A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women
Abstract
:1. Introduction
2. COVID-19 in Children
3. COVID-19 in Pregnant Women
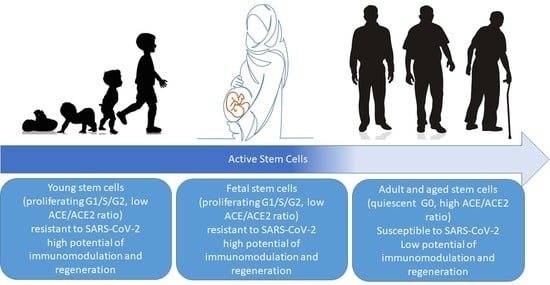
4. What Makes Children and Pregnant Women More Tolerant to COVID-19?
4.1. Active Versus Quiescent Stem Cells
How Do Proliferating Active Stem Cells Resist the SARS-Cov-2 Infection?
4.2. ACE/ACE2 Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O′neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Bolaño-Ortiz, T.R.; Camargo-Caicedo, Y.; Puliafito, S.E.; Ruggeri, M.F.; Bolaño-Diaz, S.; Pascual-Flores, R.; Saturno, J.; Ibarra-Espinosa, S.; Mayol-Bracero, O.L.; Torres-Delgado, E.; et al. Spread of SARS-CoV-2 through Latin America and the Caribbean region: A look from its economic conditions, climate and air pollution indicators. Environ. Res. 2020, 191, 109938. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. Soc. 2020, 55, 2000607. [Google Scholar] [CrossRef] [Green Version]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Hancock, A.S.; Stairiker, C.J.; Boesteanu, A.C.; Monzón-Casanova, E.; Lukasiak, S.; Mueller, Y.M.; Stubbs, A.P.; García-Sastre, A.; Turner, M.; Katsikis, P.D. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate immune response of human alveolar type ii cells infected with severe acute respiratory syndrome–coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Karlsen, A.P.H.; Wiberg, S.; Laigaard, J.; Pedersen, C.; Rokamp, K.Z.; Mathiesen, O. A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment. PLoS ONE 2020, 15, e0237903. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Afarid, M.; Sanie-Jahromi, F. Mesenchymal stem cells and COVID-19: Cure, prevention, and vaccination. Stem Cells Int. 2021, 2021, 6666370. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.S.; Guo, F.M.; Zhang, X.W.; Chang, W.; Peng, F.; Qiu, H.B.; Yang, Y. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J. Cell. Biochem. 2019, 120, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- Bernard, O.; Jeny, F.; Uzunhan, Y.; Dondi, E.; Terfous, R.; Label, R.; Sutton, A.; Larghero, J.; Vanneaux, V.; Nunes, H.; et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L360–L371. [Google Scholar] [CrossRef]
- Chen, T.L.; Han, Z.H.; Wang, W.J.; Meng, J.G. Coculture with bone marrow-derived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharide-stimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin-1 modulating the Toll-like receptor-4 signal pathway. Mol. Med. Rep. 2019, 19, 1891–1902. [Google Scholar]
- Qin, H.; Zhao, A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: From basic to clinics. Protein Cell 2020, 11, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Muraca, M.; Pessina, A.; Pozzobon, M.; Dominici, M.; Galderisi, U.; Lazzari, L.; Parolini, O.; Lucarelli, E.; Perilongo, G.; Baraldi, E. Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia? J. Control. Release 2020, 325, 135–140. [Google Scholar] [CrossRef]
- Rao, V.; Thakur, S.; Rao, J.; Arakeri, G.; Brennan, P.A.; Jadhav, S.; Sayeed, M.S.; Rao, G. Mesenchymal stem cells-bridge catalyst between innate and adaptive immunity in COVID 19. Med. Hypotheses 2020, 143, 109845. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Ware, L.B.; Matthay, M.A. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014, 2, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, R.; Spohn, G.; Bechtel, M.; Bojkova, D.; Baer, P.C.; Kuçi, S.; Seifried, E.; Ciesek, S.; Cinatl, J. Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem Cell Rep. 2021, 16, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.J.; Beaty, D.E.; Fruhwirth, L.L.; Chaves, A.P.L.; Riordan, N.H. Dodging COVID-19 infection: Low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J. Transl. Med. 2021, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021, 10, 636–642. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Goolaerts, A.; Howard, J.P.; Lee, J.W. Mesenchymal stem cells for acute lung injury: Preclinical evidence. Crit. Care Med. 2010, 38 (Suppl. 10), S569. [Google Scholar] [CrossRef]
- Laffey, J.G.; Matthay, M.A. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef]
- Matthay, M.A. Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S54–S57. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, B.P.; Djonov, V.; Volarevic, V. Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of SARS-CoV-2-induced acute respiratory distress syndrome. Anal. Cell. Pathol. 2020, 2020, 1939768. [Google Scholar] [CrossRef]
- Obendorf, J.; Fabian, C.; Thome, U.H.; Laube, M. Paracrine stimulation of perinatal lung functional and structural maturation by mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.J.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar]
- Wang, L.; Li, Y.; Xu, M.; Deng, Z.; Zhao, Y.; Yang, M.; Liu, Y.; Yuan, R.; Sun, Y.; Zhang, H.; et al. Regulation of inflammatory cytokine storms by mesenchymal stem cells. Front. Immunol. 2021, 12, 3055. [Google Scholar]
- Rawat, S.; Gupta, S.; Mohanty, S. Mesenchymal stem cells modulate the immune system in developing therapeutic interventions. Immune Response Act. Immunomodul. 2019. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between mesenchymal stem cells and B-cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 135, 999–1002. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, Y. Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J. Med. Virol. 2020, 92, 564–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.-I.; Hu, Y.-L.; Chen, P.-Y.; Huang, Y.-C.; Hsueh, P.-R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020, 53, 371. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Z.; Zhai, W.; Zheng, Y.; Liu, H.; Wang, Y.; Wu, E.; Xiong, F.; Ma, Y. Comparison of the clinical manifestations between different age groups of patients with overseas imported COVID-19. PLoS ONE 2020, 15, e0243347. [Google Scholar] [CrossRef]
- McGoogan, J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020, 323, 1239–1242. [Google Scholar]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COVID, C.; Bialek, S.; Gierke, R.; Hughes, M.; McNamara, L.A.; Pilishvili, T.; Skoff, T. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 422. [Google Scholar]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Team CC-R; Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar]
- COVID-19 National Emergency Response Center; Epidemiology and Case Management Team; Korea Centers for Disease Control and Prevention. Coronavirus disease-19: The first 7755 cases in the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, M.-J. Coronavirus disease 2019 in children: Current status. J. Chin. Med. Assoc. 2020, 83, 527–533. [Google Scholar] [CrossRef]
- Zheng, F.; Liao, C.; Fan, Q.-H.; Chen, H.-b.; Zhao, X.-g.; Xie, Z.-g.; Li, X.L.; Chen, C.X.; Lu, X.X.; Liu, Z.S.; et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, K.; Yun, Y.; Wang, X.; Yang, G.; Zheng, Y.; Lin, C.; Wang, L.F. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2020, 58, E007. [Google Scholar]
- Sun, D.; Li, H.; Lu, X.-X.; Xiao, H.; Ren, J.; Zhang, F.-R.; Liu, Z.-S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: A single center’s observational study. World J. Pediatr. 2020, 16, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Shekerdemian, L.S.; Mahmood, N.R.; Wolfe, K.K.; Riggs, B.J.; Ross, C.E.; McKiernan, C.A.; Heidemann, S.M.; Kleinman, L.C.; Sen, A.I.; Hall, M.W.; et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020, 174, 868. [Google Scholar] [CrossRef] [PubMed]
- Paret, M.; Lighter, J.; Pellett, M.R.; Raabe, V.N.; Shust, G.F.; Ratner, A.J. Severe acute respiratory syndrome coronavirus 2 infection (COVID-19) in febrile infants without respiratory distress. Clin. Infect. Dis. 2020, 71, 2243–2245. [Google Scholar] [CrossRef]
- See, K.; Liew, S.M.; Ng, D.C.; Chew, E.; Khoo, E.M.; Sam, C.; Sheena, D.; Zahilah Filzah, Z.; Chin, S.Y.; Lee, P.Y.; et al. COVID-19: Four paediatric cases in Malaysia. Int. J. Infect. Dis. 2020, 94, 125–127. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Song, X.; Delaney, M.; Bell, M.; Smith, K.; Pershad, J.; Hahn, A.; Hamdy, R.; Hanisch, B.; Harik, N.; et al. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J. Pediatr. 2020, 7, S338. [Google Scholar]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174, 722–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parri, N.; Lenge, M.; Buonsenso, D. Children with COVID-19 in pediatric emergency departments in Italy. N. Engl. J. Med. 2020, 383, 187–190. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Della Gatta, A.N.; Rizzo, R.; Pilu, G.; Simonazzi, G. COVID19 during pregnancy: A systematic review of reported cases. Am. J. Obstet. Gynecol. 2020, 223, 36–41. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Dashraath, P.; Jeslyn, W.J.L.; Karen, L.M.X.; Min, L.L.; Sarah, L.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Pierce-Williams, R.A.; Burd, J.; Felder, L.; Khoury, R.; Bernstein, P.S.; Avila, K.; Penfield, C.A.; Roman, A.S.; DeBolt, C.A.; Stone, J.L.; et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: A US cohort study. Am. J. Obstet. Gynecol. MFM 2020, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Hantoushzadeh, S.; Shamshirsaz, A.A.; Aleyasin, A.; Seferovic, M.D.; Aski, S.K.; Arian, S.E.; Pooransari, P.; Ghotbizadeh, F.; Aalipour, S.; Soleimani, Z.; et al. Maternal death due to COVID-19 disease. Am. J. Obstet. Gynecol. 2020, 223, 109.e1–109.e16. [Google Scholar] [CrossRef] [PubMed]
- Zlochiver, V.; Tilkens, B.; Perez Moreno, A.C.; Aziz, F.; Jan, M.F. COVID-19 deliveries: Maternal features and neonatal outcomes. J. Patient-Cent. Res. Rev. 2021, 8, 286–289. [Google Scholar]
- Rosen, H.; Bart, Y.; Zlatkin, R.; Ben-Sira, L.; Ben Bashat, D.; Amit, S.; Cohen, C.; Regev-Yochay, G.; Yinon, Y. Fetal and perinatal outcome following first and second trimester COVID-19 infection: Evidence from a prospective cohort study. J. Clin. Med. 2021, 10, 2152. [Google Scholar] [CrossRef]
- Wang, C.-L. Impact of COVID-19 on Pregnancy. Int. J. Med Sci. 2021, 18, 763. [Google Scholar] [CrossRef]
- Rajewska, A.; Mikołajek-Bedner, W.; Lebdowicz-Knul, J.; Sokołowska, M.; Kwiatkowski, S.; Torbé, A. COVID-19 and pregnancy–where are we now? A Rev. J. Perinat. Med. 2020, 48, 428–434. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Liu, D.; Fan, H.; Wang, Y.; Hu, Y.; Hou, Y. Progesterone enhances immunoregulatory activity of human mesenchymal stem cells Via PGE 2 and IL-6. Am. J. Reprod. Immunol. 2012, 68, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lissauer, D.; Eldershaw, S.A.; Inman, C.F.; Coomarasamy, A.; Moss, P.A.; Kilby, M.D. Progesterone promotes maternal–fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 2015, 45, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Leeper, C.; Lutzkanin, A. Infections during pregnancy. Prim. Care Clin. Off. Pract. 2018, 45, 567–586. [Google Scholar] [CrossRef]
- Vissing, N.H.; Chawes, B.L.; Rasmussen, M.A.; Bisgaard, H. Epidemiology and risk factors of infection in early childhood. Pediatrics 2018, 141, e20170933. [Google Scholar] [CrossRef] [Green Version]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and older adults: What we know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Neumann-Podczaska, A.; Al-Saad, S.R.; Karbowski, L.M.; Chojnicki, M.; Tobis, S.; Wieczorowska-Tobis, K. COVID 19-clinical picture in the elderly population: A qualitative systematic review. Aging Dis. 2020, 11, 988. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Rumman, M.; Dhawan, J.; Kassem, M. Concise review: Quiescence in adult stem cells: Biological significance and relevance to tissue regeneration. Stem Cells 2015, 33, 2903–2912. [Google Scholar] [CrossRef]
- Liu, M.; Lei, H.; Dong, P.; Fu, X.; Yang, Z.; Yang, Y.; Ma, J.; Liu, X.; Cao, Y.; Xiao, R. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplant. 2017, 26, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.E.; Mezzapelle, R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Rossi, D.J.; Bryder, D.; Weissman, I.L. Hematopoietic stem cell aging: Mechanism and consequence. Exp. Gerontol. 2007, 42, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagers, A.J.; Conboy, I.M. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell 2005, 122, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Slutsky, S.G.; Joseph, N.M.; He, S.; Pardal, R.; Krishnamurthy, J.; Sharpless, N.; Morrison, S.J. Increasing p16 INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006, 443, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Khosrotehrani, K.; Bianchi, D.W. Multi-lineage potential of fetal cells in maternal tissue: A legacy in reverse. J. Cell Sci. 2005, 118, 1559–1563. [Google Scholar] [CrossRef] [Green Version]
- Verfaillie, C.M.; Pera, M.F.; Lansdorp, P.M. Stem cells: Hype and reality. Hematology 2002, 2002, 369–391. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J. Exp. Med. 1983, 157, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.A.; Deamond, S.F.; Ts′o, P.O. In vitro senescence of Syrian hamster mesenchymal cells of fetal to aged adult origin. Inverse relationship between in vivo donor age and in vitro proliferative capacity. Mech. Ageing Dev. 1986, 34, 151–173. [Google Scholar] [CrossRef]
- Van Zant, G.; Liang, Y. The role of stem cells in aging. Exp. Hematol. 2003, 31, 659–672. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Fisk, N.M. Fetal stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 853–875. [Google Scholar] [CrossRef]
- Samara, A.; Herlenius, E. Is there an effect of fetal mesenchymal stem cells in the mother–fetus dyad in COVID-19 pregnancies and vertical transmission? Front. Physiol. 2021, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Warr, M.R.; Passegué, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011, 195, 709–720. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.V.; Brooks, G. The mammalian cell cycle. Cell Cycle Control. 2005, 296, 113–153. [Google Scholar]
- Fan, Y.; Sanyal, S.; Bruzzone, R. Breaking bad: How viruses subvert the cell cycle. Front. Cell. Infect. Microbiol. 2018, 8, 396. [Google Scholar] [CrossRef]
- Simabuco, F.M.; Tamura, R.E.; Pavan, I.C.B.; Morale, M.G.; Ventura, A.M. Molecular mechanisms and pharmacological interventions in the replication cycle of human coronaviruses. Genet. Mol. Biol. 2021, 44. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Chen, Y.; Qi, S.; Shi, D.; Feng, L.; Sun, D. A mini-review on cell cycle regulation of coronavirus infection. Front. Vet. Sci. 2020, 7, 943. [Google Scholar] [CrossRef]
- Harrison, S.M.; Dove, B.K.; Rothwell, L.; Kaiser, P.; Tarpey, I.; Brooks, G.; Hiscox, J.A. Characterisation of cyclin D1 down-regulation in coronavirus infected cells. FEBS Lett. 2007, 581, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Huang, Y.; Dai, M.; Zhao, X.; Du, Q.; Dong, F.; Wang, L.; Huo, R.; Zhang, W.; Xu, X.; et al. Transmissible gastroenteritis virus infection induces cell cycle arrest at S and G2/M phases via p53-dependent pathway. Virus Res. 2013, 178, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, J.; Shan, Y.; Yao, Z.; Dong, B.; Chen, B.; Zhao, Z.; Wang, S.; Chen, J.; Cong, Y. SARS coronavirus 7a protein blocks cell cycle progression at G0/G1 phase via the cyclin D3/pRb pathway. Virology 2006, 346, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Shan, Y.; Zhao, Z.; Chen, J.; Cong, Y. G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells. Virol. J. 2005, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-J.; Makino, S. Murine coronavirus replication induces cell cycle arrest in G0/G1 phase. J. Virol. 2004, 78, 5658–5669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Wu, H.; Huang, J.; Xu, Y.; Yang, F.; Zhang, Q.; Xu, X. Porcine epidemic diarrhea virus through p53-dependent pathway causes cell cycle arrest in the G0/G1 phase. Virus Res. 2018, 253, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Yu, Y.; Tang, N. Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res. 2020, 30, 708–710. [Google Scholar] [CrossRef]
- Chugh, R.M.; Bhanja, P.; Norris, A.; Saha, S. Experimental models to study COVID-19 effect in stem cells. Cells 2021, 10, 91. [Google Scholar] [CrossRef]
- Huang, K.; Kang, X.; Wang, X.; Wu, S.; Xiao, J.; Jinling, X.; Wu, X.; Zhang, W. Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol. Med. Rep. 2015, 11, 1685–1692. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. ACE/ACE2 ratio: A key also in 2019 coronavirus disease (COVID-19)? Front. Med. 2020, 7, 335. [Google Scholar] [CrossRef]
- Amati, E.; Perbellini, O.; Rotta, G.; Bernardi, M.; Chieregato, K.; Sella, S.; Rodeghiero, F.; Ruggeri, M.; Astori, G. High-throughput immunophenotypic characterization of bone marrow-and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: Identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res. Ther. 2018, 9, 1–11. [Google Scholar]
- Barzegar, M.; Wang, Y.; Eshaq, R.S.; Yun, J.W.; Boyer, C.J.; Cananzi, S.G.; White, L.A.; Chernyshev, O.; Kelley, R.E.; Minagar, A.; et al. Human placental mesenchymal stem cells improve stroke outcomes via extracellular vesicles-mediated preservation of cerebral blood flow. EBioMedicine 2021, 63, 103161. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Lu, X.; Zhang, C.; Huang, L.; Wang, X.; Duan, F.; Liang, H.; Chen, P.; Zeng, L.; et al. Clinical analysis and pluripotent stem cells-based model reveal possible impacts of ACE2 and lung progenitor cells on infants vulnerable to COVID-19. Theranostics 2021, 11, 2170. [Google Scholar] [CrossRef]
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev. Rep. 2020, 16, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel, D.; Mishra, R.; Soni, P.; Seetharaman, R.; Mahmood, A.; Srivastava, A. Stem cells therapy as a possible therapeutic option in treating COVID-19 patients. Stem Cell Rev. Rep. 2021, 17, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Michalopoulos, E.; Chatzistamatiou, T.; Stavropoulos-Giokas, C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J. Stem Cells 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiao, H.; Yin, X. Engineered human mesenchymal stem cells as new vaccine platform for COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
| Population under Study | Date of Data Gathering | Number of Patients under Study | Outcome | First Author (Ref) |
|---|---|---|---|---|
| China | Updated through 11 February 2020 | 965 (aged ≤19 years) | Only one death occurred in a person aged ≤19 years | Wu Z et al. [10] |
| China | 28 January–26 February 2020 (with follow-up to 8 March 2020) | 171 (aged <16 years) | Milder clinical courses and asymptomatic infections were found in children. | Lu X et al. [45] |
| United States | 12 February–2 April 2020 | 2572 (aged <18 years) out of 149,082 COVID-19 patients | Three deaths were reported in this analysis. Most of the hospitalizations occurred in infants (aged <1 year). Whereas severe cases of COVID-19 are rare in children, serious illness resulting in hospitalization still occurs. | [46] |
| China | From 17 January to 1 March 2020 | 36 (aged <16 years) | All pediatric patients manifested mild or moderate symptoms of infection. | Qiu H et al. [47] |
| China | From 1 February till 10 February 2020 | 35 (aged from 1 month to 14 years) | Children were reported to be as sensitive to COVID-19 as adults, while clinical outcomes were more favorable in children. Children under 3 years of age had the highest risk of developing serious illness and need more intensive medical care than other children. | Zheng F et al. [51] |
| China | from 16 January to 6 February 2020 | 15 (aged from 4 to 14 years) | Small nodular ground-glass opacities were the main findings of the early chest CT images of children with 2019-nCoV infection. | Feng K et al. [52] |
| China | From 24 January to 24 February | 8 severe cases (aged from 2 month to 15 years) | Common symptoms were polypnea, fever, cough, and cytokine storm. Imaging changes were multiple patch-like shadows and ground-glass opacity. | Sun D et al. [53] |
| North America | 14 March–3 April 2020 (with follow-up to 10 April 2020) | 48 (aged 4.2–16.6 years) | Severe illness was far less frequent in children than in adults. | Shekerdemian LS et al. [54] |
| New York City | Over a 1-week period in late March 2020 | 2 infants | Clinical course was benign in both infants. | Paret M et al. [55] |
| Malaysia | until end of February 2020 | 4 (aged 1.7–11 years) | Mild or asymptomatic presentation in children was reported. | See KC et al. [56] |
| Washington, DC | 15 March–30 April 2020 | 177 (children and young adults) | The risk of being hospitalized was higher in the youngest (<1 year) and oldest children/young adults (15–25 years of age). | DeBiasi RL et al. [57] |
| China | January–February 2020 | 33 infants (born to mothers with COVID-19, including 3 neonates with COVID-19) | Mild clinical symptoms were seen in 33 neonates with or at risk of COVID-19. Outcomes were favorable. | Zeng L et al. [58] |
| Nationwide case series (reported to the Chinese Center for Disease Control and Prevention) | 16 January–8 February 2020 | 2135 (aged 2–13 years) | More than 90% of all patients were asymptomatic, mild, or moderate cases. Young children, particularly infants, were vulnerable to infection. | Dong Y et al. [59] |
| Italy | 3 March–27 March | 100 (aged <18 years) | Most of the infants presented with mild disease. Severe and critical cases were diagnosed in patients with (10) conditions. No deaths were reported. | Parri N et al. [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanie-Jahromi, F.; NejatyJahromy, Y.; Jahromi, R.R. A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women. Int. J. Mol. Sci. 2021, 22, 11787. https://doi.org/10.3390/ijms222111787
Sanie-Jahromi F, NejatyJahromy Y, Jahromi RR. A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women. International Journal of Molecular Sciences. 2021; 22(21):11787. https://doi.org/10.3390/ijms222111787
Chicago/Turabian StyleSanie-Jahromi, Fatemeh, Yaser NejatyJahromy, and Rahim Raoofi Jahromi. 2021. "A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women" International Journal of Molecular Sciences 22, no. 21: 11787. https://doi.org/10.3390/ijms222111787
APA StyleSanie-Jahromi, F., NejatyJahromy, Y., & Jahromi, R. R. (2021). A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women. International Journal of Molecular Sciences, 22(21), 11787. https://doi.org/10.3390/ijms222111787