Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy
Abstract
:1. Introduction
2. Results
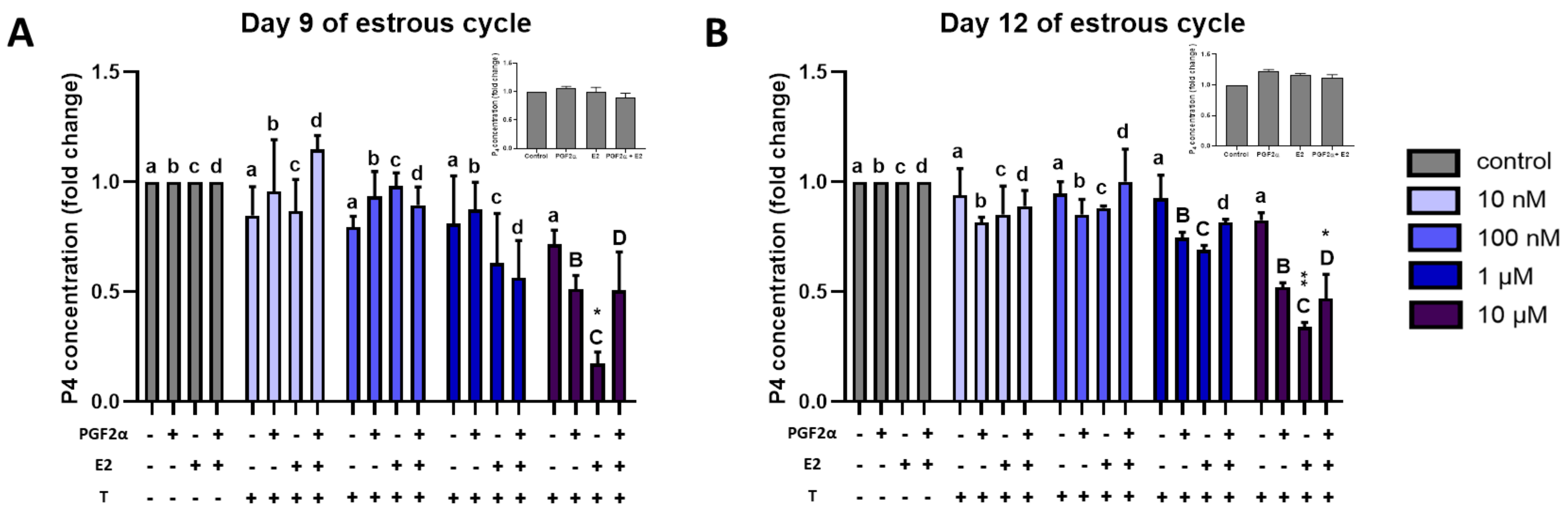
2.1. Progesterone Concentrations
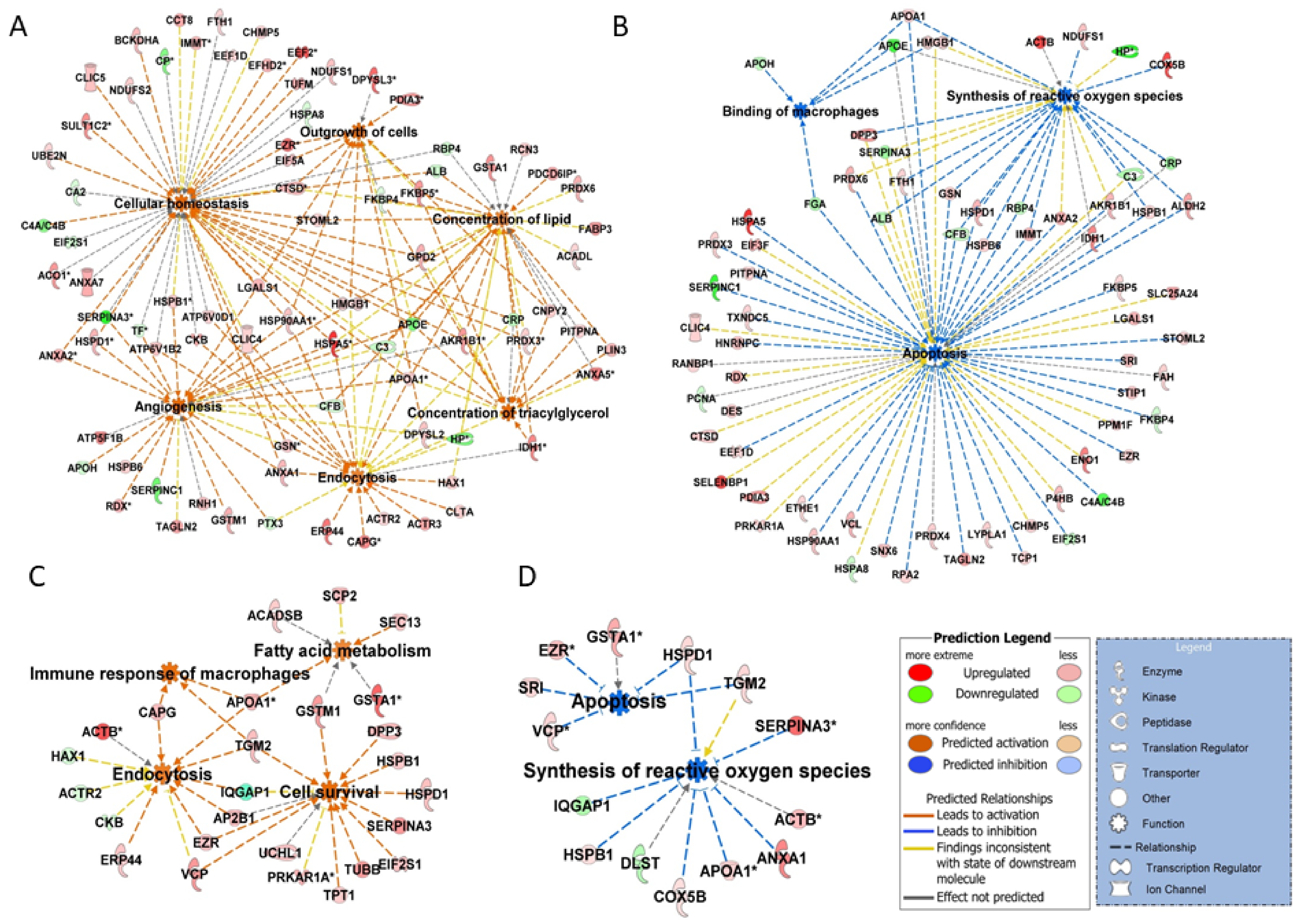
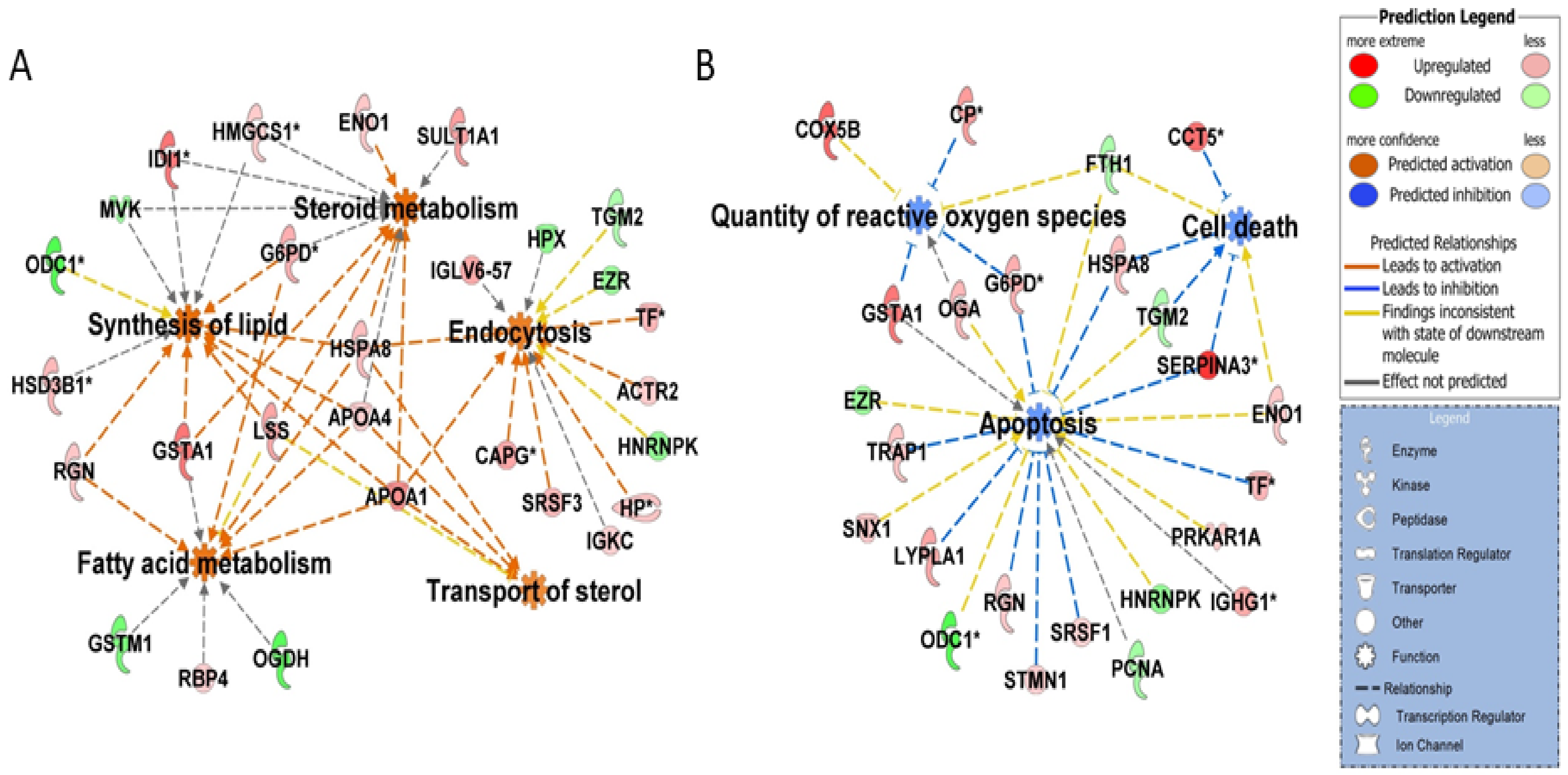
2.2. Proteomic Changes Associated with Growth and Development of CL on Days 3, 9, and 12 of the Estrous Cycle
2.3. Proteomic Changes Associated with Regression of CL on Day 15 of the Estrous Cycle
2.4. Proteomic Changes Associated with Rescue of CL Function on Day 15 of Pregnancy
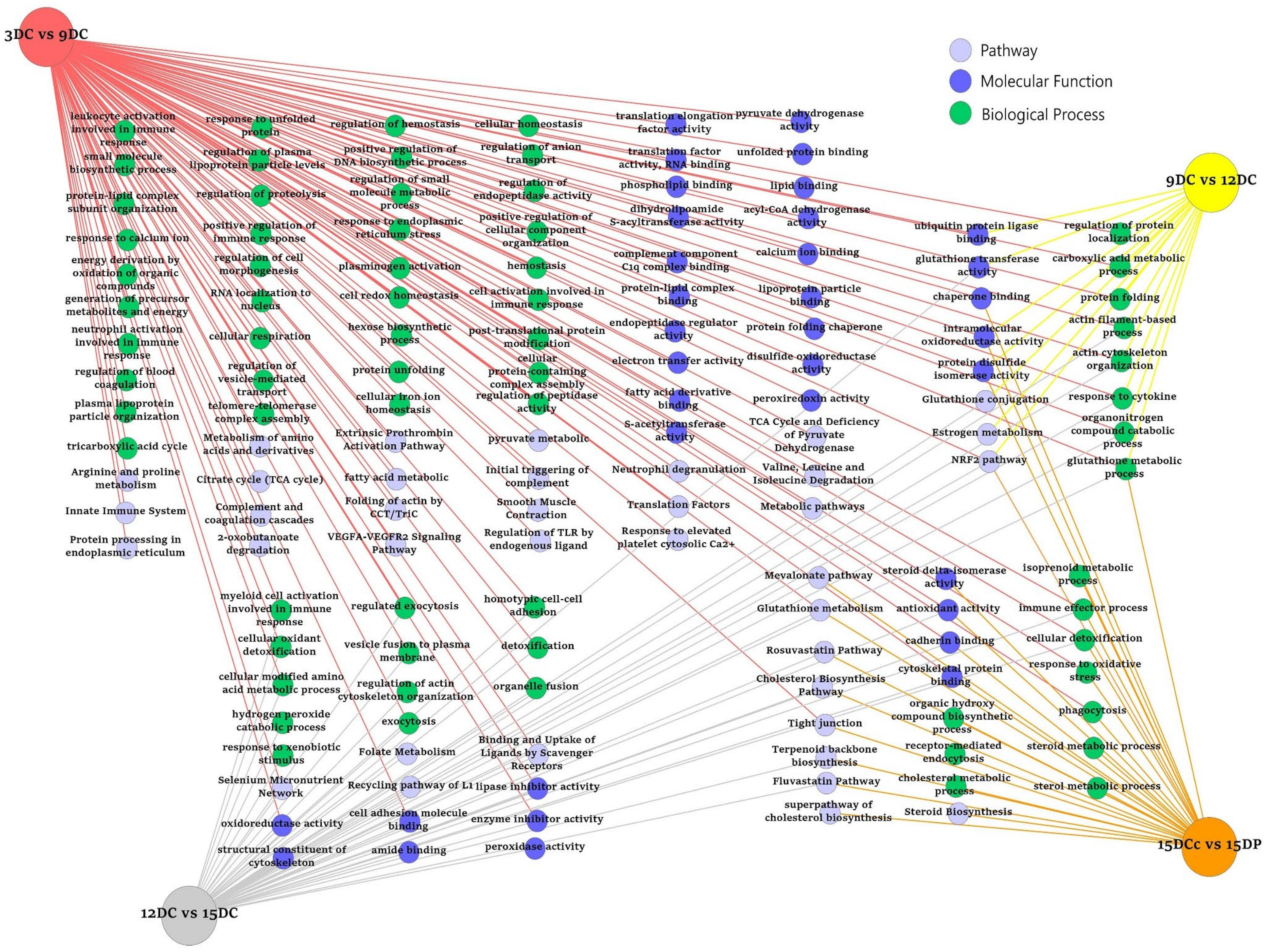
2.5. ToppCluster Analysis
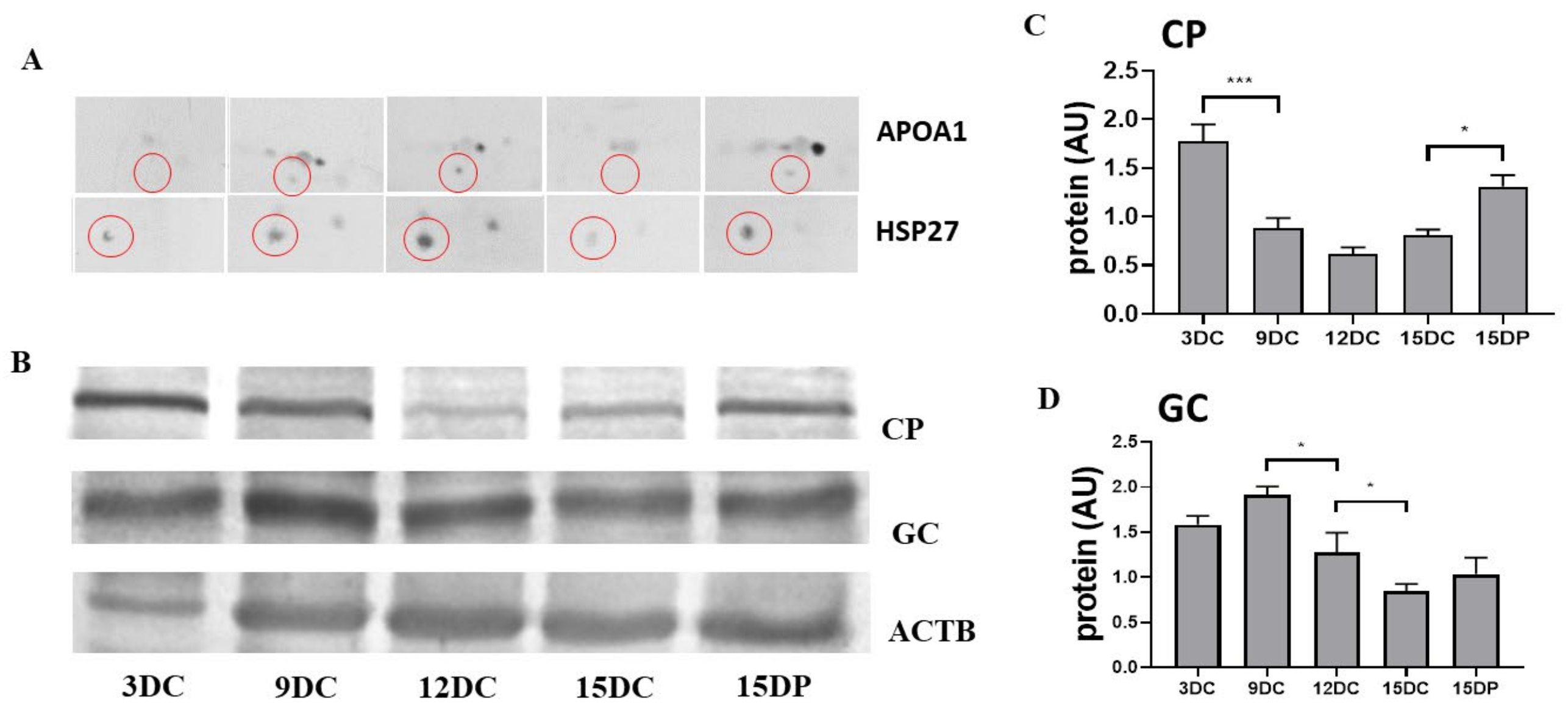
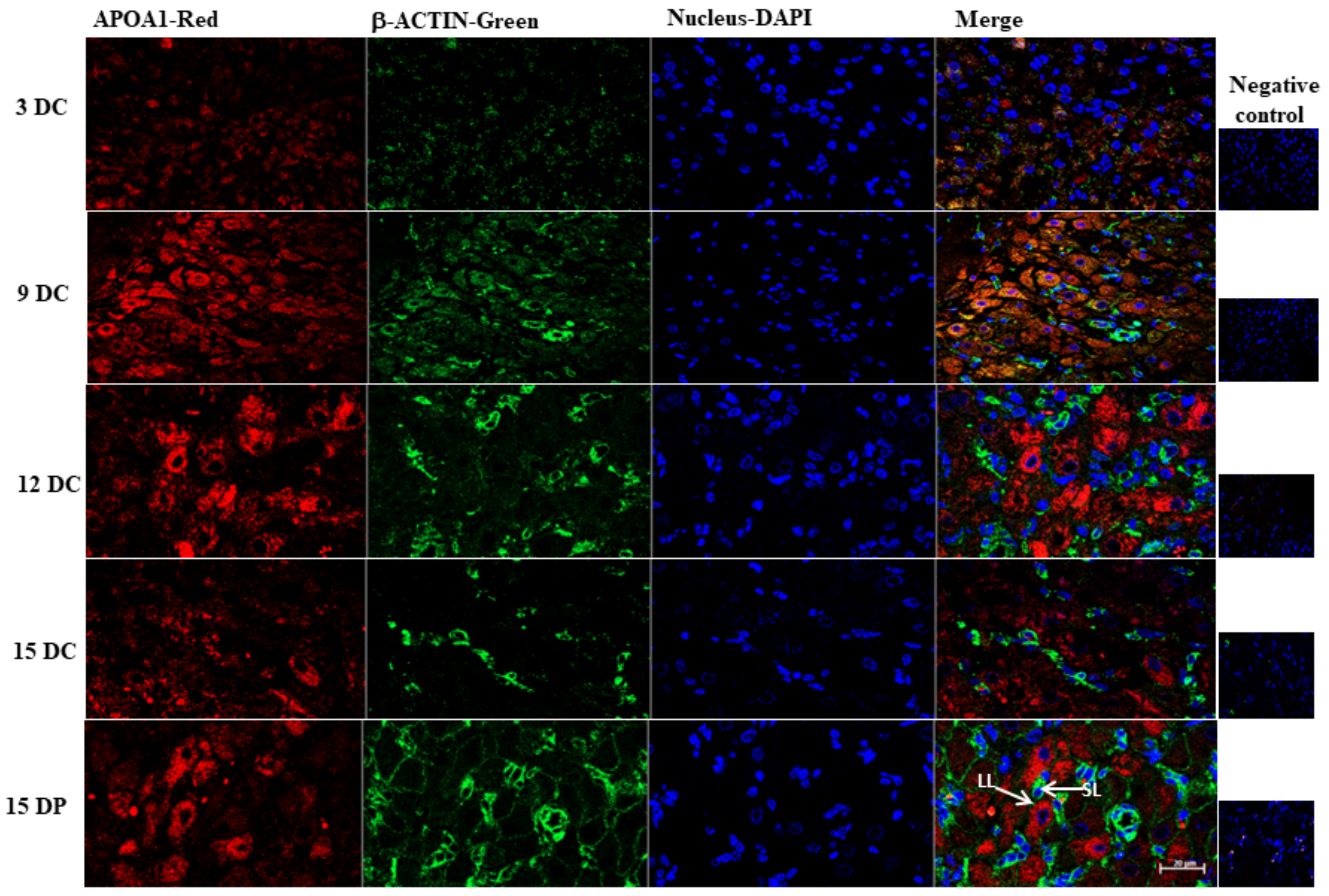
2.6. Validation of 2DE Results
2.7. Effect of Triclosan on P4 Secretion by Luteal Explants
3. Discussion
3.1. Steroid Biosynthesis
3.2. RhoA Signaling
3.3. Estrogen Metabolism
3.4. Oxidation Stress
3.5. Cellular Homeostasis and Apoptosis
3.6. Angiogenesis
4. Materials and Methods
4.1. Animals
4.2. Blood and Corpus Luteum Collection
4.3. In Vitro Study
4.4. Steroid Hormone Assays
4.5. Protein Extraction
4.6. Two-Dimensional Gel Electrophoresis (2DE)
4.7. Image and Data Analyses
4.8. Excision of 2D-Gel Spots, Tryptic Digestion, and MALDI-TOF/TOF Analysis
4.9. In Silico Functional Analysis
4.10. Western Blot
4.11. Immunofluorescence
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziecik, A.J.; Przygrodzka, E.; Moza Jalali, B.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, R57–R67. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Csapo, A.I.; Pulkkinen, M.O.; Ruttner, B.; Sauvage, J.P.; Wiest, W.G. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am. J. Obstet. Gynecol. 1972, 112, 1061–1067. [Google Scholar] [CrossRef]
- Murdoch, W.J.; Van Kirk, E.A. Luteal dysfunction in ewes induced to ovulate early in the follicular phase. Endocrinology 1998, 139, 3480–3484. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, F.; Noble, L.S. Endocrinology of recurrent pregnancy loss. Semin. Reprod. Med. 2006, 24, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Morris, D.G. Embryonic and early fetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.; Bouwman, E.G.; Chen, T.Y.; Koopmanschap, R.E.; Soede, N.M. Temporary undernutrition during early gestation, corpora lutea morphometrics, ovarian progesterone secretion and embryo survival in gilts. Reprod. Fertil. Dev. 2017, 29, 1349. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.A. Lipid metabolism and in vitro production of progesterone and prostaglandin F during induced regression of porcine corpora lutea. Prostaglandins 1980, 20, 73–85. [Google Scholar] [CrossRef]
- Hughes, C.H.K.; Bosviel, R.; Newman, J.W.; Pate, J.L. Luteal lipids regulate progesterone production and may modulate immune cell function during the estrous cycle and pregnancy. Front. Endocrinol. 2019, 10, 662. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Kaczmarek, M.M.; Kaczynski, P.; Ziecik, A.J. Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction 2016, 151, 135–147. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel insights on the corpus luteum function: Role of vaspin on porcine luteal cell angiogenesis, proliferation and apoptosis by activation of GRP78 receptor and MAP3/1 kinase pathways. Int. J. Mol. Sci. 2020, 21, 6823. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Witek, K.J.; Kaczmarek, M.M.; Andronowska, A.; Ziecik, A.J. Expression of factors associated with apoptosis in the porcine corpus luteum throughout the luteal phase of the estrous cycle and early pregnancy: Their possible involvement in acquisition of luteolytic sensitivity. Theriogenology 2015, 83, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, L.M.; D’Annibale, M.A.; Swing, S.E.; Gadsby, J.E. Expression of genes associated with apoptosis in the porcine corpus luteum during the oestrous cycle. Reprod. Domest. Anim. 2013, 48, 755–761. [Google Scholar] [CrossRef]
- Sugino, N.; Okuda, K. Species-related differences in the mechanism of apoptosis during structural luteolysis. J. Reprod. Dev. 2007, 53, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Pitzel, L.; Ludemann, S.; Wuttke, W. Secretion and gene expression of metalloproteinases and gene expression of their inhibitors in porcine corpora lutea at different stages of the luteal phase. Biol. Reprod. 2000, 62, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuttke, W.; Pitzel, L.; Knoke, I.; Theiling, K.; Jarry, H. Immune-endocrine interactions affecting luteal function in pigs. J. Reprod. Fertil. Suppl. 1997, 52, 19–29. [Google Scholar] [PubMed]
- Zmijewska, A.; Franczak, A.; Kotwica, G. Role of interleukin-1β in the regulation of porcine corpora lutea during the late luteal phase of the cycle and during pregnancy. Acta. Vet. Hung. 2012, 60, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Hehnke, K.E.; Christenson, L.K.; Ford, S.P.; Taylor, M. Macrophage infiltration into the porcine corpus luteum during prostaglandin F2α-induced luteolysis. Biol. Reprod. 1994, 50, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehnke-Vagnoni, K.E.; Clark, C.L.; Taylor, M.J.; Ford, S.P. Presence and localization of tumor necrosis factor α in the corpus luteum of nonpregnant and pregnant pigs. Biol. Reprod. 1995, 53, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Sakumoto, R.; Sakabe, Y.; Miyake, M.; Okano, A.; Okuda, K. Tumour necrosis factor-α receptors are present in the corpus luteum throughout the oestrous cycle and during the early gestation period in pigs. Reprod. Dom. Anim. 2002, 37, 105–110. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wiltbank, M.C. Acquisition of luteolytic capacity: Changes in prostaglandin F2a regulation of steroid hormone receptors and estradiol biosynthesis in pig corpora lutea. Biol. Reprod. 2004, 70, 1333–1339. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W.W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins 1977, 14, 397–400. [Google Scholar] [CrossRef]
- Likszo, P.; Skarzynski, D.J.; Moza Jalali, B. Proteomic analysis of porcine preovulatory follicle differentiation into corpus luteum. Front. Endocrinol. 2019, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Arianmanesh, M.; McIntosh, R.; Lea, R.G.; Fowler, P.A.; Al-Gubory, K.H. Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. J. Endocrinol. 2011, 210, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, K.W.; Han, D.W.; Lee, H.C.; Yang, B.C.; Chung, H.K.; Shim, M.R.; Choi, M.S.; Jo, E.B.; Jo, Y.M.; et al. Protein profile in corpus luteum during pregnancy in Korean native cows. Asian-Australas. J. Anim. Sci. 2012, 25, 1540–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Fernandez, R.; Martinez-Galisteo, E.; Gaytan, F.; Barcena, J.A.; Snchez-Criado, J.E. Changes in the proteome of functional and regressing corpus luteum during pregnancy and lactation in the rat. Biol. Reprod. 2008, 79, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Christenson, L.K.; Devoto, L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrifield, C.J.; Qualmann, B.; Kessels, M.M.; Almers, W. Neural wiskott aldrich syndrome protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 2004, 83, 13–18. [Google Scholar] [CrossRef]
- Yarar, D.; Waterman-Storer, C.M.; Schmid, S.L. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell. 2005, 16, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.M.; Raimondi, A.; Paradise, S.; Shen, H.; Mesaki, K.; Ferguson, A.; Destaing, O.; Ko, G.; Takasaki, J.; Cremona, O.; et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell. 2009, 17, 811–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannian, J.D.; Christianson, H.; Flynn, S.; Kurz, S.G. Loss of low-density lipoprotein utilization by regressing porcine luteal cells: Effects of protein kinase C activation. Biol. Reprod. 1995, 52, 793–797. [Google Scholar] [CrossRef] [Green Version]
- Ndikum-Moffor, F.M.; Simmen, R.C.M.; Fields, P.A.; Katoh, N.; Oikawa, S.; Buhi, W.C.; Rollyson, M.K.; Chang, S.T.; Fields, M.J. Synthesis and messenger ribonucleic acid expression of polipoproteins E and A-I by the bovine corpus luteum during the estrous cycle and pregnancy. Biol. Reprod. 1997, 56, 745–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seedorf, U.; Ellinghaus, P.; Roch Nofer, J. Sterol carrier protein-2. Biochim. Biophys. Acta 2000, 1486, 45–54. [Google Scholar] [CrossRef]
- McLean, M.P.; Puryear, T.K.; Khan, I.; Azhar, S.; Billheimer, J.T.; Orly, J.; Gibori, G. Estradiol regulation of sterol carrier protein-2 independent of cytochrome P450 side-chain cleavage expression in the rat corpus luteum. Endocrinology 1989, 125, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.M.; Woodford, J.K.; Moncecchi, D.; Myers-Payne, S.C.; McLean, L.R.; Billheimer, J.T.; Schroeder, F. Cholesterol interaction with recombinant human sterol carrier protein-2. Lipids 1995, 30, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Horsch, M.; Kastenmüller, G.; Möller, G.; Kumar, P.; Prehn, C.; Laumen, H.; Hauner, H.; Hrabĕ de Angelis, M.; Beckers, J.; et al. Metabolic switch during adipogenesis: From branched chain amino acid catabolism to lipid synthesis. Arch. Biochem. Biophys. 2016, 589, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbott, H.A.; Plewes, M.R.; Krause, C.; Hou, X.; Zhang, P.; Rizzo, W.B.; Jennifer, R.; Cupp, A.S.; Davis, J.S. Formation and characterization of lipid droplets of the bovine corpus luteum. Sci. Rep. 2020, 10, 11287. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.L.; Słomczyńska, M. The cytoskeleton proteins and LH-regulated steroidogenesis of porcine luteal cells. Folia Histochem. Cytobiol. 1996, 34, 35–39. [Google Scholar]
- Shen, W.J.; Zaidi, S.K.; Patel, S.; Cortez, Y.; Ueno, M.; Azhar, R.; Azhar, S.; Kraemer, F.B. Ablation of vimentin results in defective steroidogenesis. Endocrinology 2012, 153, 3249–3257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhang, Z.; Shen, W.J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.F.; Almahbobi, G. Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc. Res. Tech. 1997, 36, 463–479. [Google Scholar] [CrossRef]
- Sewer, M.B.; Li, D. Regulation of steroid hormone biosynthesis by the cytoskeleton. Lipids 2008, 43, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Zowalaty, A.E.; Li, R.; Zheng, Y.; Lydon, J.P.; DeMayo, F.J.; Ye, X. Deletion of RhoA in progesterone receptor-expressing cells leads to luteal insufficiency and infertility in female mice. Endocrinology 2017, 158, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Pedersen, L.G.; Petrotchenko, E.; Shevtsov, S.; Gorokhov, A.; Kakuta, Y.; Pedersen, L.C. Structure and function of sulfotransferases. Arch. Biochem. Biophys. 2001, 390, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Gardner, M.L.; First, N.L.; Casida, L.E. Effect of exogenous estrogen on corpus luteum maintenance in gilts. J. Anim. Sci. 1963, 22, 132–134. [Google Scholar] [CrossRef]
- Kaczynski, P.; Baryla, M.; Goryszewska, E.; Waclawik, A. Estradiol-17β regulates expression of luteal dna methyltransferases and genes involved in the porcine corpus luteum function in vivo. Int. J. Mol. Sci. 2021, 22, 3655. [Google Scholar] [CrossRef] [PubMed]
- Tekpetey, F.R.; Armstrong, D.T. Steroidogenic response of rat and pig luteal cells to estradiol-17b and catecholestrogens in vitro. Biol. Reprod. 1991, 45, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, J.; Hao, F.; Yuan, Y.; Li, J.-Y.; Lu, W.; Zhou, T.-Y. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta. Pharmacol. Sin. 2016, 37, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, M.O.; Li, W.; Summerlot, D.P.; Rowland-Faux, L.; Wood, C.E. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ. Int. 2010, 36, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Basini, G.; Bussolati, S.; Bertini, S.; Quintavalla, F.; Grasselli, F. Evaluation of triclosan effects on cultured swine luteal cells. Animals 2021, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Devoto, L.; Henríquez, S.; Kohen, P.; Strauss, J.F. The significance of estradiol metabolites in human corpus luteum physiology. Steroids 2017, 123, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. BioMed. 2012, 25, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J. Knockout mouse models for peroxiredoxins. Antioxidants 2020, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Park, S.J.; Koo, D.B.; Lee, S.R.; Kong, I.K.; Ryoo, J.W.; Park, Y.; Chang, K.-T.; Lee, D.-S. Progesterone production is affected by unfolded protein response (UPR) signaling during the luteal phase in mice. Life Sci. 2014, 113, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Brannian, J.; Zhao, Y.; Burbach, J.A. Localization of lipid peroxidation-derived protein epitopes in the porcine corpus luteum. Biol. Reprod. 1997, 57, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrionl. 2005, 3, 43. [Google Scholar]
- Sugino, N.; Nakamura, Y.; Takeda, O.; Ishimatsu, M.; Kato, H. Changes in activities of superoxide dismutase and lipid peroxide in corpus luteum during pregnancy in rats. J. Reprod. Fertil. 1993, 97, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, D.G.; Seong, J.B.; Lee, H.S.; Kwon, O.S.; Kang, B.S.; Park, J.W.; Lee, S.R.; Lee, D.S. Peroxiredoxin I maintains luteal function by regulating unfolded protein response. Reprod. Biol. Endocrinol. 2018, 16, 79. [Google Scholar] [CrossRef]
- Sugino, N.; Takiguchi, S.; Kashida, S.; Karube, A.; Nakamura, Y.; Kato, H. Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol. Hum. Reprod. 2000, 6, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kim, D.H.; Yang, S.G.; Kim, D.Y. Improved effect of a mitochondria-targeted antioxidant on hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells. BMC Pharmacol. Toxicol. 2021, 22, 7. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Huang, Y.; Zhang, Y.; Zeng, X.; Yan, M.; Xia, Z.; Lai, D. DPP3/CDK1 contributes to the progression of colorectal cancer through regulating cell proliferation, cell apoptosis, and cell migration. Cell Death Dis. 2021, 12, 529. [Google Scholar]
- Fujiwara, H.; Maeda, M.; Imai, K.; Fukuoka, M.; Yasuda, K.; Takakura, K.; Mori, T. Human luteal cells express dipeptidyl peptidase IV on the cell surface. J. Clin. Endocrinol. Metab. 1992, 75, 1352–1357. [Google Scholar]
- Aikawa, S.; Deng, W.; Liang, X.; Yuan, J.; Bartos, A.; Sun, X.; Dey, S. Uterine deficiency of high-mobility group box-1 (HMGB1) protein causes implantation defects and adverse pregnancy outcomes. Cell Death Differ. 2020, 27, 1489–1504. [Google Scholar] [CrossRef]
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.-J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am. J. Physiol. Cell Physiol. 2010, 299, C1267–C1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Burbach, J.A.; Roby, K.F.; Terranova, P.F.; Brannian, J.D. Macrophages are the major source of tumor necrosis factor α in the porcine corpus luteum. Biol. Reprod. 1998, 59, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kamada, D.; Shirasuna, K.; Matsui, M.; Shimizu, T.; Kida, K.; Berisha, B.; Schams, D.; Miyamoto, A. Effect of local neutralization of basic fibroblast growth factor or vascular endothelial growth factor by a specific antibody on the development of the corpus luteum in the cow. Mol. Reprod. Dev. 2008, 75, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Zalman, Y.; Klipper, E.; Farberov, S.; Mondal, M.; Wee, G.; Folger, J.K.; Smith, G.W.; Meidan, R. Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biol. Reprod. 2012, 86, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.K.; Maalouf, S.W.; Liu, W.-S.; Pate, J.L. Molecular profiling demonstrates modulation of immune cell function and matrix remodeling during luteal rescue. Biol. Reprod. 2019, 100, 581–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hajjar, K.A. The annexin A2 system and angiogenesis. Biol. Chem. 2016, 397, 1005–1016. [Google Scholar] [CrossRef]
- Sarangdhar, M.A.; Allam, R. Angiogenin (ANG)-ribonuclease inhibitor (RNH1) system in protein synthesis and disease. Int. J. Mol. Sci. 2021, 22, 1287. [Google Scholar] [CrossRef]
- Wojciak-Stothard, B.; Abdul-Salam, V.B.; Lao, K.H.; Tsang, H.; Irwin, D.C.; Lisk, C.; Loomis, Z.; Stenmark, K.R.; Edwards, J.C.; Yuspa, S.H.; et al. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation 2014, 129, 1770–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Xu, S. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sini. 2016, 48, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmeyer, H.U. Methoden der Enzymatischen Analyse; Verlag Chemie GmbH: Weinheim, Germany, 1974. [Google Scholar]
- Kaimal, V.; Bardes, E.E.; Tabar, S.C.; Jegga, A.G.; Aronow, B.J. ToppCluster: A multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic. Acids. Res. 2010, 38, W96–W102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, M.; Morawska-Pucinska, E.; Krawczynski, K.; Kiewisz, J.; Ziecik, A.J.; Blitek, A. Effect of oestrus synchronization with PGF2α/eCG/hCG on luteal P4 synthesis in early pregnant gilts. Reprod. Domest. Anim. 2014, 49, 1034–1042. [Google Scholar] [CrossRef] [PubMed]


| Top Molecular and Cellular Functions | p-Value | No. of Molocules | Canonical Pathways | p-Value | No. of Molecules | |
|---|---|---|---|---|---|---|
| 3DC vs. 9DC | Cellular Compromise | 5.36 × 104–3.99 × 1020 | 48 | Acute Phase Response Signaling | 3.41 × 1015 | 18 |
| Post-Translation Modification | 4.01 × 104–2.13 × 1017 | 28 | Xenobiotic Metabolism PXR Signaling Pathway | 2.11 × 109 | 13 | |
| Cell Death and Survival | 4.80 × 104–9.20 × 1017 | 105 | LXR/RXR Activation | 3.66 × 109 | 11 | |
| Protein Folding | 2.44 × 1016–2.44 × 1016 | 17 | RHOA Signaling | 3.54 × 108 | 10 | |
| Protein Synthesis | 4.03 × 104–9.63 × 1016 | 61 | Clathrin-mediated Endocytosis Signaling | 2.15 × 106 | 10 | |
| 9DC vs. 12DC | Cellular Funtion and Maintenance | 4.93 × 103–1.45 × 107 | 38 | NRF2-mediated Oxidative Stress Response | 4.00 × 106 | 7 |
| Cell Death and Survival | 4.83 × 103–1.83 × 107 | 34 | Akryl Hydrocarbon Receptor Signaling | 5.06 × 106 | 6 | |
| Cell-to-Cell Signaling and Interaction | 5.50 × 103–3.67 × 107 | 25 | Remodeling of Epithelial Adhrerns Junctions | 3.74 × 105 | 4 | |
| Post-Translation Modification | 2.75 × 103–1.80 × 106 | 13 | Glutathione-mediated Detoxification | 9.35 × 105 | 3 | |
| Protein Folding | 1.80 × 106–1.80 × 106 | 6 | Xenobiotic Metabolism PXR Signaling Pathway | 1.84 × 104 | 5 | |
| 12DC vs. 15DC | Cell Death and Survival | 1.64 × 103–3.81 × 1011 | 43 | Xenobiotic Metabolism PXR Signaling Pathway | 6.77 × 109 | 9 |
| Cellular Funtion and Maintenance | 1.68 × 103–1.82 × 109 | 28 | Glutathione Redox Reactions I | 1.16 × 108 | 5 | |
| Cellular Compromise | 9.49 × 104–2.57 × 108 | 23 | Remodeling of Epithelial Adhrerns Junctions | 6.15 × 108 | 6 | |
| Cell-to-Cell Signaling and Interaction | 1.39 × 103–2.72 × 108 | 26 | LPS/IL-1 Mediated Inhibition of RXR Function | 7.17 × 108 | 9 | |
| Small Molecule Biochemistry | 1.24 × 103–2.72 × 108 | 27 | Clathrin-mediated Endocytosis Signaling | 2.06 × 106 | 7 | |
| 15DC vs. 15DP | Cell Death and Survival | 7.19 × 103–7.49 × 1011 | 51 | Acute Phase Response Signaling | 6.58 × 1011 | 11 |
| Lipid Metabolism | 7.19 × 103–1.00 × 107 | 19 | LXR/RXR Activation | 1.49 × 108 | 8 | |
| Small Molecule Biochemistry | 7.19 × 103–1.00 × 107 | 42 | RHOA Signaling | 3.28 × 107 | 7 | |
| Free Radical Scavenging | 7.02 × 103–1.47 × 106 | 17 | Clathrin-mediated Endocytosis Signaling | 4.87 × 107 | 8 | |
| Protein Synthesis | 2.43 × 103–2.05 × 106 | 24 | Superwathwy of Choresterol Biosynthesis | 3.48 × 106 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likszo, P.; Skarzynski, D.J.; Moza Jalali, B. Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy. Int. J. Mol. Sci. 2021, 22, 11740. https://doi.org/10.3390/ijms222111740
Likszo P, Skarzynski DJ, Moza Jalali B. Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy. International Journal of Molecular Sciences. 2021; 22(21):11740. https://doi.org/10.3390/ijms222111740
Chicago/Turabian StyleLikszo, Pawel, Dariusz Jan Skarzynski, and Beenu Moza Jalali. 2021. "Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy" International Journal of Molecular Sciences 22, no. 21: 11740. https://doi.org/10.3390/ijms222111740
APA StyleLikszo, P., Skarzynski, D. J., & Moza Jalali, B. (2021). Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy. International Journal of Molecular Sciences, 22(21), 11740. https://doi.org/10.3390/ijms222111740







