Abstract
The physiological balance between excitation and inhibition in the brain is significantly affected in Alzheimer’s disease (AD). Several neuroactive compounds and their signaling pathways through various types of receptors are crucial in brain homeostasis, among them glutamate and γ-aminobutyric acid (GABA). Activation of microglial receptors regulates the immunological response of these cells, which in AD could be neuroprotective or neurotoxic. The novel research approaches revealed the complexity of microglial function, including the interplay with other cells during neuroinflammation and in the AD brain. The purpose of this review is to describe the role of several proteins and multiple receptors on microglia and neurons, and their involvement in a communication network between cells that could lead to different metabolic loops and cell death/survival. Our review is focused on the role of glutamatergic, GABAergic signaling in microglia–neuronal cross-talk in AD and neuroinflammation. Moreover, the significance of AD-related neurotoxic proteins in glutamate/GABA-mediated dialogue between microglia and neurons was analyzed in search of novel targets in neuroprotection, and advanced pharmacological approaches.
1. Introduction
Alzheimer’s disease (AD) is the major cause of cognitive impairment and dementia, which affects about 50 million people, and its prevalence was estimated to triple worldwide by 2050 [1]. The majority of AD cases are late-onset sporadic AD (SAD), whereas about 5–10% are early-onset familiar AD (FAD). Despite many years of intensive research and significant advances, we are still far from understanding of the pathogenesis SAD, but several genetic and environmental risk factors have been identified [2,3,4]. The amyloid cascade hypothesis, which dominated the field for about 30 years, suggested that the accumulation of amyloid β (Aβ) is a primary factor responsible for the pathological alterations [5]. Alternative hypotheses proposed other possible AD initiating factors, such as metabolic imbalance, infections, and toxins [2,6,7]. These factors, together with Aβ peptides and other proteins with altered conformation, affect several neurotransmitters and growth factors-dependent signaling pathways and communication networks between neurons, microglia, astrocytes, and other cells, leading to synaptic apoptosis and neuronal death.
However, all therapeutic strategies, including those based on anti-Aβ tactics, have failed to produce a reasonable effect, and to date there is no cure for AD. The inhibitors of acetylcholinesterase (AChE), and also an antagonist of the NMDA receptor (memantine), are commonly prescribed medications for AD, but these drugs do not stop the progression of the disease. Inhibitors of AChE, such as donepezil, galantamine, or rivastigmine, increase the level of acetylcholine, leading to normalization of cholinergic signaling, which is impaired in AD. Memantine blocks excessive glutamatergic signaling in the AD brain. Therefore, these drugs slow down the memory decline and maintain the ability to perform daily functions by stabilization of impaired neurotransmission in AD.
The imbalance between excitation and inhibition significantly contributes to AD pathology [8,9,10]. Alterations of the levels of glucose, glutamate, gamma-aminobutyric acid (GABA), and kynurenic acid may affect the homeostasis between major neurotransmitter systems in the brain, glutamate and GABA.
However, the function of glutamate and GABA is not limited to signal transmission between neurons, but they also play important roles in communications between neurons and microglia. Microglia, the only resident immune cells in the brain, have both immunological and non-immunological functions in the central nervous system (CNS). In a healthy brain, they play a prominent role in development, plasticity, cognition, and homeostasis. However, depletion, dysfunction, or undesirable activation of microglia may severely impair several signaling pathways, molecular processes, and communication with neurons, astrocytes, and as a consequence learning, memory, and other essential cognitive functions [11]. The growing body of evidence indicates their involvement in various aspects of the pathomechanism of AD. The novel research tools and experimental approaches reveal the heterogeneity of the microglial population, the complexity of these cells’ function, and dysfunction, and highlight the crucial role of neurotransmitters in tuning up microglial function [12,13,14,15,16,17]. Microglia express several receptors for neurotransmitters and neuroactive compounds, among them receptors for glutamate, GABA, acetylcholine, dopamine, adrenalin, ATP, and adenosine [18,19]. The aim of this article is to review the current knowledge on the relationship between microglia and neurons in the pathomechanism of AD. We will focus on the role of glutamatergic and GABAergic neurotransmitter systems in cross-talk between these cells in the brain, and on its impairment in the progression of AD. Moreover, based on this knowledge, the novel pharmacological therapeutic approaches will be presented.
2. The Role of Microglia in the Brain
Microglia arise from erythromyeloid precursors originating from the yolk sac in early embryogenesis [20,21]. The microglia migrate via the circulatory system and penetrate the developing CNS according to a specific spatiotemporal pattern, and in humans, this process was observed during the first two trimesters of gestation [22]. The analysis of rodent development demonstrated that in the postnatal period the number of microglial cells in the brain initially increases, and then their population begins declining, due to developmental apoptosis and decreased proliferation [23].
In a healthy brain, microglia play a key role in shaping and fine-tuning brain circuits; they control homeostasis and development via scavenging cellular debris, secretion of trophic factors, and monitoring synaptic development and activity. During perinatal development, microglia regulate neurogenesis and control synaptic density (synaptic pruning). In the adult brain, by secreting insulin-like growth factor 1 for cortical layer V, microglia provide neurotrophic support to neurons. Microglia represent 5–12% of all glial cells in rodent brains and 0.5–16% in humans, depending on the brain region [24,25,26]. It is important to underline that microglia are engaged also in learning and memory processes through the production and liberation of brain-derived growth or neurotrophic factors as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), and colony-stimulating factor 1 (CSF1) (Figure 1) [27,28]. Additionally, microglia accumulate in brain regions with a high density of neuronal precursors and significantly contribute to the maintenance of embryonic progenitors [21].
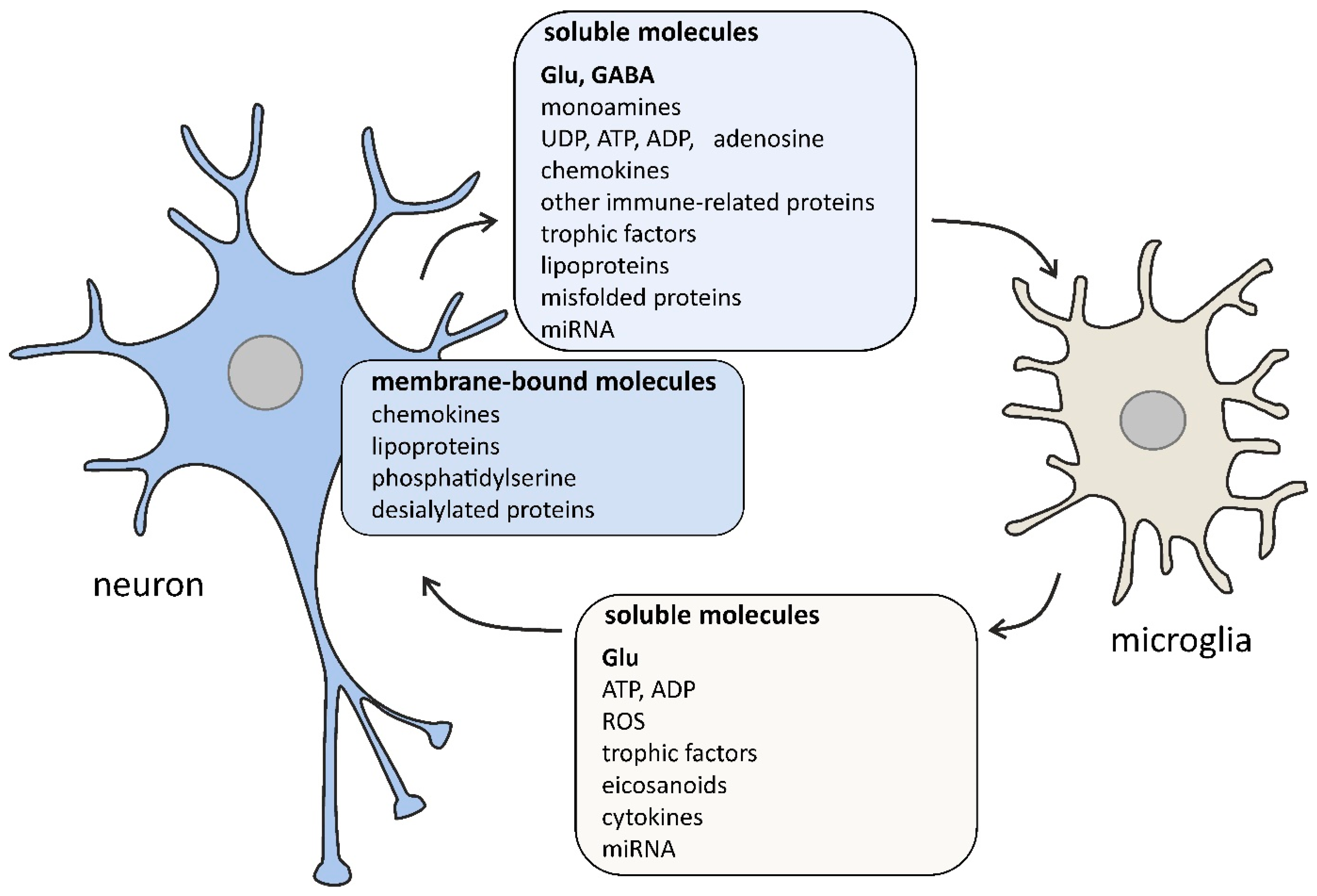
Figure 1.
The selected signaling molecules, both cell surface-bound and soluble, that are involved in the cross-talk between neurons and microglia. The bidirectional communication between neurons and microglia is mediated by numerous molecules. Neurons affect microglial function by classical (glutamate, GABA, dopamine, serotonin, ATP) and non-classical neurotransmitters (UDP, ADP, adenosine), chemokines (membrane-bound and soluble CX3CL1), membrane-bound proteins (CD200, CD22, CD34, CD47), complement system components (C1q, C3), trophic factors (colony-stimulating factor-1, transforming growth factor β), lipoproteins (apolipoprotein E), membrane-bound phosphatidylserine, desialylated membrane proteins, misfolded proteins (amyloid β, α-synuclein), and miRNA (e.g., miR-124 and miR-9). Microglia affect neurons by releasing gliotransmitters (glutamate, ATP, ADP), reactive oxygen species (ROS; e.g., nitric oxide, superoxide), eicosanoids (prostaglandin E2), cytokines (interleukin 1β, tumor necrosis factor α), trophic factors (brain-derived neurotrophic factor), and miRNA (e.g., miR-146a). Some signaling pathways occur only under pathological conditions.
Genomic, epigenomic, and transcriptomic analyses revealed that microglia are very different from other glial cells and tissue macrophages [29,30]. The signaling factors derived from the tissue microenvironment promote the specialized phenotype in the residing microglia population. The recent transcriptome-wide studies on human brain tissues based on analysis of transcriptomes of single cells shed light on the heterogeneity of the microglial population. Olah and co-workers identified some separate human microglial subpopulations (clusters) with specific gene expression patterns [12]. This microglial cluster architecture was present in all (17) tested persons. Importantly, one of these subpopulations (clusters) was significantly affected in AD, which suggests that a small fraction of brain microglia may be directly involved in the pathomechanism of AD [12]. Additionally, these data suggest also the need for understanding the specific function of each separate cluster of microglia and its contribution to the pathomechanism of AD. Moreover, because of phenotypic diversity, targeting microglia in AD is challenging and it is necessary to find an approach that does not suppress microglia overall, but instead modulates specific subpopulations or pathways. Adaptation of microglia/macrophages to local tissue microenvironment, including neuron-derived signal molecules, was shown to be mediated by epigenetic changes in chromatin [31]. These epigenetic mechanisms comprise histone methylation and acetylation, as well as miRNA. Several approaches targeting epigenetic regulation of microglia/macrophage function were proposed, including modulation of epigenetic code readers—bromodomain and extraterminal domain (BET) proteins [32,33,34,35].
3. Microglial Phenotype Polarization during Neuroinflammation/Neurodegeneration and the Role in Intercellular Communication
In a “resting” state, microglia very vigorously infiltrate the local microenvironment by extending and retracting motile processes equipped with a vast number of surface receptors. Detection of any specific or unspecific stimuli related to infection or tissue damage stimulates microglia to execute a respective response. The standard classification of the activation phenotype of microglia was borrowed from the nomenclature of macrophages, which, after stimulation in vitro, achieved M1 or M2 phenotype, depending on the stimulus. According to this classification, M1 (classic, proinflammatory, neurotoxic) microglia produce high amounts of proinflammatory mediators (cytokines, chemokines, and eicosanoids, including prostaglandins) and reactive oxygen/nitrogen species. Therefore, when overactivated, M1 microglia may be dangerous for own host’s tissues. On the contrary, M2 (alternative, anti-inflammatory, neuroprotective) microglia release anti-inflammatory cytokines and produce enzymes, such as arginase, which are necessary for tissue repair and regeneration. Later, additional variants were added to this list, such as M2a, M2b, M2c, and also M3, which has a role in the anti-inflammatory defense and tissue repair and can be stimulated by macrophage colony-stimulating factor (MCSF), interleukin-4 (IL-4) and IL-34 [36,37]. Recent studies analyzing transcriptomic and proteomic profiles clearly demonstrated that even this classification is a great oversimplification and is not relevant in categorizing microglia in vivo. In response to factors present in the tissue, activated microglia do not form steady subsets, but rather represent a broader functional repertoire of complex, even mixed, interpenetrating states, with M1-like and M2-like being just some near-border options [38,39].
The analysis of AD patients suggests that distinct neuroinflammatory microglial populations exist within the brain and that the neuroinflammatory status changes as the disease develops [12,40]. Microglia activation in neuroinflammation and AD is mediated by several complex signaling pathways [41,42]. Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) have been demonstrated to mediate microglia activation and immune response via different pattern-recognition receptors (PRRs), including Toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs). Microglia, through complex mechanisms, are involved in the non-autonomous clearance of protein aggregates in the brain. This function of microglia is significantly changed in brain aging and AD and may lead to proteinopathy. Accumulation of Aβ and several other proteins with altered conformations is responsible for disturbances in communication between microglia and neurons and also with other cells in the brain. These processes lead to neuroinflammation/neurodegeneration, cell death, mood disorders, and severe dementia [42,43].
4. The Role of Aβ Peptides and Other Proteins with Altered Conformations in the Neuron–Microglia Dialogue in AD and Neuroinflammation
The neuropathological hallmarks of AD are the presence of extracellular senile plaques, which are mainly built of aggregates of Aβ and the neuronal presence of neurofibrillary tangles (NFT), consisting mostly of the hyperphosphorylated microtubule-associated protein (MAP) Tau. However, in AD brains, other proteinopathies exist with high frequency [44] and may also influence neurotransmitters signaling, including glutamate and GABA, and their role in the microglia–neuron communication/interaction [45,46,47,48]. Aβ peptides significantly influence glutamatergic and GABAergic neurotransmission and the level of these compounds and may be responsible for disturbances of learning and memory processes [49,50]. The proteins most frequently involved in various forms of dementia are amyloid-β (Aβ), MAP Tau, α-synuclein (α-Syn), and TAR DNA-binding protein 43 (TDP-43). These proteins may act intracellularly and also extracellularly, and their interaction could potentiate toxicity and may disturb the microglia–neuron dialogue and evoke additional effects, as a release of several cytokines, trophic factors, free radicals, and also altered proteins. These processes form some type of metabolic vicious circle—for example, neuron-derived extracellular Tau can induce microglia-mediated neuronal death [51]. Interaction of extracellular Aβ with α-Syn leads to irreversible molecular alterations in cells and to neuronal cells death [52]. There are several suggestions and indications on the role of altered proteins α-Syn and Tau in the transmission of protein pathology from cells to cells similarly to prions [53,54].
Moreover, these peptides and other altered proteins affect the concentration and signaling pathways of several neurotransmitters, such as serotonin, dopamine, purines, and acetylcholine, which at an early stage may influence mood and induce apathy, agitation, or depression. These symptoms are, in many cases, the first alterations in neurodegenerative disorders, including Alzheimer’s or Parkinson’s disease [55,56]. However, the significance of altered proteins in microglia–neuron communication during AD is not fully elucidated. Moreover, the relations between proteins with altered conformation and the glutamate and GABA in microglia–neuron cross-talk in AD should be better understood.
Microglia play a crucial role in removing an excessive amount of Aβ and other altered proteins in AD. They may contribute to both the metabolism of Aβ and neuroinflammation. The presence of “activated” microglia in AD was originally reported by Alois Alzheimer in his report on Auguste D. in 1907 [57]. It was later hypothesized that the microglia are contributors to the pathomechanism of this disease via an autotoxic loop in which activation of microglia is primarily triggered by Aβ plaque deposition. This initial activation leads to further tissue damage, followed again by microglial activation, and thus, the process would continue in a vicious circle. The role of an altered amount of glutamate and GABA in such an autotoxic loop seems to be important.
The interest in microglia-powered neuroinflammation has increased in recent years, mainly because of genome-wide association studies (GWAS) done on large cohorts, counting thousands of patients, identifying new loci associated with the risk of developing a sporadic form of AD (ATXN1, CD33, CLU, CR1, PICALM, BIN1, CD2AP, MS4A6A/MS4A4E, EPHA1, ABCA7, SHIP1, TREM2, and an unidentified locus on chromosome 14 (GWA_14q31.2)) [58,59,60,61,62,63]. The effect of virtually all of these genetic factors on the pathomechanism of AD is associated with the regulation of the microglial function. For example, the triggering receptor expressed on myeloid cells 2 (TREM2) is involved in the phagocytosis of not only apoptotic neurons but also living neurons and synapses in neurodegeneration [64]. It was suggested that inhibition of microglial phagocytosis is sufficient to prevent inflammation-related neuronal death [65]. Recently, galectin-3 was found to be a specific novel endogenous TREM2 ligand [66]. Based on a study in the AD animal model, it was also suggested that TREM2 is responsible for the switch of microglia cells from the basal homeostatic and the intermediate state to disease-associated state and itself is an AD risk gene [67].
The TREM2 receptor is also engaged in the clearance of Aβ and other pathological proteins; therefore, the alteration in its function impacts the microglia-driven response to Aβ and could be important in microglia–neuron interaction (Figure 2). In AD brain and in transgenic models of AD, microglia accumulate around Aβ plaques, but this does not occur in TREM2-deficient or TREM2-haploinsufficient mice [68,69,70]. However, TREM2 deficiency decreases the expression of several genes necessary for microglial activation, including inflammatory cytokines, trophic factors, and proteins related to phagocytosis. Importantly, the significant impact of systemic inflammation on cognitive decline and on glutamate and GABA level and transmission in AD was also shown [71,72,73,74,75]. Moreover, the data suggest that systemic factors, such as tumor necrosis factor α (TNF-α), may significantly contribute to neuroinflammatory signaling in the brain [76]. Some data support the hypothesis of a possible role of infection/inflammation in the etiopathogenesis of AD [77,78,79]. The association was demonstrated between the frequency of several bacterial and viral infections and the risk of AD [80]. Recently, Porphyromonas gingivalis, the keystone pathogen in chronic periodontitis, was suggested to be directly involved in the etiopathogenesis of sporadic AD through the release of neurotoxic gingipains [81,82].
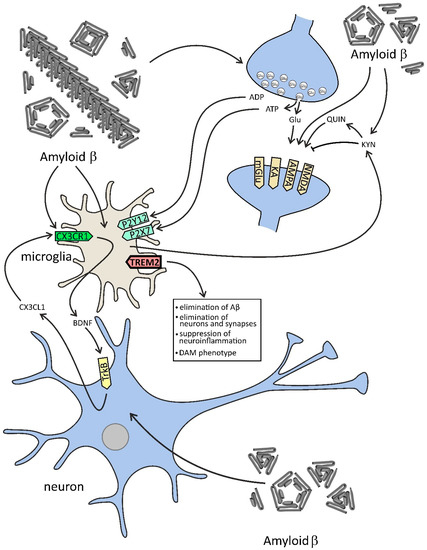
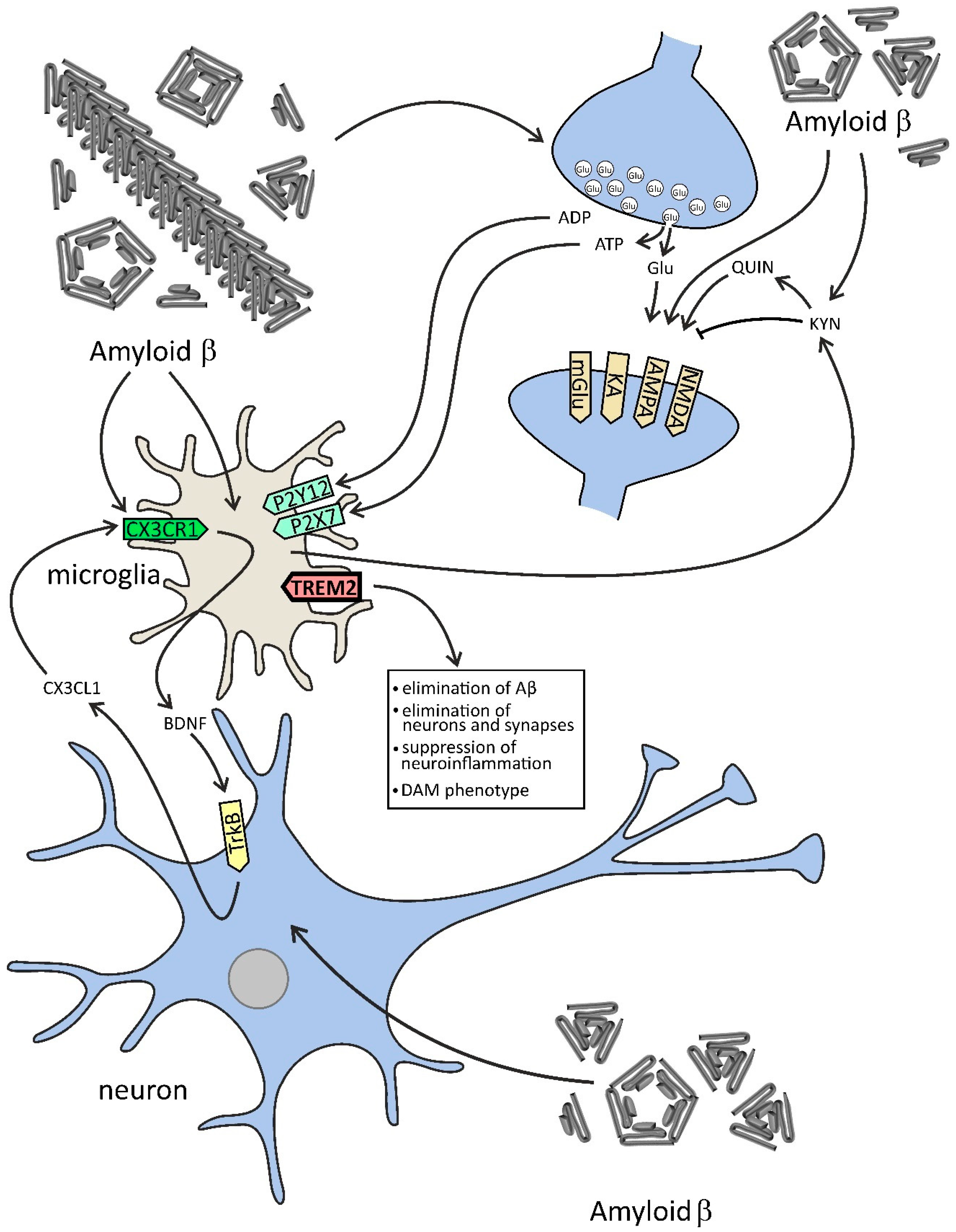
Figure 2.
The effect of Aβ on selected components of microglia–neuron dialogue in AD. In this figure, the effect of Aβ on neurons and microglia is demonstrated. Aβ peptides evoke the release of glutamate and ATP from neurons, which activates neuronal NMDA and other receptors and in microglial cells exerts a significant effect on purinergic receptors. Aβ-activated microglia change tryptophan metabolism toward the kynurenine (KYN) pathway, which leads to an increase in the level of KYN and other molecules affecting glutamatergic signaling, such as quinolinic acid (QUIN), which may evoke excitotoxicity. Microglia concomitantly release neurotrophic factors, among them brain-derived neurotrophic factor (BDNF), leading to stimulation of tropomyosin receptor kinase B (TrkB) and release of chemokine (C-X3-C motif) ligand 1 (CX3CL1). The proper function of microglia is dependent on the TREM2 (triggering receptor expressed on myeloid cells 2) receptor, which is engaged in the clearance of Aβ and other pathological proteins, but also in the elimination of neurons and synapses, and in the suppression of neuroinflammation. TREM2 is also responsible for the switch of microglia phenotype to disease-associated state (disease-associated microglia, DAM).
5. Glutamatergic Signaling in Microglia–Neuron Communication in AD
The recent hypothesis of homeostasis network collapse suggests that the imbalance between excitation and inhibition in the CNS, leading to dysregulation of neuronal networks, might be an exacerbating or even causative factor in the etiopathology of AD [83,84,85,86]. In parallel with changes in the balance between inhibition and excitation of neurons, dysregulation of microglial function may occur. It is obvious that microglial cells, to efficiently perform their homeostatic function, are equipped with a set of receptors. Several receptors are necessary for continuously surveying the CNS for changes in homeostasis, including proteostasis, for detecting the presence of invading pathogens or the harmful processes inside the CNS. A comprehensive list of receptors expressed in microglia has been published recently [18,19,78].
Glial cells express many receptors for neurotransmitters and actively respond to neuronal activity. Recent data indicate that neurons and glia share some components of signaling pathways and the cross-talk between them plays a prominent role in the development and function of the CNS (Figure 1) [87,88].
Glutamate, the most important neurotransmitter in CNS, is a nonessential amino acid that is synthesized in neurons and glial cells from glucose and α-ketoglutarate and is easily distributed throughout the brain. Its level in CNS is not affected by peripheral organs because it does not cross the blood–brain barrier (BBA). The average concentration of glutamate in the brain is 10–12 mM, and in a synaptic vesicle, it exceeds 100 mM. However, the concentration of glutamate in the synaptic cleft is in the range of 0.6 µM up to 10 µM during presynaptic neuronal depolarization; therefore, in the brain’s extracellular compartment, it must be maintained at a low micromolar level. The gradient of glutamate in different cerebral compartments is coordinated by specific transporters and enzymes that are responsible for its metabolism.
Glutamate is the precursor of GABA, the other crucial neurotransmitter with opposite functions. The balance between these two neurotransmitter-mediated signaling pathways is fundamental for appropriate brain function. Glutamate, under physiological conditions, is released by a mechanism dependent on various types of stimuli from synaptic vesicles and transduces the information via specific ionotropic and metabotropic receptors. Several diverse types of signaling pathways lead the molecular information to the nucleus, and through the regulation of gene transcription, glutamate may influence the metabolism and function of the brain. One of the most important roles of glutamatergic transmission is regulation of cognition, learning, and memory through activation of long-term potentiation (LTP) or long-term depression (LTD) by calcium-dependent liberation of nitric oxide and arachidonic acid, and its different metabolites, as eicosanoids and docosanoids [89,90]. Recently, the molecular control of glutamatergic NMDA receptor-dependent GABAergic plasticity was described [91].
However, neuroinflammation and AD lead to aberrant neurotransmitters’ release not only from neuronal synapses but also by microglia and astrocytes (Figure 3 and Table 1). The excess amount of glutamate is rapidly removed by glial cells and by excitatory amino acid transporters (EAAT1, EAAT2). In astrocytes, glutamate is transformed to glutamine, which is released and taken up by neuronal presynaptic part and metabolized to glutamate, which is subsequently accumulated in synaptic vesicles by vesicular glutamate transporters (VGLUT1/VGLUT2), and then released in the process of neurotransmission in very well operated tri- or tetrapartite synapse. The most important glutamate transporter is GLT-1/EAAT2, which is responsible for about 90% of forebrain uptake of glutamate and its homeostasis in the adult brain [92].
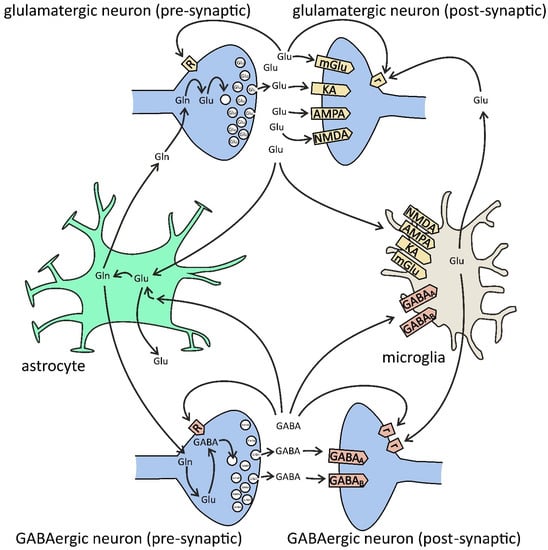
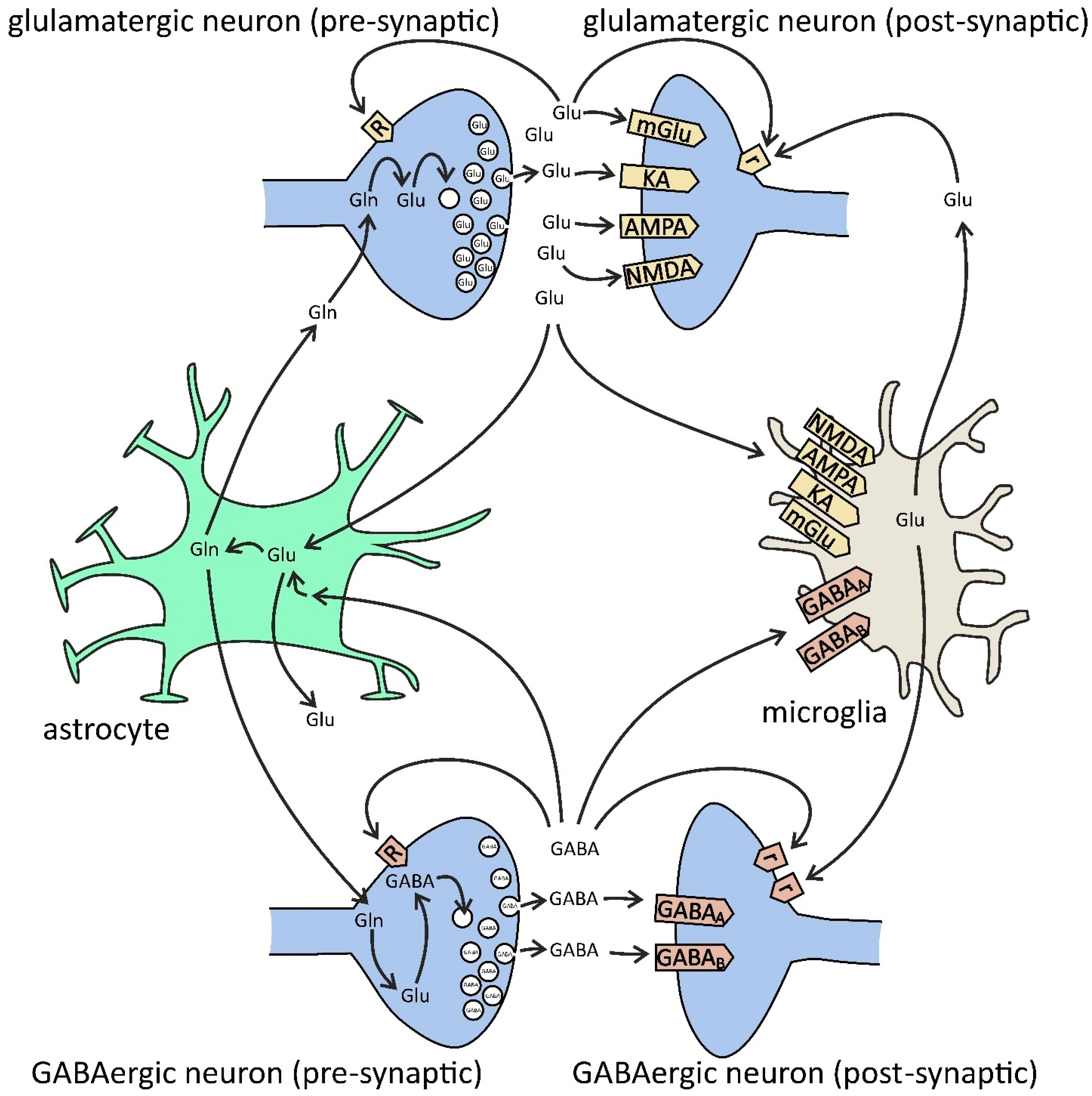
Figure 3.
Glutamate (Glu) and GABA in glia–neuron cross-talk. Presynaptic [R] and extrasynaptic [r] receptors. Glutamate is synthesized from glutamine (Gln) by phosphate-activated glutaminase (PAG) and then is packed into synaptic vesicles by vesicular glutamate transporters (VGLUT1/2). After release into the synaptic cleft, glutamate binds to neuronal postsynaptic receptors for glutamate (ionotropic N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), kainate (KA), and metabotropic mGluR1/5), to presynaptic receptors (ionotropic NMDA, AMPA, KA, and metabotropic mGluR7), and extrasynaptic receptors (mGluR2/3/4/8), but also to microglial receptors (NMDA, AMPA, KA, and mGluR2–8). Glutamate is also released by microglia via gap-junction hemichannels (e.g., Cx32) and by astrocytes via NMDA and purinergic P2X7 receptors, plasma membrane glutamate transporters, anion transporters, gap-junction hemichannels, and exocytosis. The major uptake of glutamate is performed by astrocytes via excitatory amino acid transporters (EAAT1/2). In astrocytes, glutamate is converted to glutamine by the glutamine synthetase (GS) pathway. Then, glutamine is transferred from astrocytes back to the neurons. GABA is synthesized from glutamate by glutamate decarboxylase (GAD) and then is packed into synaptic vesicles by the vesicular GABA transporter (VGAT). After release into the synaptic cleft, GABA binds to neuronal ionotropic GABAA (pre-, post-, and extrasynaptic), GABAc (pre- and postsynaptic), and metabotropic GABAB (pre-, post-, and extrasynaptic) receptors, but also to microglial GABAA/B. Both presynaptic nerve terminals and surrounding glial cells are responsible for the uptake of GABA from extracellular space. In glia, GABA is converted to glutamine, and then it is transferred back to the neurons and converted to glutamate, and finally to GABA.

Table 1.
Alterations of glutamatergic system components in human AD.
Glutamate released from synaptic vesicles exerts its role through activation of several ionotropic and metabotropic glutamate receptors localized mainly in the postsynaptic part of synapsis. However, its excessive release, also from glial cells, may affect several extrasynaptic receptors in neurons and microglia, and it may contribute to neuronal excitotoxicity and neurodegenerative processes in AD [18,97,98,99,100,101,102,103,104]. Recently, Szepesi et al. also highlighted a crucial role of changes in microglia–neuron dialogue in AD [105], but Bukke et al. underlined the dual role of glutamatergic transmission in AD [104]. Recent data showed that activated microglia release glutamate together with ATP, which exerts an effect through stimulation of multiple receptor-mediated signaling, including purinergic receptors, such as P2X4 and P2X7 [106,107]. Moreover, ATP could be released from microglia by lipopolysaccharide (LPS), lysophosphatidic acid, and high intracellular calcium [Ca2+] level, and all these events may alter neurotransmission processes. Activation of glutamate receptors (GluRs) in microglia plays a significant role in the regulation of the inflammatory response and could be a promising target in therapeutic strategy in AD. In this neurodegenerative disease, the microglial release of glutamate may be evoked by secreted soluble Aβ precursor protein (sAPP) and Aβ peptides by TNF-α and may occur concomitantly with excessive stimulation of glutamate release from synaptic vesicles in the presynaptic part of nerve endings. The massive release of glutamate leads to over-stimulation of pre- and postsynaptic glutamatergic receptors, both ionotropic (AMPA, Kainate, NMDA) and metabotropic mGluR1 and mGluR5 (group I), mGluR2 and mGluR3 (group II), and mGluR4,6,8 (group III) [18,78]. Extrasynaptic glutamate receptors were suggested to significantly contribute to degenerative processes by triggering the expression of Tau protein and by suppression of transcription of the cAMP-response element binding (CREB) protein [103].
Dysfunction of clearance of glutamate together with its excessive release by microglia leads to excitotoxicity and aberrant extrasynaptic signaling and synaptic dysfunction, and then to synaptosis, neuronal death, and behavioral alterations [43]. Glutamate exerts pro- and anti-inflammatory effects depending on the type of receptor(s) that are involved, the amount of glutamate, and the type of cells engaged in the regulation of its homeostasis. Aβ peptides could be important triggers of glutamate release in AD. Additionally, the other toxic proteins such as α-synuclein (α-Syn), which accumulates in the neurons in 50–60% of AD cases, could be released into extracellular space by Aβ and may induce oxidative stress and additionally may exert an effect on glutamatergic signaling and cells death pathway(s) [53,54,108]. It has been known for a long time that GluRs in synapses play a crucial role in brain function in learning and memory and in the regulation of mood. However, the role of glutamatergic receptors (GluRs) in microglia in physiological conditions is not fully understood. It seems that GluRs in microglia are mainly responsible for the modulation of these cells′ response by altering their morphology and motility, generation of reactive oxygen species (ROS), and release of cytokines, leading probably to modification of neuronal function and synaptic transmission [109]. Microglia may influence glutamatergic neurotransmission mainly through AMPA receptor and presynaptic mGlu2/mGlu3 [110]. Recent studies have indicated the role of neuron–microglia interaction in the function and maturation of glutamatergic synapse and demonstrated how microglia influence synaptic organizations and activity [111,112]. Moreover, Perez-Rodriguez described the role of microglia in neurogenesis in the adult brain [113]. Despite all studies, the physiological function of microglial cells and their interplay with neurons in the brain is not fully elucidated. However, in neuroinflammation and in AD excessive glutamate and ATP release activate the extrasynaptic receptor (NMDA), purinergic P2 × 7 cation channel receptor and also other purinergic receptors, which could be consequently responsible for the atrophy of dendritic spines and for synaptic and neuronal degeneration [43,114]. Moreover, activated microglia may change tryptophan metabolism toward the kynurenine (KYN) pathway, which leads to an increase in the level of KYN, an antagonist of the NMDA receptor, and other molecules affecting glutamatergic signaling, such as quinolinic acid (QUIN), an agonist of the NMDA receptor (Figure 2) [115,116,117]. Furthermore, astrocytes produce more KYN, while microglia produce more QUIN [117]. The QUIN may evoke excitotoxicity even in physiological concentration [118,119]. The neurotoxic effect of QUIN may be additive or synergistic with neuroinflammation or excitotoxicity and can also evoke gliotoxicity [119,120,121].
It is postulated that an enhancement of glutamate level in the extrasynaptic area stimulates extrasynaptic NMDA receptor subunit GluN 2B, leading to alteration of Ca2+ ions homeostasis and to long-term depression (LTD), and consequently to dysregulation of synaptic function [104,122]. Moreover, these events may evoke alterations of APP expression and activation of the amyloidogenic pathway of APP metabolism in direction to Aβ liberation and oligomerization [104,123]. It is now accepted that Aβ oligomers are responsible for the alteration of synapses and for cognition impairment [124,125,126,127]. The synaptic function is highly dependent on microglia and astrocytes. Microglia cells constantly scan the brain milieu, neuronal cells, and synapses. Activated microglia are engaged in removing damaged neurons and altered–dysfunctional synapses, and this process is termed “synaptic stripping.” There are many factors by which microglia sense neurons and synaptic alterations/injury. Among them, chemokines and ATP play an important role. Moreover, the complement pathway, the part of the innate immune system, is significantly involved in microglia in the removal of pathogens and is also engaged in the elimination of synapses during neuroinflammation/neurodegeneration [128,129,130]. However, microglia concomitantly play a crucial physiological role in learning and memory by stimulation of novel learning-related synapse formation by the brain-derived neurotrophic factor (BDNF) receptor-mediated signaling pathway and probably also by other factors such as nerve growth factor (NGF) and glial cell-derived neurotrophic factor (GDNF) [27,129].
Microglial cells are significantly altered in brain aging and may contribute to age-associated cognitive alteration. Microglia senescence may be responsible for changes in synaptic communication and plasticity and consequently for alteration of cognitive functions. Microglia, through a variety of multiple receptors, including glutaminergic and GABA receptors and other receptors for neurotransmitters, neuropeptides, and neuromodulators, can sense neuronal activity. Microglia express adrenergic, dopaminergic, bradykinin, endothelin-1, histamine, and substance P receptors. Stimulation of these receptors evokes liberation of several cytokines and other bioactive compounds from microglia and affects the movement of these cells and phagocytosis [131]. In pathological conditions, microglia remove synapses from neuronal cells. However, the involvement of microglia in the brain depends on the type of pathology. For example, in prion disease, activation of microglia does not lead to synaptic stripping, but in AD this process probably could play an important role in some brain regions [132]. Additionally, the role of neurotransmitters in the modulation of microglial activity in AD is not elucidated.
The previous data demonstrated that neurotransmitter level (GABA and glutamate) and Aβ, and also hyperphosphorylated Tau protein, are not significantly altered in the brain cortex, hippocampus, and retina at an early stage in the experimental model of AD (3×Tg mouse model) [133]. However, this study simultaneously demonstrated a few differential changes of microglia in retina and brain regions, which means different profiles in microglia branching at this early stage of AD (4-month-old AD-Tg mouse) with hypertrophy of microglia in the hippocampus and atrophic morphology of microglia in the retina. This and other studies did not answer the question, what kind of alterations of microglia could be evoked in AD pathology? Our recent data on the very early molecular changes in the AD-Tg mouse model indicated significant alteration in the expression of genes for enzymes involved in antioxidative defense and mitochondrial function [134]. The latest study by Styr and Slutsky [85] suggested that failures in firing stability and imbalance between firing homeostasis and synaptic plasticity in cortico-hippocampal circuits activate the driving force of early-phase AD, which may then lead to memory impairment.
6. GABAergic Signaling in Microglial Dialogue with Neurons in AD and Neuroinflammation
Alterations of glucose, glutamate level, and their metabolism may significantly affect gamma-aminobutyric acid (GABA), which is a major inhibitory neurotransmitter in the brain. Inhibitory neurons are crucial for the correct control and coordination of neuronal networks within and between various brain regions. The imbalance between excitation and inhibition, leading to variations in the activity of neural populations, was suggested to be a potential mechanism of cognitive dysfunction significantly contributing to the pathomechanism of AD [8,9,10].
The dysfunction of GABAergic signaling in human AD has been usually overlooked, even if impairment of inhibitory neurons and alterations in EEG, GABA levels, GABA receptors, etc., have been reported in AD patients and in experimental models (Table 2) [8,135].

Table 2.
Alterations of GABAergic system components in human AD.
However, the results did not give conclusive and consistent results. For example, studies on post-mortem levels of GABA, distribution, and activity of glutamic acid decarboxylase (GAD) often lead to contradictory results, likely because of significant limitations related to post-mortem analysis. Reduced GABA uptake due to loss of cortical and hippocampal GABA terminals in the AD brain was reported [142,143]. The levels of GABA receptors (GABAA, GABAB) and transporters (GAT1, GAT2, GAT3, BGT1) change in a region- and layer-specific manner [135,144]. In general, it seems that in AD an inhibition of GABAergic signaling in many brain structures occurs.
However, the role of GABA in the brain appears much beyond the controlling function of neural circuits and networks, because glial cells, including microglia, are also GABAceptive cells. Microglia express GABA metabolism-related enzymes and functional ionotropic and metabotropic GABA receptors A–C [145,146,147,148]. However, some studies suggested that the effects of neurotransmitters (glutamate, GABA) on microglia may not be direct, because local application of neurotransmitters does not evoke electrical responses in microglia [149,150]. As an alternative, it was suggested that the effect is mediated indirectly via extracellular ATP and purinergic receptors [151].
Data on the effect of GABA on microglial activity are not consistent. It was demonstrated that GABA suppresses the reactive response of microglia to the proinflammatory stimuli, leading to inhibition of pathways mediated by nuclear factor kappa B (NF-κB) and p38 MAP kinase [146,147]. Moreover, studies on mouse cortex in vivo have demonstrated that ramified microglia respond to current levels of GABAergic neurotransmission, as a surface application of the GABA receptor blocker, bicuculline, significantly increases microglial processes motility [152]. On the contrary, GABA evokes activation of microglia via the nucleotide oligomerization domain (NOD)-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome and NF-κB signaling pathways, leading to a significant increase in the mRNA and protein levels of TNF-α, IL-6, and IL-1β in microglial cell line BV2 [153].
The connection between neuronal activity and microglial activity in the pathomechanism of AD was demonstrated in 5×FAD mice, a well-established model of AD [154]. 5×FAD mice have reduced power in gamma oscillations during hippocampal sharp-wave ripples. Artificial stimulation of gamma oscillations in the hippocampus upregulated microglial genes related to phagocytosis, migration, and cell adhesion, evoked morphological transformation of microglia, increased co-localization of microglia and Aβ, and triggered Aβ peptide uptake by microglia. This effect was strictly dependent on GABAergic neurotransmission, because pre-treatment of 5×FAD mice with GABAA antagonist, picrotoxin, completely canceled the beneficial effects of gamma oscillations. Therefore, GABA signaling seems to be essential for the activation of Aβ uptake by microglia.
7. Therapeutic Compounds Affecting Glutamate/GABA Neurotransmission in Microglial–Neuron Communication
In AD, the amyloid cascade hypothesis has gained a central position and dominated the field for the last three decades [5]. The fundamental principle of this hypothesis is that the accumulation of Aβ is the initial pathological event that triggers Tau pathology, mitochondrial dysfunction, neuroinflammation, and oxidative stress, leading finally to neurodegeneration and dementia [155,156,157]. However, AD is a multifaceted disorder with very complex pathomechanism, and its etiology remains not fully understood. Despite thousands of preclinical studies and clinical trials, the therapeutic possibilities are still very limited. No new treatment has been introduced into clinical practice since 2003. Therapeutic strategies based on an anti-Aβ approach have not yielded satisfactory results. The recent accelerated approval of aducanumab (a monoclonal antibody directed against soluble and insoluble forms of amyloid β) by the United States Food and Drug Administration (FDA) opened the floodgates to new disease-modifying drugs, but using aducanumab faced criticism and is considered controversial [158,159,160,161].
Early studies demonstrated that long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) declined the risk of developing AD [162,163,164]. However, the clinical trials on NSAIDs gave inconclusive results, showing protective effects, no effects, or even detrimental effects [165,166,167,168,169,170]. Data seem to suggest that anti-inflammatory therapy may be beneficial if introduced at an early stage of the disease, before symptom onset; however, it may be detrimental at a later stage, after cognitive impairment develops [169,170]. Additional factors, such as the rate of cognitive decline, type of NSAID (cyclooxygenase-2 inhibitor vs. non-specific), genetic background, general health, and lifestyle of the patient, may also be important.
Currently, acetylcholinesterase inhibitors and NMDA receptor antagonist are commonly prescribed medications for AD. Inhibitors of acetylcholinesterase (rivastigmine, donepezil, galantamine) have been approved for the treatment of mild and moderately severe AD. These drugs slow the development of the clinical symptoms, but cannot stop the progression of the disease. Memantine, an NMDA receptor antagonist, has been approved for the treatment of patients with moderate and severe disease. Moreover, combination therapy with acetylcholinesterase inhibitor and memantine has been proposed, but the clinical relevance of its effect is uncertain [171]. Memantine is used in the treatment of AD to inhibit NMDA receptor-mediated excitotoxicity. It preferentially acts on extrasynaptic NMDA receptors containing the GluN2A subunit [103]. Moreover, memantine inhibits the LPS-induced production of ROS and proinflammatory factors. Importantly, glia-mediated neurotrophic and anti-inflammatory effects of memantine are NMDA receptor-independent. Rosi and co-workers demonstrated in a rat model that memantine at therapeutically relevant doses inhibits the activation of microglia and reduces LPS-induced neuroinflammation [172]. In the model of Aβ toxicity in vivo, memantine significantly reduced changes of glial marker proteins and activation of inducible nitric oxide synthase (iNOS) [173]. The effect of memantine on inwardly rectifying K+ Kir2.1 channels was demonstrated in BV2 microglial cells, which was suggested to be one of the important mechanisms underlying memantine’s actions on microglial cells [174]. By contrast, memantine did not influence the activation of microglial cells in culture, suggesting that microglia–neuron communication is necessary for the anti-inflammatory effect of memantine [175]. Similarly, in rodent microglial cells stimulated with TNF-α, memantine did not affect the production of nitric oxide (NO), Ca2+ elevation, expression of inflammation-related genes, or phagocytic activity [176]. Memantine reduced Aβ-evoked phosphorylation of Tau protein in vitro [177]. In addition, memantine caused neurotrophic effects through the increased production of glial cell line-derived neurotrophic factor (GDNF) [178]. This effect on the GDNF level was evoked by inhibiting the activity of cellular histone deacetylase, leading to histone hyperacetylation.
Other drugs that are NMDA receptor blockers, such as ifenprodil, fluoxetine, and desipramine, have not been approved for AD treatment. However, novel drugs are still developed to attenuate glutamatergic dysfunction in AD, among them riluzole. The clinical trial on riluzole (NCT01703117), an inhibitor of both glutamate release and postsynaptic glutamate receptor signaling, met the primary and the secondary outcome measures, showing a strong correlation between riluzole treatment, cognitive measures, and brain metabolism [179]. In transgenic mouse models of AD, riluzole modulated the activity of small conductance and Ca2+-activated K+ channels (SK channels), decreased Aβ levels (oligomers and plaques) and Tau pathology (total level and phosphorylation), and reversed changes in gene expression [180,181,182]. Importantly, analysis of gene expression patterns showed that riluzole impacts some immune-related pathways implicated in AD, including expression of canonical gene markers for microglia (e.g., Clec7a and IRF7) [181]. Another compound, BMS-984923, an mGluR5 silent allosteric modulator, is now in a phase I trial (NCT04805983). The action of BMS-984923 is not limited to modulating mGluR5-dependent calcium signaling. It recovered Aβ oligomer-dependent impairment of intracellular signaling, synaptic plasticity, Tau pathology (total level and phosphorylation), and synaptic loss in a mouse transgenic model of AD [183]. However, this drug did not impact Aβ load and microgliosis. Therefore, these glia-related effects of novel pharmacological compounds targeting glutamate signaling should be considered and studied.
Additionally, the clinical application of GABAergic drugs should be further evaluated for the treatment of behavioral and psychological symptoms of dementia in AD, as it was mentioned many years ago [184]. The positive GABAA receptor modulating steroid allopregnanolone (APα) is currently in a phase II trial (NCT04838301). In a single dose, APα was shown to reduce Aβ generation and to promote regeneration of neurons and restore learning and memory in a 3×Tg mouse model of AD [185,186], but the chronic treatment caused memory decline in wild-type mice and accelerated dementia in selected AD mice models [187,188]. Interestingly, APα affects microglial morphology and phagocytic function, which might potentially contribute to its beneficial effect in AD [189]. It cannot be excluded that mechanisms other than modulating glutamatergic and GABAergic signaling contribute to the neuroprotective effects of the compounds described above. Moreover, current strategies and novel drug approaches for AD were described, including modulatory effects of autophagy on APP processing, as a potential treatment target for AD [190,191,192] (Table 3).

Table 3.
Pharmacological compounds affecting glutamatergic/GABAergic signaling in Alzheimer’s disease.
As mentioned in this review, the metabolism of glutamate and GABA is very complex. Glutamate links carbohydrate and amino acid metabolism via the tricarboxylic acid (TCA) cycle and plays a key role in maintaining nitrogen and ammonia homeostasis in the brain [193,194]. Moreover, glutamate through its receptor-dependent signaling is also involved in APP/Aβ and protein Tau metabolism. However, several recent studies have indicated that alterations of APP/Aβ and Tau cannot fully explain the pathomechanism of the sporadic form of AD [195]. Using quantitative metabolomics, proteomics, and lipidomics methods to analyze plasma and cerebrospinal fluid (CSF) indicated specific and close association of amino acids (including homocysteine, a non-proteinogenic amino acid) and tryptophan metabolites (kynurenic acid and quinolinic acid) with AD biomarkers: Aβ42, Tau, and phospho-Tau (Thr181). Importantly, the peripheral metabolites of the kynurenine pathway were recently suggested as potential biomarkers in neurodegenerative diseases, including AD [196]. These advanced methods could be helpful in better understanding the AD pathomechanism and in the identification of novel promising targets in therapy [197,198]. It has been proposed that metabolic impairment, including alterations of glucose metabolism, and modifications of amino acids and proteins can significantly enhance the risk of AD. Moreover, a growing body of data demonstrated the failure of mitochondrial dynamics and function in AD and alterations of glucose metabolism in the TCA cycle and in the glycolytic pathway, shifting it from oxidative phosphorylation to aerobic glycolysis [199,200]. Dyslipidemia, hypertension, abdominal obesity, and insulin resistance, which alter glucose metabolism, are accepted hallmarks of metabolic syndrome (MetS) and potential causes of AD [201]. Accordingly, type 2 diabetes mellitus-related insulin and obesity-related adiponectin have been proposed to be promising targets in the therapy of AD [192,202,203]. Several compounds improving mitochondrial function or glucose metabolism are currently in AD drug development, including metformin (NCT04098666) and tricaprilin (NCT04187547) in phase 3, and benfotiamine (NCT02292238), dapagliflozin (NCT03801642), liraglutide (NCT01843075), and S-Equol (NCT03101085) in phase 2. Some of these compounds, such as metformin, which is an insulin sensitizer and improves CNS glucose metabolism, may potentially affect glutamatergic and GABAergic signaling in AD, including microglia–neuronal cross-talk [204,205].
The compounds affecting systemic and brain metabolic disturbances together with modulators of glutamatergic, GABAergic, and other neurotransmitters-related signaling pathways could improve the therapeutic efficacy against AD.
8. Concluding Remarks and Future Challenges
The recent available data support the crucial role of microglia–neuron communication in the brain and highlight the view regarding the microglia cells as the guardians of the central nervous system in physiological conditions and as important players in AD pathology. This review summarizes the data on alterations of glutamate and GABA level and signaling in microglia–neuron interaction at the early stage of AD and in the progression of this most severe dementia. However, the limitation of this article is the insufficiency of published data, which is related to the low availability of high-quality post-mortem human samples and limited relevance of current animal models of AD [206]. Moreover, some studies present contradictory results. Analysis of samples with low post-mortem interval (PMI) from various brain structures, and various disease stages, should reduce discrepancies and enable a better understanding of the complex interplay between cells and between neurotransmitter systems in AD [135]. An additional source of bias in data concerning glutamatergic and GABAergic systems in AD is the fact that drugs that are commonly prescribed in AD impact neurotransmission, potentially including glutamatergic and GABAergic systems. Therefore, many basic questions remain not elucidated.
The fundamental question arises if the effect of glutamate and GABA on microglia is direct or is mediated by ATP and its action on several purinergic receptors. The other question that needs an answer is related to the role of ATP, concomitantly secreted with these both neurotransmitters. The question is, how may ATP modulate/alter the communication between neurons and microglia in AD? The following important question is if several microglial receptors for neurotransmitters, neuropeptides, and neuromodulators are activated during synaptic transmission and how they change in AD. Then the question arises according to the involvement of the kynurenine pathway in modulation of glutamatergic signaling and microglia–neuron communication in AD. Moreover, the role of alterations of microglia–neuron cross-talk in disturbances of cognition function should be better elucidated.
To answer these questions, a more rigorous and systematic analysis of data from human samples is necessary. Advanced non-invasive spectroscopic and imaging techniques with future improvements will provide the most adequate data on the impairment of metabolic processes and neurotransmission in AD [207,208]. In correlation with novel serum biomarkers, they will be useful for the complex evaluation of individuals at various stages of AD. There is also a need for more relevant animal models that could provide a mechanistic explanation for the impairment of glutamatergic and GABAergic signaling. Moreover, because of some differences in human and rodent physiology, advanced models in vitro could offer additional benefits.
Answering and elucidating these several questions is necessary for better understanding the dialogue between microglia and neurons in neuroinflammation and in the pathomechanism of AD and will be fundamental for elaborating better strategies in the diagnosis and therapy of AD.
Author Contributions
Conceptualization, G.A.C. and J.B.S.; writing—original draft preparation, G.A.C. and J.B.S.; writing—review and editing, G.A.C. and J.B.S.; visualization, G.A.C. and J.B.S.; funding acquisition, G.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland, grant number 2018/31/B/NZ4/01379 (G.A.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors of this study declare no conflict of interest.
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Adams, J.D. Probable Causes of Alzheimer’s Disease. Science 2021, 3, 16. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. Genomic mechanisms in Alzheimer’s disease. Brain Pathol. 2020, 30, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Moyse, E.; Haddad, M.; Benlabiod, C.; Ramassamy, C.; Krantic, S. Common Pathological Mechanisms and Risk Factors for Alzheimer’s Disease and Type-2 Diabetes: Focus on Inflammation. Curr. Alzheimer Res. 2019, 16, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [Green Version]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef]
- Ambrad Giovannetti, E.; Fuhrmann, M. Unsupervised excitation: GABAergic dysfunctions in Alzheimer’s disease. Brain Res. 2019, 1707, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Plescher, M.; Seifert, G.; Hansen, J.N.; Bedner, P.; Steinhäuser, C.; Halle, A. Plaque-dependent morphological and electrophysiological heterogeneity of microglia in an Alzheimer’s disease mouse model. Glia 2018, 66, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Jesudasan, S.J.B.; Gupta, S.J.; Churchward, M.A.; Todd, K.G.; Winship, I.R. Inflammatory Cytokine Profile and Plasticity of Brain and Spinal Microglia in Response to ATP and Glutamate. Front. Cell. Neurosci. 2021, 15, 634020. [Google Scholar] [CrossRef] [PubMed]
- Noda, M. Dysfunction of Glutamate Receptors in Microglia May Cause Neurodegeneration. Curr. Alzheimer Res. 2016, 13, 381–386. [Google Scholar] [CrossRef]
- Stolero, N.; Frenkel, D. The dialog between neurons and microglia in Alzheimer’s disease: The neurotransmitters view. J. Neurochem. 2020, 158, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Younger, D.; Murugan, M.; Rama Rao, K.V.; Wu, L.J.; Chandra, N. Microglia Receptors in Animal Models of Traumatic Brain Injury. Mol. Neurobiol. 2019, 56, 5202–5228. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, T.L.; Carrier, M.; Tremblay, M. Physiology of Microglia. Adv. Exp. Med. Biol. 2019, 1175, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Monier, A.; Adle-Biassette, H.; Delezoide, A.L.; Evrard, P.; Gressens, P.; Verney, C. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 2007, 66, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Kimyon, R.S.; De, I.; Small, A.L.; Collier, L.S.; Watters, J.J. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J. Neuroimmunol. 2015, 278, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Nicola, D.; Perry, V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neuroscientist 2015, 21, 169–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [Green Version]
- Udeochu, J.C.; Shea, J.M.; Villeda, S.A. Microglia communication: Parallels between aging and Alzheimer’s disease. Clin. Exp. Neuroimmunol. 2016, 7, 114–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef]
- Veremeyko, T.; Yung, A.W.Y.; Dukhinova, M.; Strekalova, T.; Ponomarev, E.D. The Role of Neuronal Factors in the Epigenetic Reprogramming of Microglia in the Normal and Diseased Central Nervous System. Front. Cell. Neurosci. 2019, 13, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahaya, M.A.F.; Zolkiffly, S.Z.I.; Moklas, M.A.M.; Hamid, H.A.; Stanslas, J.; Zainol, M.; Mehat, M.Z. Possible Epigenetic Role of Vitexin in Regulating Neuroinflammation in Alzheimer’s Disease. J. Immunol. Res. 2020, 2020, 9469210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.; Liang, J.; Wang, C.; Deng, Y.; Chen, Z. Epigenetic Regulation of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4956. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ventura, J.; Amo-Aparicio, J.; Navarro, X.; Penas, C. BET protein inhibition regulates cytokine production and promotes neuroprotection after spinal cord injury. J. Neuroinflamm. 2019, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudduth, T.L.; Schmitt, F.A.; Nelson, P.T.; Wilcock, D.M. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B.; Mota, M.; Pizzi, M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim. Biophys. Acta 2016, 1862, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.A.; Van Enoo, A.A.; Ikezu, T. Alzheimer’s Disease: The Role of Microglia in Brain Homeostasis and Proteopathy. Front. Neurosci. 2017, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Kwon, S.; Iba, M.; Kim, C.; Masliah, E. Immunotherapies for Aging-Related Neurodegenerative Diseases-Emerging Perspectives and New Targets. Neurotherapeutics 2020, 17, 935–954. [Google Scholar] [CrossRef] [PubMed]
- Tweedy, C.; Kindred, N.; Curry, J.; Williams, C.; Taylor, J.P.; Atkinson, P.; Randall, F.; Erskine, D.; Morris, C.M.; Reeve, A.K.; et al. Hippocampal network hyperexcitability in young transgenic mice expressing human mutant alpha-synuclein. Neurobiol. Dis. 2021, 149, 105226. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Yang, C.H.; Fang, Y.H.; Cho, K.H.; Chien, W.L.; Wang, W.T.; Wu, T.W.; Lin, C.P.; Fu, W.M.; Shen, C.K. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J. Exp. Med. 2010, 207, 1661–1673. [Google Scholar] [CrossRef] [Green Version]
- Hebron, M.; Chen, W.; Miessau, M.J.; Lonskaya, I.; Moussa, C.E. Parkin reverses TDP-43-induced cell death and failure of amino acid homeostasis. J. Neurochem. 2014, 129, 350–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammisetty, S.S.; Pedragosa, J.; Weng, Y.C.; Calon, F.; Planas, A.; Kriz, J. Age-related deregulation of TDP-43 after stroke enhances NF-κB-mediated inflammation and neuronal damage. J. Neuroinflamm. 2018, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D. Amyloid-β Impairs Synaptic Inhibition via GABA(A) Receptor Endocytosis. J. Neurosci. 2015, 35, 9205–9210. [Google Scholar] [CrossRef] [Green Version]
- Hascup, K.N.; Hascup, E.R. Soluble Amyloid-β42 Stimulates Glutamate Release through Activation of the α7 Nicotinic Acetylcholine Receptor. J. Alzheimers Dis. 2016, 53, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Borutaite, V. Extracellular tau-induced microglia-mediated neuronal death. In Proceedings of the Book of Abstracts of Virtual FENS Regional Meeting, Kraków, Poland, 25–27 August 2021; p. 31. [Google Scholar]
- Kazmierczak, A.; Strosznajder, J.B.; Adamczyk, A. alpha-Synuclein enhances secretion and toxicity of amyloid beta peptides in PC12 cells. Neurochem. Int. 2008, 53, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wilkaniec, A.; Strosznajder, J.B.; Adamczyk, A. Toxicity of extracellular secreted alpha-synuclein: Its role in nitrosative stress and neurodegeneration. Neurochem. Int. 2013, 62, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Jęśko, H.; Cieślik, M.; Gromadzka, G.; Adamczyk, A. Dysfunctional proteins in neuropsychiatric disorders: From neurodegeneration to autism spectrum disorders. Neurochem. Int. 2020, 141, 104853. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol. Aspects Med. 2015, 43-44, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, M.; Shao, H.; Zandi, P.; Lyketsos, C.G.; Welsh-Bohmer, K.A.; Norton, M.C.; Breitner, J.C.; Steffens, D.C.; Tschanz, J.T. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int. J. Geriatr. Psychiatry 2008, 23, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Psych.-Gerichtl. Med. 1907, 64, 146–148. [Google Scholar]
- Bertram, L.; Lange, C.; Mullin, K.; Parkinson, M.; Hsiao, M.; Hogan, M.F.; Schjeide, B.M.; Hooli, B.; Divito, J.; Ionita, I.; et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am. J. Hum. Genet. 2008, 83, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef]
- Hollingworth, P.; Harold, D.; Sims, R.; Gerrish, A.; Lambert, J.C.; Carrasquillo, M.M.; Abraham, R.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011, 43, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Naj, A.C.; Jun, G.; Beecham, G.W.; Wang, L.S.; Vardarajan, B.N.; Buros, J.; Gallins, P.J.; Buxbaum, J.D.; Jarvik, G.P.; Crane, P.K.; et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011, 43, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, C.A.; Popescu, A.S.; Kitchener, E.J.A.; Allendorf, D.H.; Puigdellívol, M.; Brown, G.C. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 2021, 158, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Zhao, J.W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 2011, 186, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; García-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennström, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276-1290.e1217. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Ulrich, J.D.; Finn, M.B.; Wang, Y.; Shen, A.; Mahan, T.E.; Jiang, H.; Stewart, F.R.; Piccio, L.; Colonna, M.; Holtzman, D.M. Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2. Mol. Neurodegener. 2014, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H., Jr.; Selvin, E.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology 2019, 92, e1256–e1267. [Google Scholar] [CrossRef]
- Marsland, A.L.; Gianaros, P.J.; Kuan, D.C.; Sheu, L.K.; Krajina, K.; Manuck, S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015, 48, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puris, E.; Auriola, S.; Korhonen, P.; Loppi, S.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Gynther, M. Systemic inflammation induced changes in protein expression of ABC transporters and ionotropic glutamate receptor subunit 1 in the cerebral cortex of familial Alzheimer‘s disease mouse model. J. Pharm. Sci. 2021. S0022-3549(21)00414-7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Zurek, A.A.; Lecker, I.; Yu, J.; Abramian, A.M.; Avramescu, S.; Davies, P.A.; Moss, S.J.; Lu, W.Y.; Orser, B.A. Memory deficits induced by inflammation are regulated by α5-subunit-containing GABAA receptors. Cell Rep. 2012, 2, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoi, Y.; Saito, S. An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit. Care Med. 1996, 24, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochocka, M.; Zwolinska, K.; Leszek, J. The Infectious Etiology of Alzheimer’s Disease. Curr. Neuropharm. 2017, 15, 996–1009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, D.; Zhang, B.; Zhu, J.; Zhou, Z.; Cui, L. Regulation of microglia by glutamate and its signal pathway in neurodegenerative diseases. Drug Discov. Today 2020, 25, 1074–1085. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Bu, X.L.; Yao, X.Q.; Jiao, S.S.; Zeng, F.; Liu, Y.H.; Xiang, Y.; Liang, C.R.; Wang, Q.H.; Wang, X.; Cao, H.Y.; et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 1519–1525. [Google Scholar] [CrossRef]
- Kanagasingam, S.; Chukkapalli, S.S.; Welbury, R.; Singhrao, S.K. Porphyromonas gingivalis is a Strong Risk Factor for Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2020, 4, 501–511. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frere, S.; Slutsky, I. Alzheimer’s Disease: From Firing Instability to Homeostasis Network Collapse. Neuron 2018, 97, 32–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vico Varela, E.; Etter, G.; Williams, S. Excitatory-inhibitory imbalance in Alzheimer’s disease and therapeutic significance. Neurobiol. Dis. 2019, 127, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Styr, B.; Slutsky, I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. 2018, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Fort, M.; Audinat, E.; Angulo, M.C. Central role of GABA in neuron-glia interactions. Neuroscientist 2012, 18, 237–250. [Google Scholar] [CrossRef]
- Hodo, T.W.; de Aquino, M.T.P.; Shimamoto, A.; Shanker, A. Critical Neurotransmitters in the Neuroimmune Network. Front. Immunol. 2020, 11, 1869. [Google Scholar] [CrossRef]
- Danysz, W.; Zajaczkowski, W.; Parsons, C.G. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav. Pharmacol. 1995, 6, 455–474. [Google Scholar] [CrossRef]
- Lazarewicz, J.W.; Salinska, E.; Wroblewski, J.T. NMDA receptor-mediated arachidonic acid release in neurons: Role in signal transduction and pathological aspects. Adv. Exp. Med. Biol. 1992, 318, 73–89. [Google Scholar] [CrossRef]
- Jędrzejewska-Szmek, J. Molecular control of NMDAR-dependent gabaergic plasticity. In Proceedings of the Book of Abstracts of Virtual FENS Regional Meeting, Kraków, Poland, 25–27 August 2021; p. 56. [Google Scholar]
- Robinson, M.B. The family of sodium-dependent glutamate transporters: A focus on the GLT-1/EAAT2 subtype. Neurochem. Int. 1998, 33, 479–491. [Google Scholar] [CrossRef]
- Li, S.; Mallory, M.; Alford, M.; Tanaka, S.; Masliah, E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 1997, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuba, G.; Vernay, A.; Kraftsik, R.; Tardif, E.; Riederer, B.M.; Savioz, A. Pathological reorganization of NMDA receptors subunits and postsynaptic protein PSD-95 distribution in Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Palma, E.; Bustos, B.I.; Villamán, C.F.; Alarcón, M.A.; Avila, M.E.; Ugarte, G.D.; Reyes, A.E.; Opazo, C.; De Ferrari, G.V. Overrepresentation of glutamate signaling in Alzheimer’s disease: Network-based pathway enrichment using meta-analysis of genome-wide association studies. PLoS ONE 2014, 9, e95413. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.J.; Dimayuga, E.; Morganti, J.M.; Van Eldik, L.J. Microglial-associated responses to comorbid amyloid pathology and hyperhomocysteinemia in an aged knock-in mouse model of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 274. [Google Scholar] [CrossRef]
- Sebastian Monasor, L.; Müller, S.A.; Colombo, A.V.; Tanrioever, G.; König, J.; Roth, S.; Liesz, A.; Berghofer, A.; Piechotta, A.; Prestel, M.; et al. Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. eLife 2020, 9, e54083. [Google Scholar] [CrossRef]
- Rienecker, K.D.A.; Paladini, M.S.; Grue, K.; Krukowski, K.; Rosi, S. Microglia: Ally and Enemy in Deep Space. Neurosci. Biobehav. Rev. 2021, 126, 509–514. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Cassano, T.; Pace, L.; Bedse, G.; Lavecchia, A.M.; De Marco, F.; Gaetani, S.; Serviddio, G. Glutamate and Mitochondria: Two Prominent Players in the Oxidative Stress-Induced Neurodegeneration. Curr. Alzheimer Res. 2016, 13, 185–197. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Melgar, A.; Albasanz, J.L.; Palomera-Ávalos, V.; Pallàs, M.; Martín, M. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol. Neurobiol. 2019, 56, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Wilkaniec, A.; Czapski, G.A.; Adamczyk, A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: Facts and hypotheses. J. Neurochem. 2016, 136, 222–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual, O.; Ben Achour, S.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Achour, S.; Pascual, O. Glia: The many ways to modulate synaptic plasticity. Neurochem. Int. 2010, 57, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Basilico, B.; Pagani, F.; Grimaldi, A.; Cortese, B.; Di Angelantonio, S.; Weinhard, L.; Gross, C.; Limatola, C.; Maggi, L.; Ragozzino, D. Microglia shape presynaptic properties at developing glutamatergic synapses. Glia 2019, 67, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Basilico, B.; Ferrucci, L.; Ratano, P.; Golia, M.T.; Grimaldi, A.; Rosito, M.; Ferretti, V.; Reverte, I.; Marrone, M.C.; Giubettini, M.; et al. Microglia control glutamatergic synapses in the adult mouse hippocampus. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, D.R.; Blanco-Luquin, I.; Mendioroz, M. The Participation of Microglia in Neurogenesis: A Review. Brain Sci. 2021, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Allam, S.L.; Bouteiller, J.M.; Hu, E.Y.; Ambert, N.; Greget, R.; Bischoff, S.; Baudry, M.; Berger, T.W. Synaptic Efficacy as a Function of Ionotropic Receptor Distribution: A Computational Study. PLoS ONE 2015, 10, e0140333. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Walker, A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. 2014, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.M.; Parrott, J.M.; Tuñon, A.; Delgado, J.; Redus, L.; O’Connor, J.C. Kynurenine pathway metabolic balance influences microglia activity: Targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology 2018, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zmarowski, A.; Wu, H.Q.; Brooks, J.M.; Potter, M.C.; Pellicciari, R.; Schwarcz, R.; Bruno, J.P. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur. J. Neurosci. 2009, 29, 529–538. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R. Kynurenines and Glutamate: Multiple Links and Therapeutic Implications. Adv. Pharmacol. 2016, 76, 13–37. [Google Scholar] [CrossRef] [Green Version]
- Guillemin, G.J. Quinolinic acid: Neurotoxicity. FEBS J. 2012, 279, 1355. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Becerril-Ortega, J.; Nicole, O.; Buisson, A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15927–15942. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. Amyloid β-protein and beyond: The path forward in Alzheimer’s disease. Curr. Opin. Neurobiol. 2020, 61, 116–124. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Perry, V.H.; O’Connor, V. The role of microglia in synaptic stripping and synaptic degeneration: A revised perspective. ASN Neuro. 2010, 2, e00047. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Neves, A.C.; Carecho, R.; Correia, S.C.; Carvalho, C.; Campos, E.J.; Baptista, F.I.; Moreira, P.I.; Ambrósio, A.F. Retina and Brain Display Early and Differential Molecular and Cellular Changes in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 3043–3060. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Wencel, P.L.; Wieczorek, I.; Strosznajder, J.B.; Strosznajder, R.P. Early alterations in transcription of genes coding NAD dependent- enzymes and mitochondria proteins in Alzheimer’s dis-ease animal model. Novel target(s) for neuroprotection. In Proceedings of the Book of Abstracts of Virtual FENS Regional Meeting, Kraków, Poland, 25–27 August 2021; p. 306. [Google Scholar]
- Govindpani, K.; Calvo-Flores Guzmán, B.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrer, T.E.; Palpagama, T.H.; Waldvogel, H.J.; Synek, B.J.L.; Turner, C.; Faull, R.L.; Kwakowsky, A. Impaired expression of GABA transporters in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. Neuroscience 2017, 351, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Ikonomovic, M.D.; Grayson, D.R.; Sheffield, R.; Armstrong, D.M. Immunohistochemical study of GABAA receptor alpha1 subunit in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Brain Res. 1998, 799, 148–155. [Google Scholar] [CrossRef]
- Rissman, R.A.; Mishizen-Eberz, A.J.; Carter, T.L.; Wolfe, B.B.; De Blas, A.L.; Miralles, C.P.; Ikonomovic, M.D.; Armstrong, D.M. Biochemical analysis of GABA(A) receptor subunits alpha 1, alpha 5, beta 1, beta 2 in the hippocampus of patients with Alzheimer’s disease neuropathology. Neuroscience 2003, 120, 695–704. [Google Scholar] [CrossRef]
- Limon, A.; Reyes-Ruiz, J.M.; Miledi, R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. USA 2012, 109, 10071–10076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakiri, M.; Mizukami, K.; Ikonomovic, M.D.; Ishikawa, M.; Hidaka, S.; Abrahamson, E.E.; DeKosky, S.T.; Asada, T. Changes in hippocampal GABABR1 subunit expression in Alzheimer’s patients: Association with Braak staging. Acta Neuropathol. 2005, 109, 467–474. [Google Scholar] [CrossRef]
- Chu, D.C.; Penney, J.B., Jr.; Young, A.B. Cortical GABAB and GABAA receptors in Alzheimer’s disease: A quantitative autoradiographic study. Neurology 1987, 37, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Cowburn, R.; Barton, A.; Reynolds, G.; Dodd, P.; Wester, P.; O’Carroll, A.M.; Lofdahl, E.; Winblad, B. A disorder of cortical GABAergic innervation in Alzheimer’s disease. Neurosci. Lett. 1987, 73, 192–196. [Google Scholar] [CrossRef]
- Simpson, M.D.; Cross, A.J.; Slater, P.; Deakin, J.F. Loss of cortical GABA uptake sites in Alzheimer’s disease. J. Neural Transm. 1988, 71, 219–226. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABA(A) receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.J.; Deuchars, J.; Davies, C.H.; Pangalos, M.N. GABA B receptor subunit expression in glia. Mol. Cell. Neurosci. 2003, 24, 214–223. [Google Scholar] [CrossRef]
- Kuhn, S.A.; van Landeghem, F.K.; Zacharias, R.; Färber, K.; Rappert, A.; Pavlovic, S.; Hoffmann, A.; Nolte, C.; Kettenmann, H. Microglia express GABA(B) receptors to modulate interleukin release. Mol. Cell. Neurosci. 2004, 25, 312–322. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; McGeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Schmidt, M. GABA(C) receptors in retina and brain. Results Probl. Cell Differ. 2008, 44, 49–67. [Google Scholar] [CrossRef]
- Fontainhas, A.M.; Wang, M.; Liang, K.J.; Chen, S.; Mettu, P.; Damani, M.; Fariss, R.N.; Li, W.; Wong, W.T. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS ONE 2011, 6, e15973. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Zhuo, M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J. Neurophysiol. 2008, 99, 2026–2032. [Google Scholar] [CrossRef]
- Wong, W.T.; Wang, M.; Li, W. Regulation of microglia by ionotropic glutamatergic and GABAergic neurotransmission. Neuron Glia Biol. 2011, 7, 41–46. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, L.; Xu, B.; Yuan, J.; Li, S.; Lian, S.; Chen, Y.; Guo, J.; Yang, H. GABA-mediated activated microglia induce neuroinflammation in the hippocampus of mice following cold exposure through the NLRP3 inflammasome and NF-κB signaling pathways. Int. Immunopharmacol. 2020, 89, 106908. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E. FDA Approval of Aduhelm Paves a New Path for Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 2714–2715. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Armstrong, R.A. A critical analysis of the ‘amyloid cascade hypothesis’. Folia Neuropathol. 2014, 52, 211–225. [Google Scholar] [CrossRef]
- Mullard, A. FDA approval for Biogen’s aducanumab sparks Alzheimer disease firestorm. Nat. Rev. Drug Discov. 2021, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- in t’ Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef] [Green Version]
- Côté, S.; Carmichael, P.H.; Verreault, R.; Lindsay, J.; Lefebvre, J.; Laurin, D. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2012, 8, 219–226. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol. Aging 2007, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schafer, K.A.; Grundman, M.; Pfeiffer, E.; Sano, M.; Davis, K.L.; Farlow, M.R.; Jin, S.; Thomas, R.G.; Thal, L.J. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 2003, 289, 2819–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekely, C.A.; Thorne, J.E.; Zandi, P.P.; Ek, M.; Messias, E.; Breitner, J.C.; Goodman, S.N. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: A systematic review. Neuroepidemiology 2004, 23, 159–169. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Silverman, D.H.; Ercoli, L.M.; Miller, K.J.; Lavretsky, H.; Bookheimer, S.Y.; Huang, S.C.; Barrio, J.R.; Phelps, M.E. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: Randomized controlled study. Am. J. Geriatr. Psychiatry 2008, 16, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, M.P.; Carriba, P.; Masgrau, R.; Pujol, A.; Galea, E. Staging anti-inflammatory therapy in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 142. [Google Scholar] [CrossRef] [Green Version]
- Breitner, J.C.; Baker, L.D.; Montine, T.J.; Meinert, C.L.; Lyketsos, C.G.; Ashe, K.H.; Brandt, J.; Craft, S.; Evans, D.E.; Green, R.C.; et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Leoutsakos, J.M.; Muthen, B.O.; Breitner, J.C.; Lyketsos, C.G. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: Findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. Int. J. Geriatr. Psychiatry 2012, 27, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Glinz, D.; Gloy, V.L.; Monsch, A.U.; Kressig, R.W.; Patel, C.; McCord, K.A.; Ademi, Z.; Tomonaga, Y.; Schwenkglenks, M.; Bucher, H.C.; et al. Acetylcholinesterase inhibitors combined with memantine for moderate to severe Alzheimer’s disease: A meta-analysis. Swiss Med. Wkly. 2019, 149, w20093. [Google Scholar] [CrossRef] [Green Version]
- Rosi, S.; Vazdarjanova, A.; Ramirez-Amaya, V.; Worley, P.F.; Barnes, C.A.; Wenk, G.L. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience 2006, 142, 1303–1315. [Google Scholar] [CrossRef]
- Arif, M.; Chikuma, T.; Ahmed, M.M.; Nakazato, M.; Smith, M.A.; Kato, T. Effects of memantine on soluble Alphabeta(25-35)-induced changes in peptidergic and glial cells in Alzheimer’s disease model rat brain regions. Neuroscience 2009, 164, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Chang, H.F.; Wu, S.N. The inhibition of inwardly rectifying K+ channels by memantine in macrophages and microglial cells. Cell. Physiol. Biochem. 2013, 31, 938–951. [Google Scholar] [CrossRef]
- Rosi, S.; Ramirez-Amaya, V.; Vazdarjanova, A.; Esparza, E.E.; Larkin, P.B.; Fike, J.R.; Wenk, G.L.; Barnes, C.A. Accuracy of hippocampal network activity is disrupted by neuroinflammation: Rescue by memantine. Brain 2009, 132, 2464–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakawa-Hirachi, T.; Mizoguchi, Y.; Ohgidani, M.; Haraguchi, Y.; Monji, A. Effect of memantine, an anti-Alzheimer’s drug, on rodent microglial cells in vitro. Sci. Rep. 2021, 11, 6151. [Google Scholar] [CrossRef]
- Song, M.S.; Rauw, G.; Baker, G.B.; Kar, S. Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur. J. Neurosci. 2008, 28, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Tzeng, N.S.; Qian, L.; Wei, S.J.; Hu, X.; Chen, S.H.; Rawls, S.M.; Flood, P.; Hong, J.S.; Lu, R.B. Novel neuroprotective mechanisms of memantine: Increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology 2009, 34, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.C.; Mao, X.; Dowd, K.; Tsakanikas, D.; Jiang, C.S.; Meuser, C.; Andrews, R.D.; Lukic, A.S.; Lee, J.; Hampilos, N.; et al. Riluzole, a glutamate modulator, slows cerebral glucose metabolism decline in patients with Alzheimer’s disease. Brain 2021. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, H.C.; Weitzner, D.S.; Rudy, C.C.; Hickman, J.E.; Libell, E.M.; Speer, R.R.; Gerhardt, G.A.; Reed, M.N. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. J. Neurochem. 2015, 135, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Gray, J.D.; Larson, C.S.; Kazim, S.F.; Soya, H.; McEwen, B.S.; Pereira, A.C. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl. Psychiatry 2018, 8, 153. [Google Scholar] [CrossRef]
- Cao, Y.J.; Dreixler, J.C.; Couey, J.J.; Houamed, K.M. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur. J. Pharmacol. 2002, 449, 47–54. [Google Scholar] [CrossRef]
- Haas, L.T.; Salazar, S.V.; Smith, L.M.; Zhao, H.R.; Cox, T.O.; Herber, C.S.; Degnan, A.P.; Balakrishnan, A.; Macor, J.E.; Albright, C.F.; et al. Silent Allosteric Modulation of mGluR5 Maintains Glutamate Signaling while Rescuing Alzheimer’s Mouse Phenotypes. Cell Rep. 2017, 20, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Lanctôt, K.L.; Herrmann, N.; Mazzotta, P.; Khan, L.R.; Ingber, N. GABAergic function in Alzheimer’s disease: Evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can. J. Psychiatry 2004, 49, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, J.M.; Irwin, R.W.; Yao, J.; Liu, L.; Brinton, R.D. Allopregnanolone promotes regeneration and reduces β-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE 2011, 6, e24293. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Liu, L.; Wang, J.M.; Irwin, R.W.; Yao, J.; Chen, S.; Henry, S.; Thompson, R.F.; Brinton, R.D. Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol. Aging 2012, 33, 1493–1506. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, S.K.; Johansson, M.; Backstrom, T.; Nitsch, R.M.; Wang, M. Brief but chronic increase in allopregnanolone cause accelerated AD pathology differently in two mouse models. Curr. Alzheimer Res. 2013, 10, 38–47. [Google Scholar] [CrossRef]
- Bengtsson, S.K.; Johansson, M.; Bäckström, T. Long-term continuous allopregnanolone elevation causes memory decline and hippocampus shrinkage, in female wild-type B6 mice. Horm. Behav. 2016, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jolivel, V.; Brun, S.; Binamé, F.; Benyounes, J.; Taleb, O.; Bagnard, D.; De Sèze, J.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Microglial Cell Morphology and Phagocytic Activity Are Critically Regulated by the Neurosteroid Allopregnanolone: A Possible Role in Neuroprotection. Cells 2021, 10, 698. [Google Scholar] [CrossRef]
- Ghai, R.; Nagarajan, K.; Arora, M.; Grover, P.; Ali, N.; Kapoor, G. Current Strategies and Novel Drug Approaches for Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Islam, T.; Zaman, T.; Shahjaman, M.; Karim, M.R.; Huq, F.; Quinn, J.M.W.; Holsinger, R.M.D.; Gov, E.; Moni, M.A. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer’s disease: Insights from a systems biomedicine perspective. Genomics 2020, 112, 1290–1299. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement. 2021, 7, e12179. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 2014, 11, 13–30. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.; Jeitner, T.M. Central Role of Glutamate Metabolism in the Maintenance of Nitrogen Homeostasis in Normal and Hyperammonemic Brain. Biomolecules 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullane, K.; Williams, M. Alzheimer’s disease (AD) therapeutics—2: Beyond amyloid—Re-defining AD and its causality to discover effective therapeutics. Biochem. Pharmacol. 2018, 158, 376–401. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef] [PubMed]
- van der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, J.M.; Trushina, E. Application of Metabolomics in Alzheimer’s disease. Front. Neurol. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, P.J.; Trushina, E. Mitochondrial dynamics and transport in Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 98, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Raichle, M.E. Brain aerobic glycolysis functions and Alzheimer’s disease. Clin. Transl. Imaging 2015, 3, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.A.; Cisternas, P.; Arrese, M.; Barja, S.; Inestrosa, N.C. Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog. Neurobiol. 2014, 121, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6419. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Li, D.; Chen, H.S.; Huang, J.G.; Xu, J.F.; Zhu, W.W.; Chen, J.G.; Wang, F. Metformin produces anxiolytic-like effects in rats by facilitating GABA(A) receptor trafficking to membrane. Br. J. Pharmacol. 2019, 176, 297–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhou, T.; Guo, A.M.; Chen, W.B.; Lin, D.; Liu, Z.Y.; Fei, E.K. Metformin Ameliorates Lipopolysaccharide-Induced Depressive-Like Behaviors and Abnormal Glutamatergic Transmission. Biology 2020, 9, 359. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Preclinical Models of Alzheimer’s disease: Relevance and Translational Validity. Curr. Protoc. Pharmacol. 2019, 84, e57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straathof, M.; Meerwaldt, A.E.; De Feyter, H.M.; de Graaf, R.A.; Dijkhuizen, R.M. Deuterium Metabolic Imaging of the Healthy and Diseased Brain. Neuroscience 2021, 474, 94–99. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, R.S.; Mecca, A.P.; Chen, M.K.; Naganawa, M.; Toyonaga, T.; Lu, Y.; Godek, T.A.; Harris, J.E.; Bartlett, H.H.; Banks, E.R.; et al. Association of Aβ deposition and regional synaptic density in early Alzheimer’s disease: A PET imaging study with [(11)C]UCB-J. Alzheimers Res. Ther. 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).