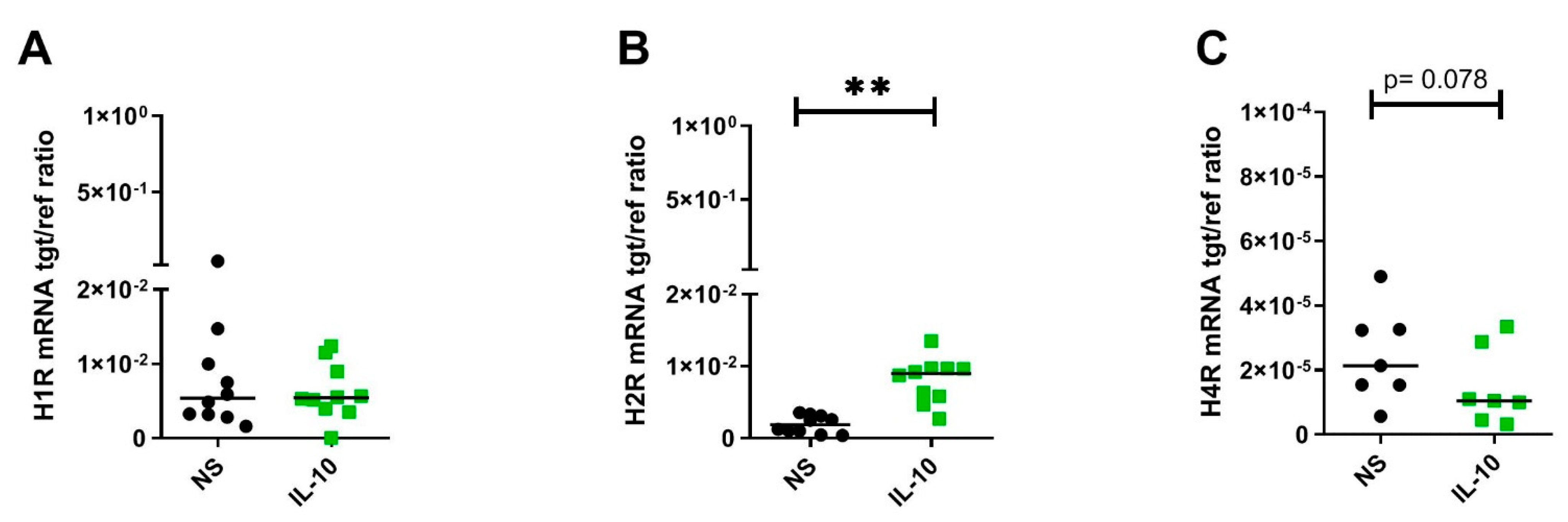
2.2. IL-10 Significantly Upregulates H2R but Downregulates H4R mRNA Expression by Trend in Human M2 Macrophages
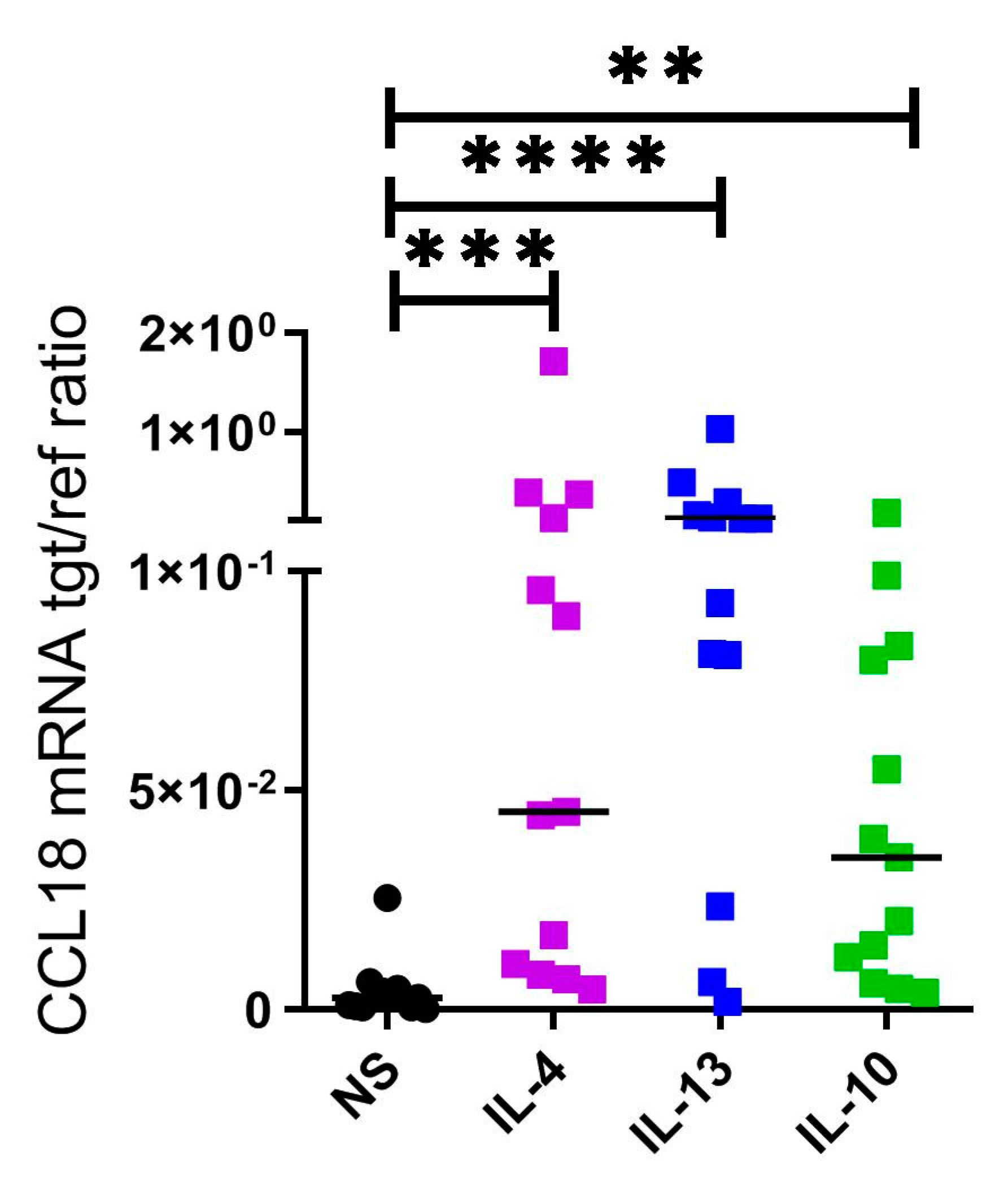
2.3. CCL18 mRNA Expresion Is Upregulated by Th2 Cytokines IL-4, IL-13 and by IL-10 in Human M2 Macrophages
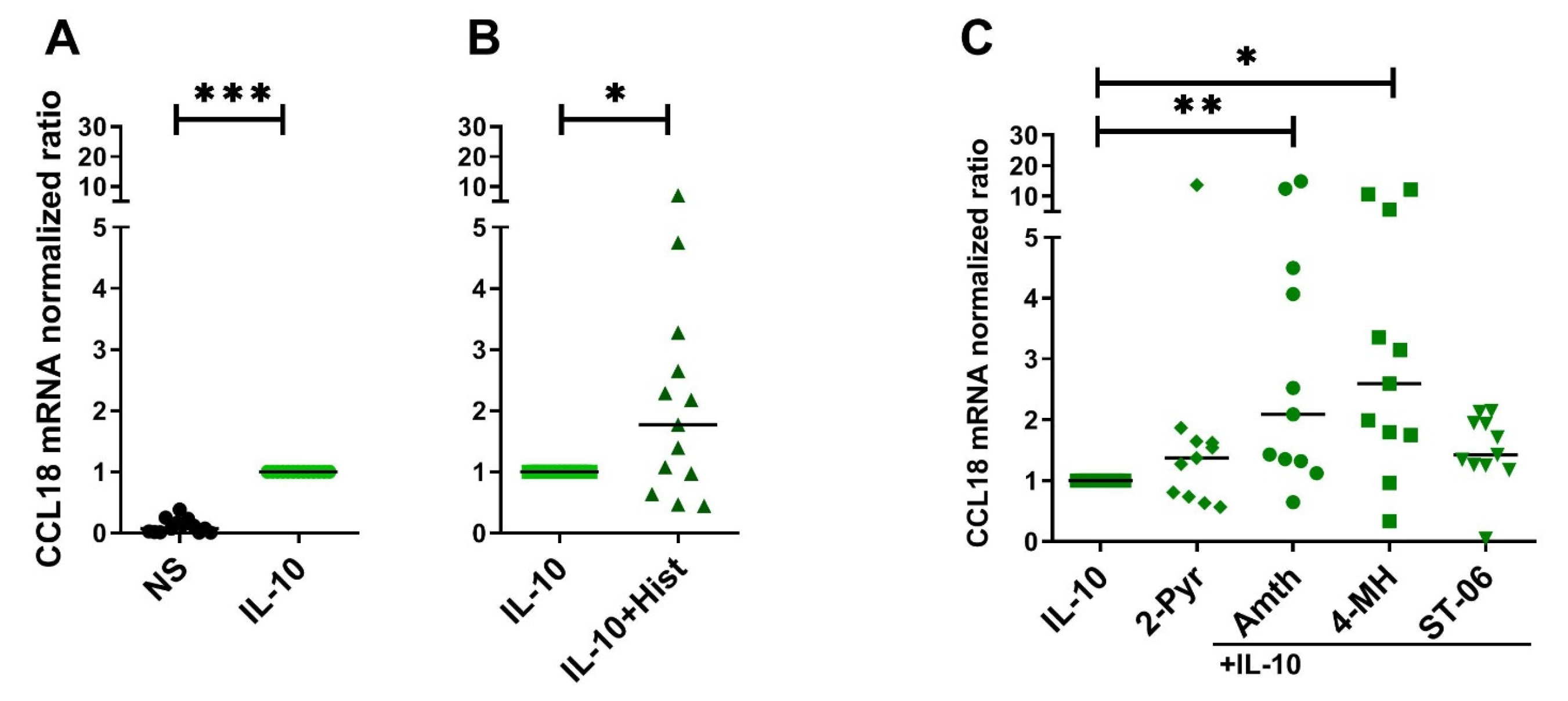
2.4. Histamine Increases CCL18 mRNA Expression by Stimulating the H2R in IL-10-Activated Human M2 Macrophages
2.5. Histamine Increases CCL18 Protein Production by Stimulating the H2R in IL-10-Activated Human M2 Macrophages
2.6. The Potentiating Effect of Histamine on CCL18 Protein Production in IL-10-Activated M2 Macrophages Was Inhibited by Preincubating the Cells with the Selective H2R Antagonist
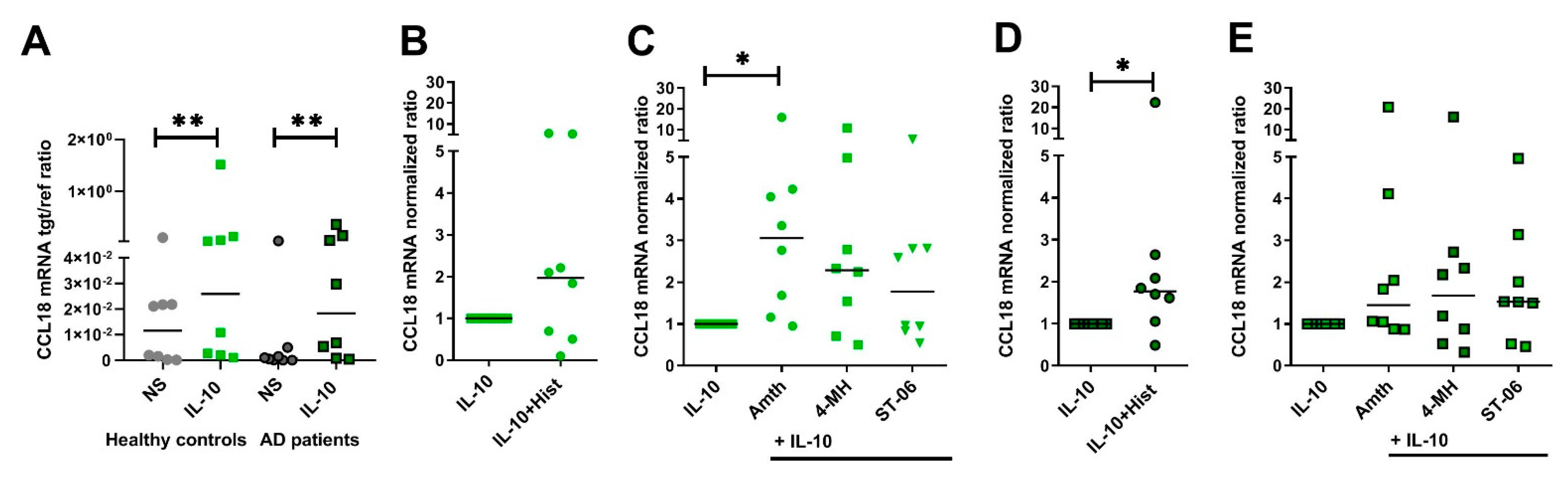
2.7. Upregulation of CCL18 mRNA Expression in Response to H2R Stimulation Is More Pronounced in Human IL-10-Activatd M2 Macrophages from Healthy Control Persons When Compared to Cells from AD Patients
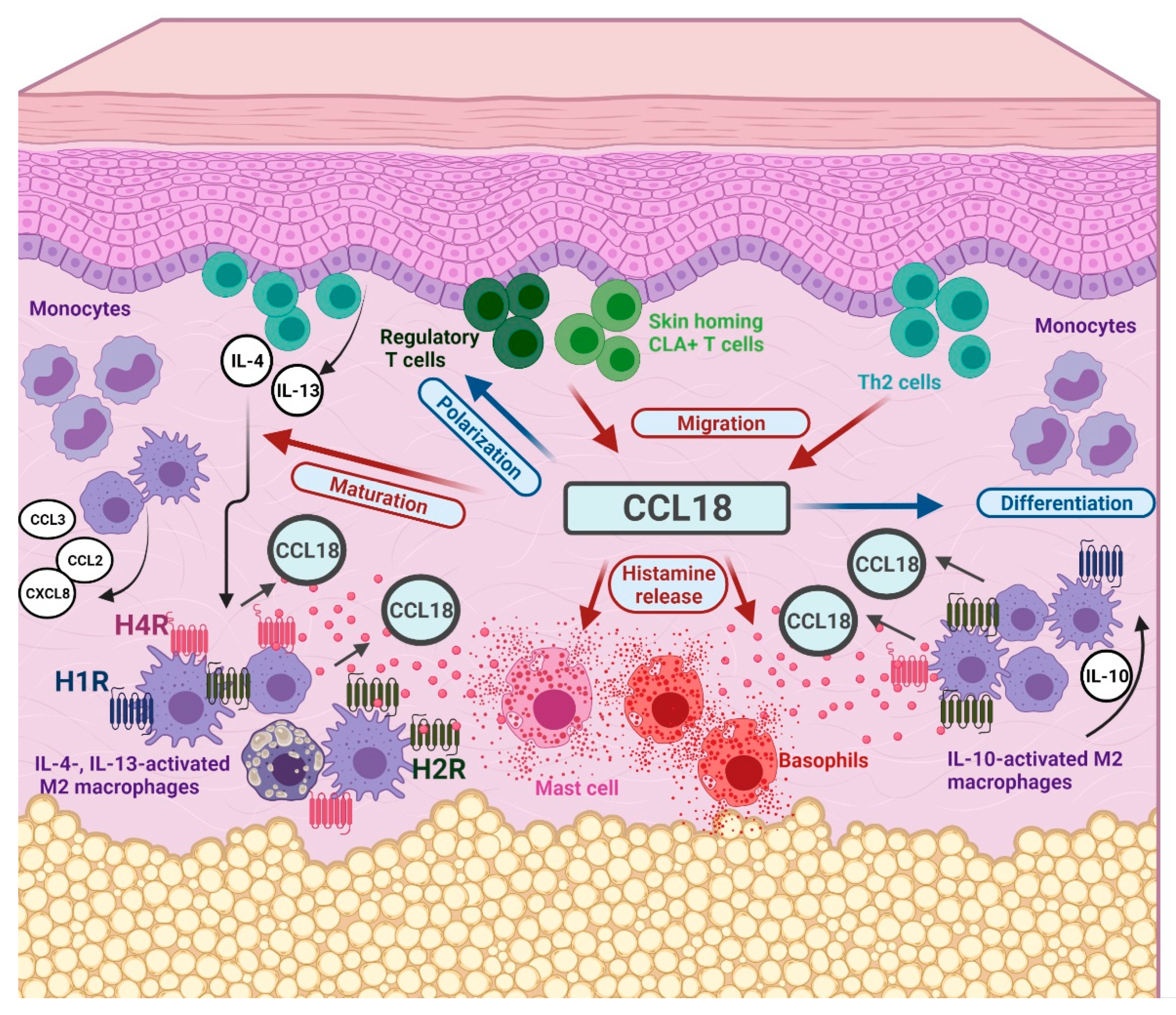
3. Discussion
4. Materials and Methods
4.1. Isolation of Monocyte Derived Macrophages
4.2. Cell Culture, Differentiation of M2 Macrophages
4.3. Histamine Receptor Ligands
4.4. Stimulation of M2 Macrophages
4.5. Quantitative Real Time (RT)-PCR
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chenivesse, C.; Tsicopoulos, A. CCL18—Beyond Chemotaxis. Cytokine 2018, 109, 52–56. [Google Scholar] [CrossRef]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of Human CCR8 as a CCL18 Receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef]
- Azzaoui, I.; Yahia, S.A.; Chang, Y.; Vorng, H.; Morales, O.; Fan, Y.; Delhem, N.; Ple, C.; Tonnel, A.B.; Wallaert, B.; et al. CCL18 Differentiates Dendritic Cells in Tolerogenic Cells Able to Prime Regulatory T Cells in Healthy Subjects. Blood 2011, 118, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Gunther, C.; Bello-Fernandez, C.; Kopp, T.; Kund, J.; Carballido-Perrig, N.; Hinteregger, S.; Fassl, S.; Schwarzler, C.; Lametschwandtner, G.; Stingl, G.; et al. CCL18 is Expressed in Atopic Dermatitis and Mediates Skin Homing of Human Memory T Cells. J. Immunol. 2005, 174, 1723–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.O.; Cho, S.I.; Chung, B.Y.; Ahn, H.K.; Park, C.W.; Lee, C.H. Expression of CCL1 and CCL18 in Atopic Dermatitis and Psoriasis. Clin. Exp. Dermatol. 2012, 37, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsi, A.; Gombert, M.; Dieu-Nosjean, M.C.; Lauerma, A.; Kubitza, R.; Meller, S.; Rieker, J.; Muller, A.; Da Cunha, L.; Haahtela, A.; et al. CC Chemokine Ligand 18, an Atopic Dermatitis-Associated and Dendritic Cell-Derived Chemokine, is Regulated by Staphylococcal Products and Allergen Exposure. J. Immunol. 2004, 173, 5810–5817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Werfel, T. The Role of Leukocytes, Keratinocytes, and Allergen-Specific IgE in the Development of Atopic Dermatitis. J. Investig. Dermatol. 2009, 129, 1878–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and Molecular Immunologic Mechanisms in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Kiekens, R.C.; Thepen, T.; Oosting, A.J.; Bihari, I.C.; Van De Winkel, J.G.; Bruijnzeel-Koomen, C.A.; Knol, E.F. Heterogeneity within Tissue-Specific Macrophage and Dendritic Cell Populations during Cutaneous Inflammation in Atopic Dermatitis. Br. J. Dermatol. 2001, 145, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Gluck, S. Cutaneous Histamine Levels and Histamine Releasability from the Skin in Atopic Dermatitis and Hyper-IgE-Syndrome. Arch. Dermatol. Res. 1983, 275, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, R.L.; Gelfand, E.W.; Dunford, P.J. The Role of Histamine H1 and H4 Receptors in Allergic Inflammation: The Search for New Antihistamines. Nat. Rev. Drug Discov. 2008, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Aslan, D.; Ratz, L.; Stark, H.; Gutzmer, R.; Werfel, T. The Anaphylatoxin C3a Receptor Expression on Human M2 Macrophages is Down-Regulated by Stimulating the Histamine H4 Receptor and the IL-4 Receptor. J. Innate Immun. 2018, 10, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Jahn, M.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Expression of Histamine Receptors H2R and H4R are Predominantly Regulated Via the IL-4/IL-13 Receptor Type II on Human M2 Macrophages. Allergy 2021, 76, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Lelong, M.; Jeljeli, M.; Piquet-Pellorce, C.; Morzadec, C.; Ballerie, A.; Jouneau, S.; Jego, P.; Vernhet, L.; Batteux, F.; et al. Combined Anti-Fibrotic and Anti-Inflammatory Properties of JAK-Inhibitors on Macrophages in Vitro and in Vivo: Perspectives for Scleroderma-Associated Interstitial Lung Disease. Biochem. Pharmacol. 2020, 178, 114103. [Google Scholar] [CrossRef]
- van Lieshout, A.W.; van der Voort, R.; le Blanc, L.M.; Roelofs, M.F.; Schreurs, B.W.; van Riel, P.L.; Adema, G.J.; Radstake, T.R. Novel Insights in the Regulation of CCL18 Secretion by Monocytes and Dendritic Cells Via Cytokines, Toll-Like Receptors and Rheumatoid Synovial Fluid. BMC Immunol. 2006, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.O.; Lee, H.J.; Lee, J.H.; Wu, W.H.; Chang, N.S.; Hua, L.; Lee, M.G.; Lee, K.H. Increased Expression of CC Chemokine Ligand 18 in Extrinsic Atopic Dermatitis Patients. Exp. Dermatol. 2008, 17, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Ratz, L.; Stark, H.; Gutzmer, R.; Werfel, T. The Histamine H4 Receptor Modulates the Differentiation Process of Human Monocyte-Derived M1 Macrophages and the Release of CCL4/MIP-1beta from Fully Differentiated M1 Macrophages. Inflamm. Res. 2018, 67, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Schaper-Gerhardt, K.; Kother, B.; Wolff, L.; Kabatas, A.; Gehring, M.; Nikolouli, E.; Mommert, S.; Werfel, T.; Gutzmer, R. The H4 R is Highly Expressed on Eosinophils from AD Patients and IL-4 Upregulates Expression and Function Via the JAK/STAT Pathway. Allergy 2021, 76, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-10 Production by Effector T Cells: Th1 Cells show Self Control. J. Exp. Med. 2007, 204, 239–243. [Google Scholar] [CrossRef]
- Gros, E.; Bussmann, C.; Bieber, T.; Forster, I.; Novak, N. Expression of Chemokines and Chemokine Receptors in Lesional and Nonlesional Upper Skin of Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2009, 124, 753–760.e1. [Google Scholar] [CrossRef] [PubMed]
- Laouini, D.; Alenius, H.; Bryce, P.; Oettgen, H.; Tsitsikov, E.; Geha, R.S. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J. Clin. Invest. 2003, 112, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Vulcano, M.; Struyf, S.; Scapini, P.; Cassatella, M.; Bernasconi, S.; Bonecchi, R.; Calleri, A.; Penna, G.; Adorini, L.; Luini, W.; et al. Unique Regulation of CCL18 Production by Maturing Dendritic Cells. J. Immunol. 2003, 170, 3843–3849. [Google Scholar] [CrossRef]
- Lewis, C.V.; Vinh, A.; Diep, H.; Samuel, C.S.; Drummond, G.R.; Kemp-Harper, B.K. Distinct Redox Signalling Following Macrophage Activation Influences Profibrotic Activity. J. Immunol. Res. 2019, 2019, 1278301. [Google Scholar] [CrossRef]
- Beermann, S.; Seifert, R.; Neumann, D. Commercially available antibodies against human and murine histamine H4-receptor lack specificity. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 125–135. [Google Scholar] [CrossRef]
- Gutzmer, R.; Werfel, T.; Bäumer, W.; Kietzmann, M.; Chazot, P.L.; Leurs, R. Well characterized antihistamine 4 receptor antibodies contribute to current knowledge of the expression and biology of the human and murine histamine 4 receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 853–854, author reply 855–860. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Woods, V.M.; Chitko-McKown, C.G.; Hash, S.M.; Rice-Ficht, A.C. Interleukin-10 is Expressed by Bovine Type 1 Helper, Type 2 Helper, and Unrestricted Parasite-Specific T-Cell Clones and Inhibits Proliferation of all Three Subsets in an Accessory-Cell-Dependent Manner. Infect. Immun. 1994, 62, 4697–4708. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Zhang, X. Interleukin-10: New Perspectives on an Old Cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ferstl, R.; Frei, R.; Schiavi, E.; Konieczna, P.; Barcik, W.; Ziegler, M.; Lauener, R.P.; Chassard, C.; Lacroix, C.; Akdis, C.A.; et al. Histamine Receptor 2 is a Key Influence in Immune Responses to Intestinal Histamine-Secreting Microbes. J. Allergy Clin. Immunol. 2014, 134, 744–746.e3. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Diestel, C.; Mommert, S.; Kother, B.; Stark, H.; Wittmann, M.; Werfel, T. Histamine H4 Receptor Stimulation Suppresses IL-12p70 Production and Mediates Chemotaxis in Human Monocyte-Derived Dendritic Cells. J. Immunol. 2005, 174, 5224–5232. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Mommert, S.; Kother, B.; Werfel, T.; Gutzmer, R. The Histamine H4 Receptor is Highly Expressed on Plasmacytoid Dendritic Cells in Psoriasis and Histamine Regulates their Cytokine Production and Migration. J. Investig. Dermatol. 2011, 131, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab Progressively Improves Systemic and Cutaneous Abnormalities in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mommert, S.; Gregor, K.; Rossbach, K.; Schaper, K.; Witte, T.; Gutzmer, R.; Werfel, T. Histamine H2 Receptor Stimulation Upregulates TH2 Chemokine CCL17 Production in Human M2a Macrophages. J. Allergy Clin. Immunol. 2018, 141, 782–785.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, I.; Gao, B.; Boguniewicz, M.; Darst, M.A.; Travers, J.B.; Leung, D.Y. Distinct Patterns of Gene Expression in the Skin Lesions of Atopic Dermatitis and Psoriasis: A Gene Microarray Analysis. J. Allergy Clin. Immunol. 2003, 112, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The Chemokine CCL18 Causes Maturation of Cultured Monocytes to Macrophages in the M2 Spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borriello, F.; Granata, F.; Marone, G. Basophils and Skin Disorders. J. Investig. Dermatol. 2014, 134, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Nadai, P.; Charbonnier, A.S.; Chenivesse, C.; Senechal, S.; Fournier, C.; Gilet, J.; Vorng, H.; Chang, Y.; Gosset, P.; Wallaert, B.; et al. Involvement of CCL18 in Allergic Asthma. J. Immunol. 2006, 176, 6286–6293. [Google Scholar] [CrossRef]
- Luzina, I.G.; Todd, N.W.; Nacu, N.; Lockatell, V.; Choi, J.; Hummers, L.K.; Atamas, S.P. Regulation of Pulmonary Inflammation and Fibrosis through Expression of Integrins alphaVbeta3 and alphaVbeta5 on Pulmonary T Lymphocytes. Arthritis Rheum. 2009, 60, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Krohn, S.C.; Bonvin, P.; Proudfoot, A.E. CCL18 Exhibits a Regulatory Role through Inhibition of Receptor and Glycosaminoglycan Binding. PLoS ONE 2013, 8, e72321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; de Nadai, P.; Azzaoui, I.; Morales, O.; Delhem, N.; Vorng, H.; Tomavo, S.; Ait Yahia, S.; Zhang, G.; Wallaert, B.; et al. The Chemokine CCL18 Generates Adaptive Regulatory T Cells from Memory CD4+ T Cells of Healthy but Not Allergic Subjects. FASEB J. 2010, 24, 5063–5072. [Google Scholar] [PubMed]
- Hanifin, J.M. Diagnostic criteria for atopic dermatitis: Consider the context. Arch Dermatol. 1999, 135, 1551. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Schaper, J.T.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Histamine Down-Regulates the FCERI Alpha-Chain Expression in Human IL-4-Activated M2 Macrophages. Allergy 2021, 76, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; Kottke, T.; Tanrikulu, Y.; Proschak, E.; Weizel, L.; Schneider, E.H.; Seifert, R.; Schneider, G.; Stark, H. 2,4—Diaminopyrimidines as Histamine H4 Receptor Ligands--Scaffold Optimization and Pharmacological Characterization. Bioorg. Med. Chem. 2009, 17, 7186–7196. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Koether, B.; Werfel, T.; Stark, H.; Gutzmer, R. Profiling of Histamine H4 Receptor Agonists in Native Human Monocytes. Br. J. Pharmacol. 2013, 170, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mommert, S.; Schaper, J.T.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages. Int. J. Mol. Sci. 2021, 22, 11648. https://doi.org/10.3390/ijms222111648
Mommert S, Schaper JT, Schaper-Gerhardt K, Gutzmer R, Werfel T. Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages. International Journal of Molecular Sciences. 2021; 22(21):11648. https://doi.org/10.3390/ijms222111648
Chicago/Turabian StyleMommert, Susanne, Judith Tabea Schaper, Katrin Schaper-Gerhardt, Ralf Gutzmer, and Thomas Werfel. 2021. "Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages" International Journal of Molecular Sciences 22, no. 21: 11648. https://doi.org/10.3390/ijms222111648
APA StyleMommert, S., Schaper, J. T., Schaper-Gerhardt, K., Gutzmer, R., & Werfel, T. (2021). Histamine Increases Th2 Cytokine-Induced CCL18 Expression in Human M2 Macrophages. International Journal of Molecular Sciences, 22(21), 11648. https://doi.org/10.3390/ijms222111648