Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer
Abstract
1. Introduction
2. ALK Protein Structure and Interactions
3. Developmental Roles of ALK
3.1. Expression Patterns of ALK
3.2. Developmental Roles of ALK
4. ALK in Neuroblastoma
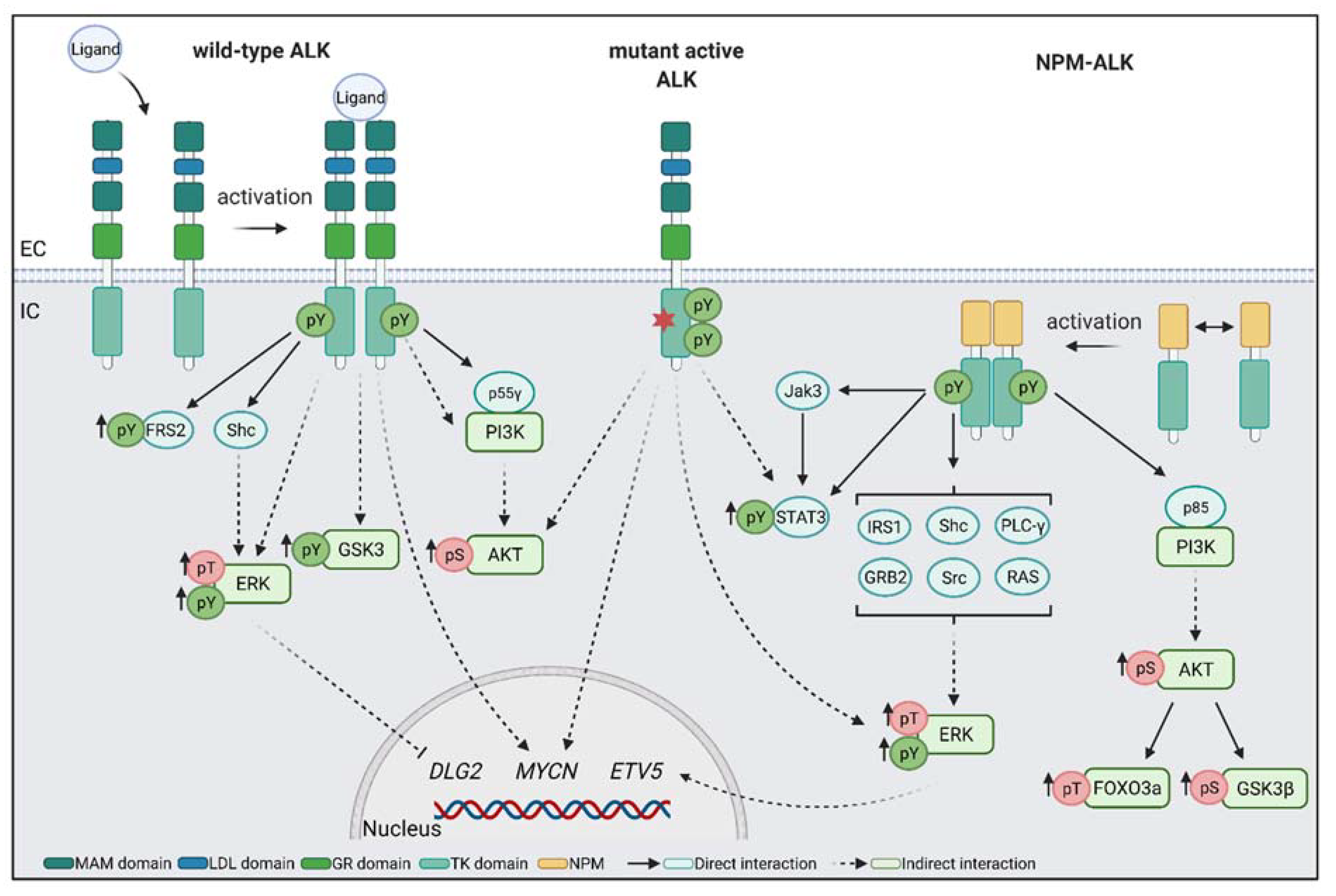
4.1. Fusion Proteins NPM–ALK and EML4–ALK Are Common in Cancer
4.2. ALK Alterations in Neuroblastoma
4.3. ALK and MYCN in Neuroblastoma
5. Downstream Targets of ALK
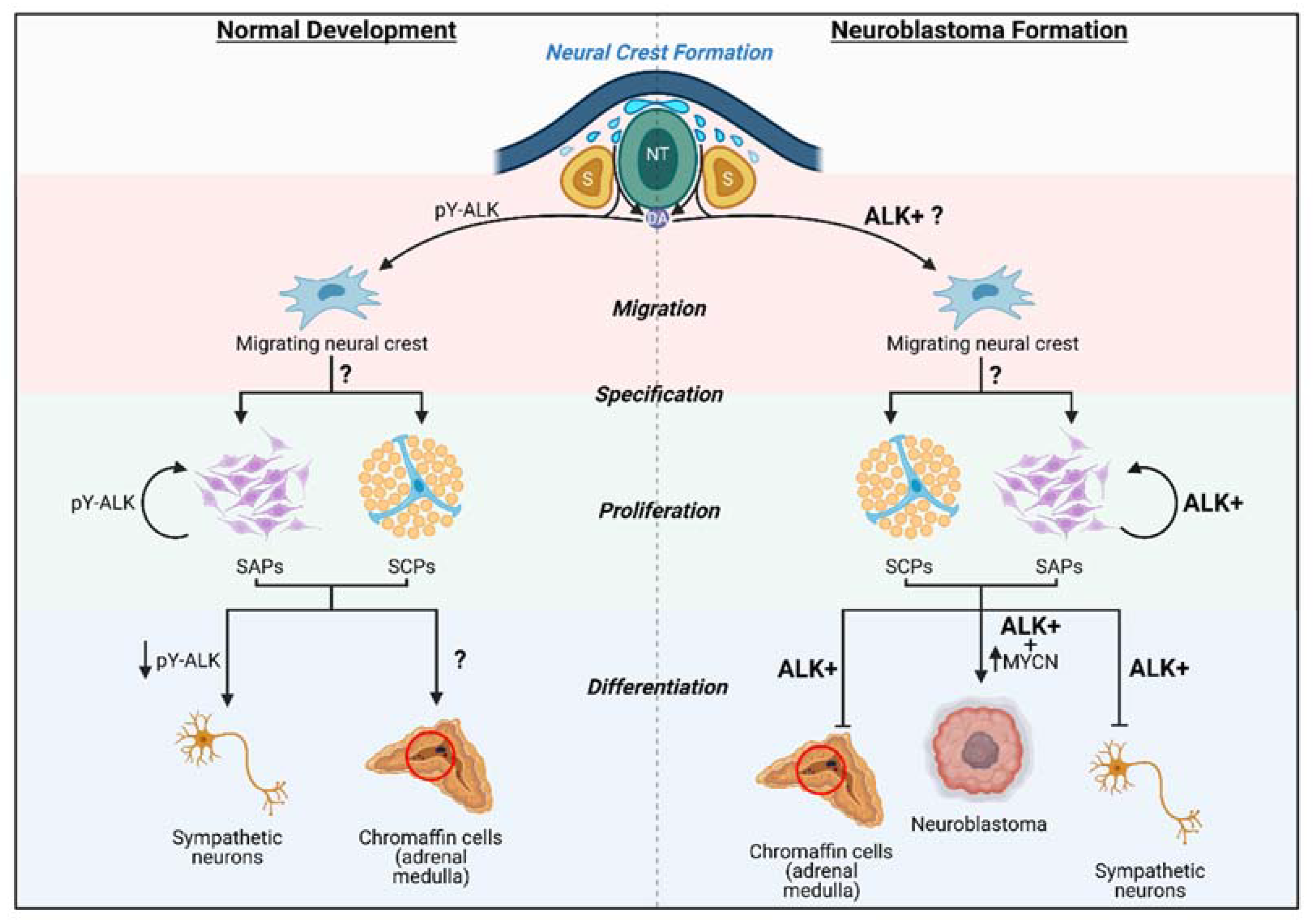
6. ALK in Neural Crest and Neuroblastoma
7. Treatment of ALK-Positive Neuroblastoma
8. Summary and Long-Term Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barr, E.; Applebaum, M. Genetic Predisposition to Neuroblastoma. Children 2018, 5, 119. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A neural crest derived embryonal malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef]
- Heck, J.E.; Ritz, B.; Hung, R.J.; Hashibe, M.; Boffetta, P. The epidemiology of neuroblastoma: A review. Paediatr. Perinat. Epidemiol. 2009, 23, 125–143. [Google Scholar] [CrossRef]
- Janoueix-Lerosey, I.; Schleiermacher, G.; Michels, E.; Mosseri, V.; Ribeiro, A.; Lequin, D.; Vermeulen, J.; Couturier, J.; Peuchmaur, M.; Valent, A.; et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J. Clin. Oncol. 2009, 27, 1026–1033. [Google Scholar] [CrossRef]
- Trochet, D.; Bourdeaut, F.; Janoueix-Lerosey, I.; Deville, A.; de Pontual, L.; Schleiermacher, G.; Coze, C.; Philip, N.; Frébourg, T.; Munnich, A.; et al. Germline Mutations of the Paired–Like Homeobox 2B (PHOX2B) Gene in Neuroblastoma. Am. J. Hum. Genet. 2004, 74, 761–764. [Google Scholar] [CrossRef]
- Tomolonis, J.A.; Agarwal, S.; Shohet, J.M. Neuroblastoma pathogenesis: Deregulation of embryonic neural crest development. Cell Tissue Res. 2018, 372, 245–262. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Prim. 2016, 2. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Look, A.T.; Hayes, F.A.; Shuster, J.J.; Douglass, E.C.; Castleberry, R.P.; Bowman, L.C.; Smith, E.I.; Brodeur, G.M. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: A pediatric oncology group study. J. Clin. Oncol. 1991, 9, 581–591. [Google Scholar] [CrossRef]
- Schwab, M.; Varmus, H.E.; Bishop, J.M.; Grzeschik, K.H.; Naylor, S.L.; Sakaguchi, A.Y.; Brodeur, G.; Trent, J. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature 1984, 308, 288–291. [Google Scholar] [CrossRef]
- Higashi, M.; Sakai, K.; Fumino, S.; Aoi, S.; Furukawa, T.; Tajiri, T. The roles played by the MYCN, Trk, and ALK genes in neuroblastoma and neural development. Surg. Today 2019, 49, 721–727. [Google Scholar] [CrossRef]
- Weiss, W.A.; Aldape, K.; Mohapatra, G.; Feuerstein, B.G.; Bishop, J.M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997, 16, 2985–2995. [Google Scholar] [CrossRef]
- George, R.E.; Sanda, T.; Hanna, M.; Fröhling, S.; II, W.L.; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef]
- Chen, Y.; Takita, J.; Choi, Y.L.; Kato, M.; Ohira, M.; Sanada, M.; Wang, L.; Soda, M.; Kikuchi, A.; Igarashi, T.; et al. Oncogenic ƒmutations of ALK kinase in neuroblastoma. Nature 2008, 455, 971–974. [Google Scholar] [CrossRef]
- Janoueix-Lerosey, I.; Lequin, D.; Brugières, L.; Ribeiro, A.; de Pontual, L.; Combaret, V.; Raynal, V.; Puisieux, A.; Schleiermacher, G.; Pierron, G.; et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 2008, 455, 967–970. [Google Scholar] [CrossRef]
- Trigg, R.; Turner, S. ALK in Neuroblastoma: Biological and Therapeutic Implications. Cancers 2018, 10, 113. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALKF1174L Mutation Potentiates the Oncogenic Activity of MYCN in Neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef]
- Heukamp, L.C.; Thor, T.; Schramm, A.; De Preter, K.; Kumps, C.; De Wilde, B.; Odersky, A.; Peifer, M.; Lindner, S.; Spruessel, A.; et al. Targeted Expression of Mutated ALK Induces Neuroblastoma in Transgenic Mice. Sci. Transl. Med. 2012, 4, 141ra91. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef]
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Bauskin, A.R. Leukocytes express a novel gene encoding a putative transmembrane protein-kinase devoid of an extracellular domain. Nature 1988, 333, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.W.; Naeve, C.; Mathew, P.; James, P.L.; Kirstein, M.N.; Cui, X.; Witte, D.P. ALK the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 1997, 14, 2175–2188. [Google Scholar] [CrossRef]
- Palmer, R.H.; Vernersson, E.; Grabbe, C.; Hallberg, B. Anaplastic lymphoma kinase: Signalling in development and disease. Biochem. J. 2009, 420, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Zondag, G.C.M.; Koningstein, G.M.; Jiang, Y.-P.; Sap, J.; Moolenaar, W.H.; Gebbink, M.F.B.G. Homophilic Interactions Mediated by Receptor Tyrosine Phosphatases μ and κ. A Critical Role for the Novel Extracellular MAM Domain. J. Biol. Chem. 1995, 270, 14247–14250. [Google Scholar] [CrossRef] [PubMed]
- Fass, D.; Blacklow, S.; Kim, P.S.; Berger, J.M. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 1997, 388, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Morris, S.W.; Turturro, F. Anaplastic Lymphoma Kinase Proteins in Growth Control and Cancer. J. Cell Physiol. 2004, 199, 330–358. [Google Scholar] [CrossRef]
- Takita, J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017, 108, 1913–1920. [Google Scholar] [CrossRef]
- Mourali, J.; Bénard, A.; Lourenço, F.C.; Monnet, C.; Greenland, C.; Moog-Lutz, C.; Racaud-Sultan, C.; Gonzalez-Dunia, D.; Vigny, M.; Mehlen, P.; et al. Anaplastic Lymphoma Kinase Is a Dependence Receptor Whose Proapoptotic Functions Are Activated by Caspase Cleavage. Mol. Cell Biol. 2006, 26, 6209–6222. [Google Scholar] [CrossRef]
- Lee, C.C.; Jia, Y.; Li, N.; Sun, X.; Ng, K.; Ambing, E.; Gao, M.; Hua, S.; Chen, C.; Kim, S.; et al. Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem. J. 2010, 430, 425–437. [Google Scholar] [CrossRef]
- Bossi, R.T.; Saccardo, M.B.; Ardini, E.; Menichincheri, M.; Rusconi, L.; Magnaghi, P.; Orsini, P.; Avanzi, N.; Borgia, A.L.; Nesi, M.; et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry 2010, 49, 6813–6825. [Google Scholar] [CrossRef]
- Roskoski, R. Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol. Res. 2013, 68, 68–94. [Google Scholar] [CrossRef]
- Donella-Deana, A.; Marin, O.; Cesaro, L.; Gunby, R.H.; Ferrarese, A.; Coluccia, A.M.L.; Tartari, C.J.; Mologni, L.; Scapozza, L.; Gambacorti-Passerini, C.; et al. Unique Substrate Specificity of Anaplastic Lymphoma Kinase (ALK): Development of Phosphoacceptor Peptides for the Assay of ALK Activity. Biochemistry 2005, 44, 8533–8542. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.; Aigner, A.; Stoica, G.E.; McDonnell, K.; Wellstein, A. Pleiotrophin Signaling through Anaplastic Lymphoma Kinase Is Rate-limiting for Glioblastoma Growth. J. Biol. Chem. 2002, 277, 14153–14158. [Google Scholar] [CrossRef]
- Yang, H.; Eriksson, T.; Vernersson, E.; Vigny, M.; Hallberg, B.; Palmer, R.H. The ligand Jelly Belly (Jeb) activates the Drosophila Alk RTK to drive PC12 cell differentiation, but is unable to activate the Mouse ALK RTK. J. Exp. Zool Part. B Mol. Dev. Evol. 2007, 308B, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Reiner, D.J.; Ailion, M.; Thomas, J.H.; Meyer, B.J.C. Elegans Anaplastic Lymphoma Kinase Ortholog SCD-2 Controls Dauer Formation by Modulating TGF-β Signaling. Curr Biol. 2008, 18, 1101–1109. [Google Scholar] [CrossRef]
- Zhang, H.; Pao, L.I.; Zhou, A.; Brace, A.D.; Halenbeck, R.; Hsu, A.W.; Bray, T.L.; Hestir, K.; Bosch, E.; Lee, E.; et al. Deorphanization of the human leukocyte tyrosine kinase (LTK) receptor by a signaling screen of the extracellular proteome. Proc. Natl. Acad. Sci. USA 2014, 111, 15741–15745. [Google Scholar] [CrossRef]
- Reshetnyak, A.V.; Murray, P.B.; Shi, X.; Mo, E.S.; Mohanty, J.; Tome, F.; Bai, H.; Gunel, M.; Lax, I.; Schlessinger, J. Augmentor α and β (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 15862–15867. [Google Scholar] [CrossRef]
- Mo, E.S.; Cheng, Q.; Reshetnyak, A.V.; Schlessinger, J.; Nicoli, S. Alk and Ltk ligands are essential for iridophore development in zebrafish mediated by the receptor tyrosine kinase Ltk. Proc. Natl. Acad. Sci. USA 2017, 114, 12027–12032. [Google Scholar] [CrossRef]
- Stoica, G.E.; Kuo, A.; Aigner, A.; Sunitha, I.; Souttou, B.; Malerczyk, C.; Caughey, D.J.; Wen, D.; Karavanov, A.; Riegel, A.T.; et al. Identification of Anaplastic Lymphoma Kinase as a Receptor for the Growth Factor Pleiotrophin. J. Biol. Chem. 2001, 276, 16772–16779. [Google Scholar] [CrossRef]
- Stoica, G.E.; Kuo, A.; Powers, C.; Bowden, E.T.; Sale, E.B.; Riegel, A.T.; Wellstein, A. Midkine Binds to Anaplastic Lymphoma Kinase (ALK) and Acts as a Growth Factor for Different Cell Types. J. Biol. Chem. 2002, 277, 35990–35998. [Google Scholar] [CrossRef]
- Nakagawara, A.; Milbrandt, J.; Muramatsu, T.; Deuel, T.F.; Zhao, H.; Cnaan, A.; Brodeur, G.M. Differential Expression of Pleiotrophin and Midkine in Advanced Neuroblastomas. Cancer Res. 1995, 55, 1792–1797. [Google Scholar] [PubMed]
- Mathivet, T.; Mazot, P.; Vigny, M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal. 2007, 19, 2434–2443. [Google Scholar] [CrossRef]
- Moog-Lutz, C.; Degoutin, J.; Gouzi, J.Y.; Frobert, Y.; Brunet-De Carvalho, N.; Bureau, J.; Créminon, C.; Vigny, M. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Biol. Chem. 2005, 280, 26039–26048. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, F.; Sjögren, C.; Birve, A.; Hedlund, L.; Eriksson, T.; Palmer, R.H. The Drosophila Midkine/Pleiotrophin Homologues Miple1 and Miple2 Affect Adult Lifespan but Are Dispensable for Alk Signaling during Embryonic Gut Formation. Singh SR, ed. PLoS ONE 2014, 9, e112250. [Google Scholar] [CrossRef]
- Perez-Pinera, P.; Zhang, W.; Chang, Y.; Vega, J.A.; Deuel, T.F. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase β/ζ signaling pathway: An alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 2007, 282, 28683–28690. [Google Scholar] [CrossRef]
- Allouche, M. ALK is a novel dependence receptor: Potential implications in development and Cancer. Cell Cycle 2007, 6, 1533–1538. [Google Scholar] [CrossRef]
- Goldschneider, D.; Mehlen, P. Dependence receptors: A new paradigm in cell signaling and cancer therapy. Oncogene 2010, 29, 1865–1882. [Google Scholar] [CrossRef]
- Lorén, C.E.; Scully, A.; Grabbe, C.; Edeen, P.T.; Thomas, J.; McKeown, M.; Hunter, T.; Palmer, R.H. Identification and characterization of DAlk: A novel Drosophila melanogaster RTK which drives ERK activation in vivo. Genes to Cells 2001, 6, 531–544. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Hurley, S.P.; Clary, D.O.; Copie, V.; Lefcort, F. Anaplastic lymphoma kinase is dynamically expressed on subsets of motor neurons and in the peripheral nervous system. J. Comp. Neurol. 2006, 495, 202–212. [Google Scholar] [CrossRef]
- Vieceli, F.M.; Bronner, M.E. Leukocyte Receptor Tyrosine Kinase interacts with secreted midkine to promote survival of migrating neural crest cells. Development 2018, 145, dev164046. [Google Scholar] [CrossRef] [PubMed]
- Iwahara, T.; Fujimoto, J.; Wen, D.; Cupples, R.; Bucay, N.; Arakawa, T.; Mori, S.; Ratzkin, B.; Yamamoto, T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997, 14, 439–449. [Google Scholar] [CrossRef]
- Vernersson, E.; Khoo, N.K.S.; Henriksson, M.L.; Roos, G.; Palmer, R.H.; Hallberg, B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr. Patterns 2006, 6, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Malagon, S.G.; Lopez Muñoz, A.M.; Doro, D.; Bolger, T.G.; Poon, E.; Tucker, E.R.; Adel Al-Lami, H.; Krause, M.; Phiel, C.J.; Chesler, L.; et al. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat. Commun. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.M.; Barrell, W.B.; Godwin, A.; Guille, M.; Liu, K.J. Anaplastic lymphoma kinase (alk), a neuroblastoma associated gene, is expressed in neural crest domains during embryonic development of Xenopus. Gene Expr. Patterns 2021, 40, 119183. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, J.; Kirschner, M. Normal Table of Xenopus Laevis (Daudin) A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; Nieuwkoop, P.D., Faber, J., Eds.; Garland Science: New York, NY, USA, 2020; ISBN 9781003064565. [Google Scholar] [CrossRef]
- Englund, C.; Lorén, C.E.; Grabbe, C.; Varshney, G.K.; Deleuil, F.; Hallberg, B.; Palmer, R.H. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature 2003, 425, 512–516. [Google Scholar] [CrossRef]
- Lorén, C.E.; Englund, C.; Grabbe, C.; Hallberg, B.; Hunter, T.; Palmer, R.H. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003, 4, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, B.; Palmer, R.H. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat. Rev. Cancer 2013, 13, 685–700. [Google Scholar] [CrossRef]
- Lee, H.H.; Norris, A.; Weiss, J.B.; Frasch, M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature 2003, 425, 507–512. [Google Scholar] [CrossRef]
- Ishihara, T.; Iino, Y.; Mohri, A.; Mori, I.; Gengyo-Ando, K.; Mitani, S.; Katsura, I. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 2002, 109, 639–649. [Google Scholar] [CrossRef]
- Kitazono, T.; Hara-Kuge, S.; Matsuda, O.; Inoue, A.; Fujiwara, M.; Ishihara, T. Multiple Signaling Pathways Coordinately Regulate Forgetting of Olfactory Adaptation through Control of Sensory Responses in Caenorhabditis elegans. J. Neurosci. 2017, 37, 10240–10251. [Google Scholar] [CrossRef]
- Wolfe, G.S.; Tong, V.W.; Povse, E.; Merritt, D.M.; Stegeman, G.W.; Flibotte, S.; van der Kooy, D. A Receptor Tyrosine Kinase Plays Separate Roles in Sensory Integration and Associative Learning in C. elegans. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Reiff, T.; Huber, L.; Kramer, M.; Delattre, O.; Janoueix-Lerosey, I.; Rohrer, H. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development 2011, 138, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.; Krauss, J.; Singh, A.P.; Nüsslein-Volhard, C. Zebrafish Leucocyte tyrosine kinase controls iridophore establishment, proliferation and survival. Pigment Cell Melanoma Res. 2016, 29, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.; Mendoza-Garcia, P.; Irion, U.; Guan, J.; Pfeifer, K.; Wiessner, S.; Serluca, F.; Singh, A.P.; Nüsslein-Volhard, C.; Palmer, R.H. ALKALs are in vivo ligands for ALK family receptor tyrosine kinases in the neural crest and derived cells. Proc. Natl. Acad. Sci. USA 2018, 115, E630–E638. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. 2016, 27, iii4–iii15. [Google Scholar] [CrossRef]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Ducray, S.P.; Natarajan, K.; Garland, G.D.; Turner, S.D.; Egger, G. The Transcriptional Roles of ALK Fusion Proteins in Tumorigenesis. Cancers 2019, 11, 1074. [Google Scholar] [CrossRef]
- Armstrong, F.; Duplantier, M.M.; Trempat, P.; Hieblot, C.; Lamant, L.; Espinos, E.; Racaud-Sultan, C.; Allouche, M.; Campo, E.; Delsol, G.; et al. Differential effects of X-ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene 2004, 23, 6071–6082. [Google Scholar] [CrossRef]
- Cazes, A.; Louis-Brennetot, C.; Mazot, P.; Dingli, F.; Lombard, B.; Boeva, V.; Daveau, R.; Cappo, J.; Combaret, V.; Schleiermacher, G.; et al. Characterization of rearrangements involving the ALK gene reveals a novel truncated form associated with tumor aggressiveness in neuroblastoma. Cancer Res. 2013, 73, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Miyake, I.; Hakomori, Y.; Shinohara, A.; Gamou, T.; Saito, M.; Iwamatsu, A.; Sakai, R. Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene 2002, 21, 5823–5834. [Google Scholar] [CrossRef]
- Okubo, J.; Takita, J.; Chen, Y.; Oki, K.; Nishimura, R.; Kato, M.; Sanada, M.; Hiwatari, M.; Hayashi, Y.; Igarashi, T.; et al. Aberrant activation of ALK kinase by a novel truncated form ALK protein in neuroblastoma. Oncogene 2012, 31, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Fransson, S.; Hansson, M.; Ruuth, K.; Djos, A.; Berbegall, A.; Javanmardi, N.; Abrahamsson, J.; Palmer, R.H.; Noguera, R.; Hallberg, B.; et al. Intragenic anaplastic lymphoma kinase ( ALK ) rearrangements: Translocations as a novel mechanism of ALK activation in neuroblastoma tumors. Genes Chromosom Cancer 2015, 54, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Weiser, D.A.; Huwe, P.J.; Park, J.H.; Krytska, K.; Ryles, H.; Laudenslager, M.; Rappaport, E.F.; Wood, A.C.; McGrady, P.W.; et al. ALK Mutations Confer Differential Oncogenic Activation and Sensitivity to ALK Inhibition Therapy in Neuroblastoma. Cancer Cell 2014, 26, 682–694. [Google Scholar] [CrossRef]
- De Brouwer, S.; De Preter, K.; Kumps, C.; Zabrocki, P.; Porcu, M.; Westerhout, E.M.; Lakeman, A.; Vandesompele, J.; Hoebeeck, J.; Van Maerken, T.; et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin. Cancer Res. 2010, 16, 4353–4362. [Google Scholar] [CrossRef]
- Stalin, L.V.; Gualandi, M.; Schulte, J.H.; Renella, R.; Shakhova, O.; Mühlethaler-Mottet, A. Expression of the neuroblastoma-associated ALK-F1174L activating mutation during embryogenesis impairs the differentiation of neural crest progenitors in sympathetic ganglia. Front. Oncol. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Pamarthy, S.; Shah, A.N.; Sagar, V.; Unno, K.; Han, H.; Yang, X.J.; Costa, R.B.; Nagy, R.J.; Lanman, R.B.; et al. Anaplastic Lymphoma Kinase Mutation ( ALK F1174C) in Small Cell Carcinoma of the Prostate and Molecular Response to Alectinib. Clin. Cancer Res. 2018, 24, 2732–2739. [Google Scholar] [CrossRef]
- Martinsson, T.; Eriksson, T.; Abrahamsson, J.; Caren, H.; Hansson, M.; Kogner, P.; Kamaraj, S.; Schönherr, C.; Weinmar, J.; Ruuth, K.; et al. Appearance of the novel activating F1174S ALK mutation in neuroblastoma correlates with aggressive tumor progression and unresponsiveness to therapy. Cancer Res. 2011, 71, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mazot, P.; Cazes, A.; Boutterin, M.C.; Figueiredo, A.; Raynal, V.; Combaret, V.; Hallberg, B.; Palmer, R.H.; Delattre, O.; Janoueix-Lerosey, I.; et al. The constitutive activity of the ALK mutated at positions F1174 or R1275 impairs receptor trafficking. Oncogene 2011, 30, 2017–2025. [Google Scholar] [CrossRef]
- Ai, X.; Niu, X.; Chang, L.; Chen, R.; Ou, S.H.I.; Lu, S. Next generation sequencing reveals a novel ALK G1128A mutation resistant to crizotinib in an ALK-Rearranged NSCLC patient. Lung Cancer 2018, 123, 83–86. [Google Scholar] [CrossRef]
- Alam, M.W.; Borenäs, M.; Lind, D.E.; Cervantes-Madrid, D.; Umapathy, G.; Palmer, R.H.; Hallberg, B. Alectinib, an Anaplastic Lymphoma Kinase Inhibitor, Abolishes ALK Activity and Growth in ALK-Positive Neuroblastoma Cells. Front. Oncol. 2019, 9, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Carén, H.; Abel, F.; Kogner, P.; Martinsson, T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem. J. 2008, 416, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Yamazaki, Y.; Chand, D.; Van Dijk, J.R.; Ruuth, K.; Palmer, R.H.; Hallberg, B. Novel mechanisms of ALK activation revealed by analysis of the Y1278S neuroblastoma mutation. Cancers 2017, 9, 149. [Google Scholar] [CrossRef]
- Schönherr, C.; Ruuth, K.; Eriksson, T.; Yamazaki, Y.; Ottmann, C.; Combaret, V.; Vigny, M.; Kamaraj, S.; Palmer, R.H.; Hallberg, B. The Neuroblastoma ALK(I1250T) Mutation Is a Kinase-Dead RTK In Vitro and In Vivo. Transl. Oncol. 2011, 4, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Yamazaki, Y.; Ruuth, K.; Schonherr, C.; Martinsson, T.; Kogner, P.; Attiyeh, E.F.; Maris, J.; Morozova, O.; Marra, M.A.; et al. Cell culture and Drosophila model systems define three classes of anaplastic lymphoma kinase mutations in neuroblastoma. Dis. Models Mech. 2013, 6, 373–382. [Google Scholar] [CrossRef]
- Kimura, S.; Hasegawa, D.; Yoshimoto, Y.; Seki, M.; Daida, A.; Sekiguchi, M.; Hirabayashi, S.; Hosoya, Y.; Kobayashi, M.; Miyano, S.; et al. Duplication of ALK F1245 missense mutation due to acquired uniparental disomy associated with aggressive progression in a patient with relapsed neuroblastoma. Oncol. Lett. 2019, 17, 3323–3329. [Google Scholar] [CrossRef]
- Cazes, A.; Lopez-Delisle, L.; Tsarovina, K.; Pierre-Eugène, C.; De Preter, K.; Peuchmaur, M.; Nicolas, A.; Provost, C.; Louis-Brennetot, C.; Daveau, R.; et al. Activated Alk triggers prolonged neurogenesis and Ret upregulation providing a therapeutic target in ALK-mutated neuroblastoma. Oncotarget 2014, 5, 2688–2702. [Google Scholar] [CrossRef]
- Ono, S.; Saito, T.; Terui, K.; Yoshida, H.; Enomoto, H. Generation of conditional ALK F1174L mutant mouse models for the study of neuroblastoma pathogenesis. Genesis 2019, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Ribeiro, D.; Arsenian-Henriksson, M.; Deller, T.; Rohrer, H. Proliferation and survival of embryonic sympathetic neuroblasts by MYCN and activated ALK signaling. J. Neurosci. 2016, 36, 10425–10439. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Nafady, A.; Takatori, A.; Kishida, S.; Ohira, M.; Suenaga, Y.; Hossain, S.; Akter, J.; Ogura, A.; Nakamura, Y.; et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci. Rep. 2013, 3, 3450. [Google Scholar] [CrossRef] [PubMed]
- Montavon, G.; Jauquier, N.; Coulon, A.; Peuchmaur, M.; Flahaut, M.; Bourloud, K.B.; Yan, P.; Delattre, O.; Sommer, L.; Joseph, J.-M.; et al. Wild-type ALK and activating ALK-R1275Q and ALK-F1174L mutations upregulate Myc and initiate tumor formation in murine neural crest progenitor cells. Oncotarget 2014, 5, 4452–4466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lee, J.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK Collaborates with MYCN in Neuroblastoma Pathogenesis. Cancer Cell 2012, 21, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Lu, M.-Y.; Yang, Y.-L.; Chou, S.-W.; Lin, D.-T.; Lin, K.-H.; Hsu, W.-M.; Jeng, Y.-M.; Jou, S.-T. The prognostic roles of and correlation between ALK and MYCN protein expression in neuroblastoma. J. Clin. Pathol. 2020, 73, 154–161. [Google Scholar] [CrossRef]
- Khudyakov, J.; Bronner-Fraser, M. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev. Dyn. 2009, 238, 716–723. [Google Scholar] [CrossRef]
- Knoepfler, P.S.; Cheng, P.F.; Eisenman, R.N. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002, 16, 2699–2712. [Google Scholar] [CrossRef]
- Kerosuo, L.; Neppala, P.; Hsin, J.; Mohlin, S.; Vieceli, F.M.; Török, Z.; Laine, A.; Westermarck, J.; Bronner, M.E. Enhanced expression of MycN/CIP2A drives neural crest toward a neural stem cell-like fate: Implications for priming of neuroblastoma. Proc. Natl. Acad. Sci. USA 2018, 115, E7351–E7360. [Google Scholar] [CrossRef]
- Bagci, O.; Tumer, S.; Olgun, N.; Altungoz, O. Copy number status and mutation analyses of anaplastic lymphoma kinase (ALK) gene in 90 sporadic neuroblastoma tumors. Cancer Lett. 2012, 317, 72–77. [Google Scholar] [CrossRef]
- Umapathy, G.; El Wakil, A.; Witek, B.; Chesler, L.; Danielson, L.; Deng, X.; Gray, N.S.; Johansson, M.; Kvarnbrink, S.; Ruuth, K.; et al. The kinase ALK stimulates the kinase ERK5 to promote the expression of the oncogene MYCN in neuroblastoma. Sci. Signal. 2014, 7, ra102. [Google Scholar] [CrossRef]
- Javanmardi, N.; Fransson, S.; Djos, A.; Umapathy, G.; Östensson, M.; Milosevic, J.; Borenäs, M.; Hallberg, B.; Kogner, P.; Martinsson, T.; et al. Analysis of ALK, MYCN, and the ALK ligand ALKAL2 (FAM150B/AUGα) in neuroblastoma patient samples with chromosome arm 2p rearrangements. Genes Chromosom Cancer 2020, 59, 50–57. [Google Scholar] [CrossRef]
- Borenäs, M.; Umapathy, G.; Lai, W.; Lind, D.E.; Witek, B.; Guan, J.; Mendoza-Garcia, P.; Masudi, T.; Claeys, A.; Chuang, T.; et al. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021, 40, 1–21. [Google Scholar] [CrossRef]
- Degoutin, J.; Vigny, M.; Gouzi, J.Y. ALK activation induces Shc and FRS2 recruitment: Signaling and phenotypic outcomes in PC12 cells differentiation. FEBS Lett. 2007, 581, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Emdal, K.B.; Pedersen, A.; Bekker-Jensen, D.B.; Lundby, A.; Claeys, S.; De Preter, K.; Speleman, F.; Francavilla, C.; Olsen, J.V. Integrated proximal proteomics reveals IRS2 as a determinant of cell survival in ALK-driven neuroblastoma. Sci. Signal. 2018, 11, eaap9752. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Ouyang, T.; Miething, C.; Morris, S.W.; Peschel, C.; Duyster, J. Nucleophosmin–anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 2000, 96, 4319–4327. [Google Scholar] [CrossRef]
- Dinsmore, C.; Dinsmore, C.J.; Soriano, P. MAPK and PI3K Signaling: At the Crossroads of Neural Crest Development MAPK and PI3K signaling: At the crossroads of neural crest development. Dev. Biol. 2020, 444, S79–S97. [Google Scholar] [CrossRef]
- Gu, T.L.; Tothova, Z.; Scheijen, B.; Griffin, J.D.; Gilliland, D.G.; Sternberg, D.W. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood 2004, 103, 4622–4629. [Google Scholar] [CrossRef] [PubMed]
- Santo, E.E.; Stroeken, P.; Sluis, P.V.; Koster, J.; Versteeg, R.; Westerhout, E.M. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013, 73, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.R.P.; Hwang, S.R.; Basrur, V.; Conlon, K.P.; Fermin, D.; Wey, E.; Murga-Zamalloa, C.; Zeng, Z.; Zu, Y.; Elenitoba-Johnson, K.S.J.; et al. NPM-ALK signals through glycogen synthase kinase 3β to promote oncogenesis. Oncogene 2012, 31, 3733–3740. [Google Scholar] [CrossRef][Green Version]
- Prasad, M.S.; Charney, R.M.; García-Castro, M.I. Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis 2019, 57, e23276. [Google Scholar] [CrossRef]
- Kuo, A.H.; Stoica, G.E.; Riegel, A.T.; Wellstein, A. Recruitment of insulin receptor substrate-1 and activation of NF-κB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene 2007, 26, 859–869. [Google Scholar] [CrossRef]
- Bai, R.; Dieter, P.; Peschel, C.; Morris, S.W.; Duyster, J. Nucleophosmin-Anaplastic Lymphoma Kinase of Large-Cell Anaplastic Lymphoma Is a Constitutively Active Tyrosine Kinase That Utilizes Phospholipase C-γ To Mediate Its Mitogenicity. Mol. Cell Biol. 1998, 18, 6951–6961. [Google Scholar] [CrossRef] [PubMed]
- Riera, L.; Lasorsa, E.; Ambrogio, C.; Surrenti, N.; Voena, C.; Chiarle, R. Involvement of Grb2 adaptor protein in nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)-mediated signaling and anaplastic large cell lymphoma growth. J. Biol. Chem. 2010, 285, 26441–26450. [Google Scholar] [CrossRef] [PubMed]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Daage, L.C.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef]
- Zamo, A.; Chiarle, R.; Piva, R.; Howes, J.; Fan, Y.; Chilosi, M.; Levy, D.E.; Inghirami, G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raghunath, P.N.; Xue, L.; Majewski, M.; Carpentieri, D.F.; Odum, N.; Morris, S.; Skorski, T.; Wasik, M.A. Multilevel Dysregulation of STAT3 Activation in Anaplastic Lymphoma Kinase-Positive T/Null-Cell Lymphoma. J. Immunol. 2002, 168, 466–474. [Google Scholar] [CrossRef]
- Sattu, K.; Hochgräfe, F.; Wu, J.; Umapathy, G.; Schönherr, C.; Ruuth, K.; Chand, D.; Witek, B.; Fuchs, J.; Li, P.; et al. Phosphoproteomic analysis of anaplastic lymphoma kinase (ALK) downstream signaling pathways identifies signal transducer and activator of transcription 3 as a functional target of activated ALK in neuroblastoma cells. FEBS J. 2013, 280, 5269–5282. [Google Scholar] [CrossRef]
- Seo, M.; Kim, J.; Suk, K. Role of the p55-gamma subunit of PI3K in ALK-induced cell migration: RNAi-based selection of cell migration regulators. Cell Adhes Migr. 2017, 11, 205–210. [Google Scholar] [CrossRef][Green Version]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef]
- Le Douarin, N.; Kalcheim, C. The Neural Crest; Cambridge University Press: Cambridge, UK, 1999; ISBN 9780521620109. [Google Scholar] [CrossRef]
- Lumb, R.; Schwarz, Q. Sympathoadrenal neural crest cells: The known, unknown and forgotten? Dev. Growth Differ. 2015, 57, 146–157. [Google Scholar] [CrossRef]
- Anderson, D.; Carnahan, J.; Michelsohn, A.; Patterson, P. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 1991, 11, 3507–3519. [Google Scholar] [CrossRef]
- Anderson, D.J.; Axel, R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell 1986, 47, 1079–1090. [Google Scholar] [CrossRef]
- Huber, K. The sympathoadrenal cell lineage: Specification, diversification, and new perspectives. Dev. Biol. 2006, 298, 335–343. [Google Scholar] [CrossRef]
- Shtukmaster, S.; Schier, M.C.; Huber, K.; Krispin, S.; Kalcheim, C.; Unsicker, K. Sympathetic neurons and chromaffin cells share a common progenitor in the neural crest in vivo. Neural Dev. 2013, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Kruepunga, N.; Hikspoors, J.P.J.M.; Hülsman, C.J.M.; Mommen, G.M.C.; Köhler, S.E.; Lamers, W.H. Development of the sympathetic trunks in human embryos. J. Anat. 2021, 239, 32–45. [Google Scholar] [CrossRef]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Adameyko, I. Schwann cell precursor: A neural crest cell in disguise? Dev. Biol. 2018, 444, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Mus, L.M.; Lambertz, I.; Claeys, S.; Kumps, C.; Van Loocke, W.; Van Neste, C.; Umapathy, G.; Vaapil, M.; Bartenhagen, C.; Laureys, G.; et al. The ETS transcription factor ETV5 is a target of activated ALK in neuroblastoma contributing to increased tumour aggressiveness. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Delloye-Bourgeois, C.; Bertin, L.; Thoinet, K.; Jarrosson, L.; Kindbeiter, K.; Buffet, T.; Tauszig-Delamasure, S.; Bozon, M.; Marabelle, A.; Combaret, V.; et al. Microenvironment-Driven Shift of Cohesion/Detachment Balance within Tumors Induces a Switch toward Metastasis in Neuroblastoma. Cancer Cell 2017, 32, 427–443.e8. [Google Scholar] [CrossRef] [PubMed]
- Siaw, J.T.; Javanmardi, N.; Van den Eynden, J.; Lind, D.E.; Fransson, S.; Martinez-Monleon, A.; Djos, A.; Sjöberg, R.-M.; Östensson, M.; Carén, H.; et al. 11q Deletion or ALK Activity Curbs DLG2 Expression to Maintain an Undifferentiated State in Neuroblastoma. Cell Rep. 2020, 32, 108171. [Google Scholar] [CrossRef]
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, Prognosis, and Treatment. Hematol. Oncol. Clin. North Am. 2010, 24, 65–86. [Google Scholar] [CrossRef]
- Wang, L.L.; Suganuma, R.; Ikegaki, N.; Tang, X.; Naranjo, A.; McGrady, P.; London, W.B.; Hogarty, M.D.; Gastier-Foster, J.M.; Look, A.T.; et al. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: A report from the Children’s Oncology Group. Cancer 2013, 119, 3718–3726. [Google Scholar] [CrossRef]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Pan, P.; Sun, H.; Xia, H.; Wang, X.; Li, Y.; Hou, T. Drug Discovery Targeting Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2019, 62, 10927–10954. [Google Scholar] [CrossRef] [PubMed]
- Schleiermacher, G.; Javanmardi, N.; Bernard, V.; Leroy, Q.; Cappo, J.; Frio, T.R.; Pierron, G.; Lapouble, E.; Combaret, V.; Speleman, F.; et al. Emergence of new ALK mutations at relapse of neuroblastoma. J. Clin. Oncol. 2014, 32, 2727–2734. [Google Scholar] [CrossRef]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Simkin, J.E.; Zhang, D.; Rollo, B.N.; Newgreen, D.F. Retinoic Acid Upregulates Ret and Induces Chain Migration and Population Expansion in Vagal Neural Crest Cells to Colonise the Embryonic Gut. Key B, ed. PLoS ONE 2013, 8, e64077. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, I.; Kumps, C.; Claeys, S.; Lindner, S.; Beckers, A.; Janssens, E.; Carter, D.R.; Cazes, A.; Cheung, B.B.; De Mariano, M.; et al. Upregulation of MAPK Negative Feedback Regulators and RET in Mutant ALK Neuroblastoma: Implications for Targeted Treatment. Clin. Cancer Res. 2015, 21, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Delisle, L.; Pierre-Eugène, C.; Louis-Brennetot, C.; Surdez, D.; Raynal, V.; Baulande, S.; Boeva, V.; Grossetête-Lalami, S.; Combaret, V.; Peuchmaur, M.; et al. Activated ALK signals through the ERK-ETV5-RET pathway to drive neuroblastoma oncogenesis. Oncogene 2018, 37, 1417–1429. [Google Scholar] [CrossRef]
- Umapathy, G.; Mendoza-Garcia, P.; Hallberg, B.; Palmer, R.H. Targeting anaplastic lymphoma kinase in neuroblastoma. Apmis 2019, 127, 288–302. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.S.; Clarke, S.; Veschi, V.; Thiele, C.J. Targeting MYCN in Pediatric and Adult Cancers. Front. Oncol. 2021, 10, 623679. [Google Scholar] [CrossRef] [PubMed]
- Knoepfler, P.S.; Kenney, A.M. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle 2006, 5, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Finkelman, H.; Martinez, A. GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Front. Mol. Neurosci. 2011, 4, 1–18. [Google Scholar] [CrossRef]
- Aboualizadeh, F.; Yao, Z.; Guan, J.; Drecun, L.; Pathmanathan, S.; Snider, J.; Umapathy, G.; Kotlyar, M.; Jurisica, I.; Palmer, R.; et al. Mapping the phospho-dependent ALK interactome to identify novel components in ALK signaling. J. Mol. Biol. 2021, 433, 167283. [Google Scholar] [CrossRef] [PubMed]
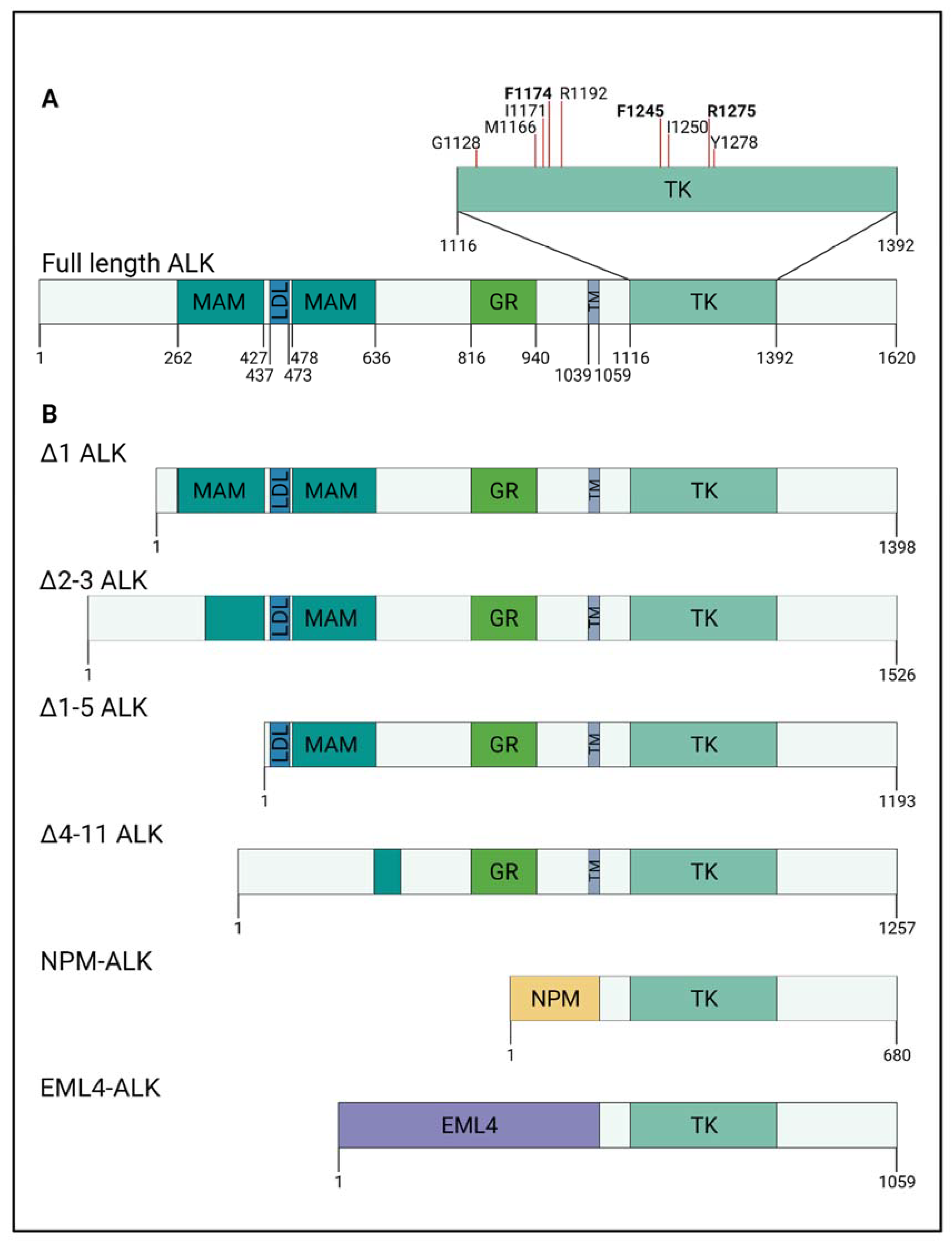
| Alteration | Affected Domain | Note | Reference | |
|---|---|---|---|---|
| Amplification | 2p23 | Full-length ALK | Ligand-dependent | [14] |
| Translocation/amplification | Δ1 | Extracellular N-terminal | Translocation to 11q14 | [75] |
| Δ2–3 | Extracellular N-terminal | Enhanced kinase activity | [74] | |
| Δ1–5 | Extracellular N-terminal | Translocation to 4q35 or 2p24 | [75] | |
| Δ4–11 | MAM and LDL domain loss | Ligand-independent kinase activity | [72,75] | |
| 3–4 exon | Extracellular N-terminal | Translocation to 2p16–2p14 region | [75] |
| Type | Mutation Site | Domain | Note | Reference | |
|---|---|---|---|---|---|
| unknown | p.(K1062M) | AAG > ATG | Kinase domain (Juxtamembrane) | Tumorigenesis in mice | [14] |
| Germline | p.(T1087I) | ACC > ATC | Kinase domain (Juxtamembrane) | unknown | [14] |
| Germline | p.(G1128A) | GGG > GCG | Kinase domain (P-loop, glycine loop) | Ligand-independent kinase activity | [8,82,83] |
| Somatic | p.(M1166R) | ATG > AGG | Kinase domain (αC helix) | Ligand-independent kinase activity | [8,83] |
| Somatic | p.(I1171N) | ATC > ACC | Kinase domain (αC helix) | Ligand-independent kinase activity | [8,83] |
| Somatic | p.(F1174I) | TCC > ATC | Kinase domain (αC helix) | Ligand-independent kinase activity | [8,82] |
| Somatic | p.(F1174L) | TTC > TTA TTC > TTG TTC > CTC | Kinase domain (αC helix) | Ligand-independent kinase activity | [4,8,13,82] |
| Somatic | p.(F1174C) | TTC > TGC | Kinase domain (αC helix) | Ligand-independent kinase activity | [4,14,79] |
| unknown | p.(F1174S) | TTC > TCC | Kinase domain (αC helix) | Ligand-independent kinase activity | [80,86,87] |
| Somatic | p.(F1174V) | TTC > GTC | Kinase domain (αC helix) | Ligand-independent kinase activity | [4,14,81] |
| Germline | p.(R1192P) | CGG > CCG | Kinase domain (β4 strand) | Ligand-independent kinase activity | [4,8,83] |
| Somatic | p.(F1245C) | TTC > TGC | Kinase domain (catalytic loop) | Ligand-independent kinase activity | [8,13,83] |
| Somatic | p.(F1245L) | TTC > TTG | Kinase domain (catalytic loop) | Ligand-independent kinase activity | [14,76,84,88] |
| Somatic | p.(F1245V) | TTC > GTC | Kinase domain (catalytic loop) | Ligand-independent kinase activity | [8,13,76] |
| Somatic | p.(I1250T) | ATT > ACT | Kinase domain (catalytic loop) | Kinase dead mutation | [8,86] |
| Somatic/Germline | p.(R1275Q) | CGA > CAA | Kinase domain (activation loop) | Ligand-independent kinase activity | [4,8,13,14] |
| Somatic | p.(Y1278S) | TAC > TCC | Kinase domain (activation loop) | Ligand-independent kinase activity | [4,83,85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulf, A.M.; Moreno, M.M.; Paka, C.; Rampasekova, A.; Liu, K.J. Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer. Int. J. Mol. Sci. 2021, 22, 11718. https://doi.org/10.3390/ijms222111718
Wulf AM, Moreno MM, Paka C, Rampasekova A, Liu KJ. Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer. International Journal of Molecular Sciences. 2021; 22(21):11718. https://doi.org/10.3390/ijms222111718
Chicago/Turabian StyleWulf, Anna M., Marcela M. Moreno, Chloé Paka, Alexandra Rampasekova, and Karen J. Liu. 2021. "Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer" International Journal of Molecular Sciences 22, no. 21: 11718. https://doi.org/10.3390/ijms222111718
APA StyleWulf, A. M., Moreno, M. M., Paka, C., Rampasekova, A., & Liu, K. J. (2021). Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer. International Journal of Molecular Sciences, 22(21), 11718. https://doi.org/10.3390/ijms222111718






