Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation
Abstract
:1. Introduction
2. Results
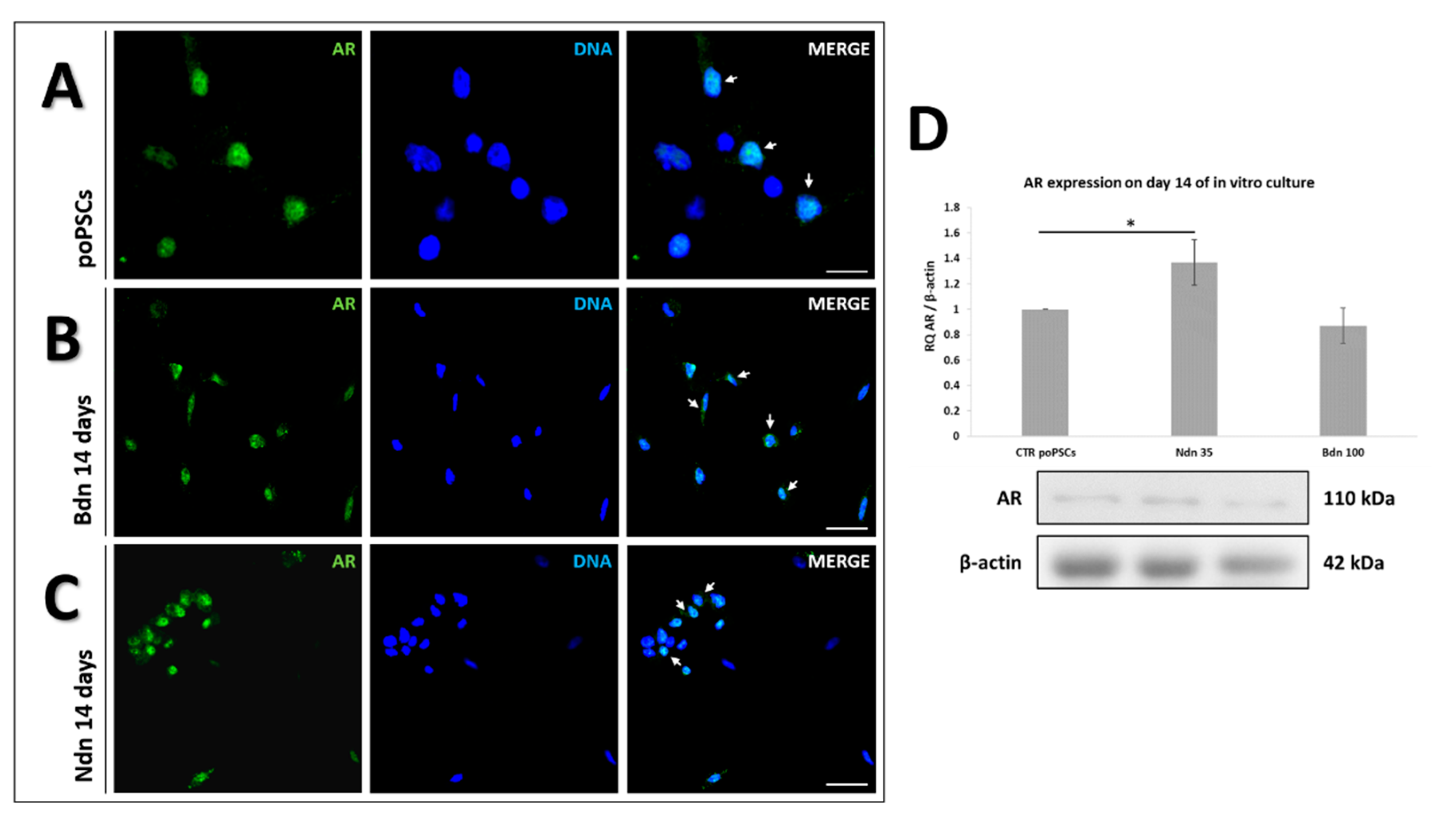
2.1. poPSCs Cultured In Vitro with or without the Presence of Boldenone and Nandrolone Express the Androgen Receptor
2.2. Nandrolone and Boldenone Affect the Proliferation of poPCS after 14-Day In Vitro Culture
2.3. Boldenone and Nandrolone Influence on poPSCs Viability, Cytotoxicity, and Apoptotic Activity
2.4. Nandrolone and Boldenone Trigger the Expression of Selected Cancer Stem Cells Markers: CD44 and CD133
2.5. Oxygen Consumption in poPSCs after 7- and 14-Day In Vitro Culture in the Presence of Boldenone and Nandrolone
2.6. Nandrolone and Boldenone Affect the Expression and Phosphorylation of Proteins within the PI3K/Akt Pathway
3. Discussion
4. Materials and Methods
4.1. Sample Collection and poPSCs Isolation
4.2. Evaluation of poPSCs Proliferation after 14-Day Exposure to Different Doses of Nandrolone or Boldenone
4.3. poPSC Culture in the Presence of Selected Doses of Nandrolone or Boldenone
4.4. ApoTox-Glo Triplex Assay
4.5. Immunofluorescence
4.6. Western Blot Analysis
4.7. Total RNA Isolation and cDNA Synthesis
4.8. Quantitative Real-Time qPCR
4.9. Seahorse Analysis
Seahorse XF Measurement of ECAR and OCR Using Seahorse XF Cell Mito Stress Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Anabolic androgenic steroids |
| AASoln | Antibiotic/antimycotic solution |
| Akt | A member of serine/threonine-specific protein kinase family (also known as protein kinase B; PKB) that plays a pivotal function in controlling the molecular balance between survival and death pathways in cells |
| AR | Androgen receptor |
| ASCs | Adult stem cells |
| Bdn | Boldenone |
| CSCs | Cancer stem cells |
| CD | Cluster of differentiation |
| ECAR | Extracellular acidification rate |
| ERK1/2 | Extracellular signal-regulated protein kinases 1 and 2; also known as p44 mitogen-activated protein (MAP) kinase (a 44-kDa isoform of MAPK) and p42 mitogen-activated protein (MAP) kinase (a 42-kDa isoform of MAPK), respectively |
| ESCs | Embryonic stem cells |
| GPCRC6A | G protein-coupled receptor family C group 6 member A; a novel membrane androgen receptor (mAR) related to the extranuclear action of androgens |
| HA | Hyaluronic acid |
| HepG2 | Human hepatocarcinoma-derived cell lines |
| IGF-I | Insulin-like growth factor-I |
| Klf-4 | Krüppel-like factor-4 (also called gut-enriched Krüppel-like factor or GKLF); an evolutionarily conserved zinc finger-containing transcription factor that regulates diverse cellular processes such as cell growth, proliferation, differentiation, apoptosis, and somatic cell reprogramming |
| mARs | Novel membrane androgen receptors (unrelated to nuclear androgen receptors) that are engaged in a broad spectrum of non-classical, cell surface-initiated androgen actions |
| MSCs | Mesenchymal stem cells |
| mTOR | Mechanistic target of rapamycin (previously known as mammalian target of rapamycin) that represents a family of serine/threonine-specific protein kinases; mammalian target of rapamycin (mTOR) kinase that has been identified as a direct target of the rapamycin-FKBP12 (FK506-binding protein 12 kDa) complex; mTOR kinase is also designated as FK506-binding protein 12-rapamycin complex-associated protein 1 (FRAP1) |
| NANOG | Homeobox-containing transcription factor whose name stems from Celtic/Irish mythical word Tír na nÓg (i.e., Tir Na Nog; The Land of the Ever-Young) |
| NDCs | Nuclear donor cells |
| Ndn | Nandrolone |
| NF-κB | Nuclear factor-κB (nuclear factor kappa-light-chain-enhancer of activated B cells); a pleiotropic inducible transcription factor that occurs in almost all cell types and is the endpoint of a series of signal transduction events that are initiated by a vast array of stimuli related to many biological processes such as cytodifferentiation, cell growth, tumorigenesis, apoptosis, inflammation, and immunity |
| NOD-SCID | Non-obese diabetic/severe combined immunodeficient mouse model |
| OCICs | Ovarian cancer initiating cells |
| OCR | Oxygen consumption rate |
| Oct-4 | Octamer-binding transcription factor-4 (also designated as POU5F1); a member of the family of POU (Pit-Oct-Unc)-domain and homeodomain transcription factors |
| oPSCs | Ovarian putative stem cells |
| OXER1 | G protein-coupled oxo-eicosanoid receptor 1; a receptor of the arachidonic acid metabolite, i.e., 5-oxoeicosatetraenoic acid (5-oxoETE); known as a novel mAR involved in the rapid effects of androgens |
| PCDs | Potentially cancerous derivatives |
| PCNA | Proliferating cell nuclear antigen |
| PCOS | Polycystic ovary syndrome |
| PI3K | Phosphatidylinositol 3-kinase; a downstream kinase activated by receptor tyrosine kinases that generates a series of phosphorylated phosphoinositides, which recruit 3-phosphoinositide-dependent protein kinase-1 (PDPK1) activity to the plasma membrane, leading to activation of Akt |
| poPSCs | Porcine ovarian putative stem cells |
| RIPA | Radioimmunoprecipitation assay buffer |
| Rex1 | Reduced expression gene 1 encoding a DNA-binding transcription factor known as reduced expression protein 1 or zinc finger protein 42 homolog |
| ROS | Reactive oxygen species |
| SCNT | Somatic cell nuclear transfer |
| Sox2 | Sex-determining region Y (SRY)-box 2; a member of the high mobility group (HMG)-box family of DNA-binding transcription factors |
| ZIP9 | Zinc transporter member 9; also designated as solute carrier family 39 member 9 (SLC39A9) or transmembrane zinc-influx transporter (Zrt)- and transmembrane iron-influx transporter (Irt)-like protein (ZIP) 9; represents both zinc (Zn2+)-iron (Fe2+) permease (ZIP) family and a novel membrane androgen receptor (mAR) family related to the extranuclear action of androgens |
References
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Tysnes, B.B. Tumor-initiating and-propagating cells: Cells that we would to identify and control. Neoplasia 2010, 12, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nat. Cell Biol. 2013, 15, 338–344. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alison, M.R.; Islam, S.; Wright, N.A. Stem cells in cancer: Instigators and propagators? J. Cell Sci. 2010, 123, 2357–2368. [Google Scholar] [CrossRef] [Green Version]
- Foster, R.; Buckanovich, R.J.; Rueda, B.R. Ovarian cancer stem cells: Working towards the root of stemness. Cancer Lett. 2013, 338, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Garson, K.; Vanderhyden, B.C. Epithelial ovarian cancer stem cells: Underlying complexity of a simple paradigm. Reproduction 2015, 149, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Nozawa-Suzuki, N.; Nagasawa, H.; Ohnishi, K.; Morishige, K.I. The inhibitory effect of hypoxic cytotoxin on the expansion of cancer stem cells in ovarian cancer. Biochem. Biophys. Res. Commun. 2015, 457, 706–711. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X. Stemness-related markers in cancer. Cancer Transl. Med. 2017, 3, 87–95. [Google Scholar]
- Basakran, N.S. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015, 36, 273–279. [Google Scholar] [CrossRef]
- Palapattu, G.S.; Wu, C.; Silvers, C.R.; Martin, H.B.; Williams, K.; Salamone, L.; Bushnell, T.; Huang, L.; Yang, Q.; Huang, J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate 2009, 69, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.M.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handgretinger, R.; Gordon, P.R.; Leimig, T.; Chen, X.; Buhring, H.J.; Niethammer, D.; Kuci, S. Biology and plasticity of CD133+ hematopoietic stem cells. Ann. N. Y. Acad. Sci. 2003, 996, 141–151. [Google Scholar] [CrossRef]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrandina, G.; Bonanno, G.; Pierelli, L.; Perillo, A.; Procoli, A.; Mariotti, A.; Corallo, M.; Martinelli, E.; Rutella, S.; Paglia, A.; et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 506–514. [Google Scholar] [CrossRef]
- Curley, M.D.; Therrien, V.A.; Cummings, C.L.; Sergent, P.A.; Koulouris, C.R.; Friel, A.M.; Roberts, D.J.; Seiden, M.V.; Scadden, D.T.; Rueda, B.R.; et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells 2009, 27, 2875–2883. [Google Scholar]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Skubitz, A.P.; Taras, E.P.; Boylan, K.L.; Waldron, N.N.; Oh, S.; Panoskaltsis-Mortari, A.; Vallera, D.A. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol. Oncol. 2013, 130, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrke, M.S.; Yesalis, C.E. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr. Opin. Pharmacol. 2004, 4, 614–620. [Google Scholar] [CrossRef]
- Simão, V.A.; Berloffa Belardin, L.; Araujo Leite, G.A.; De Almeida Chuffa, L.G.; Camargo, I.C.C. Effects of different doses of nandrolone decanoate on estrous cycle and ovarian tissue of rats after treatment and recovery periods. Int. J. Exp. Pathol. 2015, 96, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative overview of the mechanisms of action of hormones and endocrine disruptor compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.P.; Cerqueira, E.D.M.M.; Meireles, J.R.C. Chromosome damage, apoptosis, and necrosis in exfoliated cells of oral mucosa from androgenic anabolic steroids users. J. Toxicol. Environ. Health A 2015, 78, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Elks, J.; Ganellin, C.R. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies, 1st ed.; Springer Science and Business Media: Berlin/Heidelberg, Germany, 1990; pp. 44, 209, 408, 410, 640, 660. [Google Scholar]
- Handelsman, D.J. Androgen physiology, pharmacology and abuse. In Endocrinology-E-Book: Adult and Pediatric, 6th ed.; Jameson, J.L., De Groot, L.J., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010; pp. 2469–2498. [Google Scholar]
- Llewellyn, W. Part III: Drug profiles. In Anabolics; Llewellyn, W., Ed.; Molecular Nutrition LLC: Jupiter, FL, USA, 2011; pp. 739–780. [Google Scholar]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F.; et al. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Forbes, G.B. The effect of anabolic steroids on lean body mass: The dose response curve. Metabolism 1985, 34, 571–573. [Google Scholar] [CrossRef]
- Whyte, J.J.; Prather, R.S. Genetic modifications of pigs for medicine and agriculture. Mol. Reprod. Dev. 2011, 78, 879–891. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.T.; Van Thuan, N.; Kwon, D.N.; Choi, Y.J.; Kang, M.H.; Han, J.W.; Kim, T.; Kim, J.H. Identification and characterization of putative stem cells in the adult pig ovary. Development 2014, 141, 2235–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wartalski, K.; Gorczyca, G.; Wiater, J.; Tabarowski, Z.; Palus-Chramiec, K.; Setkowicz, Z.; Duda, M. Efficient generation of neural-like cells from porcine ovarian putative stem cells–morphological characterization and evaluation of their electrophysiological properties. Theriogenology 2020, 155, 256–268. [Google Scholar] [CrossRef]
- Wartalski, K.; Gorczyca, G.; Wiater, J.; Tabarowski, Z.; Duda, M. Porcine ovarian cortex-derived putative stem cells can differentiate into endothelial cells in vitro. Histochem. Cell Biol. 2021, 156, 349–362, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Connelly, M.C.; Wetmore, C.; Curran, T.; Morgan, J.I. Mouse embryos cloned from brain tumors. Cancer Res. 2003, 63, 2733–2736. [Google Scholar] [PubMed]
- Shao, G.B.; Ding, H.M.; Gao, W.L.; Li, S.H.; Wu, C.F.; Xu, Y.X.; Liu, H.L. Effect of trychostatin A treatment on gene expression in cloned mouse embryos. Theriogenology 2009, 71, 1245–1252. [Google Scholar] [CrossRef]
- Hampl, R.; Kubátová, J.; Stárka, L. Steroids and endocrine disruptors—History, recent state of art and open questions. J. Steroid Biochem. Mol. Biol. 2016, 155, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsuuw, Y.D.; Huang, F.J.; Shyr, C.R.; Chang, S.Y.; Huang, C.K.; Kang, H.Y.; Huang, K.E. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil. Steril. 2006, 85, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Lee, S.O.; Lai, K.P.; Ma, W.L.; Lin, T.H.; Tsai, M.Y.; Luo, J.; Chang, C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology 2013, 57, 1550–1563. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.M.; Chen, L.; Chang, W.C.; Su, S.Y.; Hung, Y.C.; Ma, W.L. Androgen/androgen receptor signaling in ovarian cancer: Molecular regulation and therapeutic potentials. Int. J. Mol. Sci. 2021, 22, 7748. [Google Scholar] [CrossRef]
- Chung, W.M.; Chang, W.C.; Chen, L.; Lin, T.Y.; Chen, L.C.; Hung, Y.C.; Ma, W.L. Ligand-independent androgen receptors promote ovarian teratocarcinoma cell growth by stimulating self-renewal of cancer stem/progenitor cells. Stem Cell Res. 2014, 13, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.; Jiang, L.; Liang, S.; Kwong, J.; Yang, L.; Li, Y.; Ping, Y.; Deng, Q.; Liang, Z. Nanog interaction with the androgen receptor signaling axis induce ovarian cancer stem cell regulation: Studies based on the CRISPR/Cas9 system. J. Ovarian Res. 2018, 211, 36. [Google Scholar] [CrossRef]
- Masi, M.; Garattini, E.; Bolis, M.; Di Marino, D.; Maraccani, L.; Morelli, E.; Grolla, A.A.; Fagiani, F.; Corsini, E.; Travelli, C.; et al. OXER1 and RACK1-associated pathway: A promising drug target for breast cancer progression. Oncogenesis 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Goldman, A.; Basaria, S. Adverse health effects of androgen use. Mol. Cell Endocrinol. 2018, 464, 46–55. [Google Scholar] [CrossRef]
- Estrada, M.; Varshney, A.; Ehrlich, B.E. Elevated testosterone induces apoptosis in neuronal cells. J. Biol. Chem. 2006, 281, 25492–25501. [Google Scholar] [CrossRef] [Green Version]
- Zelleroth, S.; Nylander, E.; Nyberg, F.; Grönbladh, A.; Hallberg, M. Toxic impact of anabolic androgenic steroids in primary rat cortical cell cultures. Neuroscience 2019, 397, 172–183. [Google Scholar] [CrossRef]
- Balgoma, D.; Zelleroth, S.; Grönbladh, A.; Hallberg, M.; Pettersson, C.; Hedeland, M. Anabolic androgenic steroids exert a selective remodeling of the plasma lipidome that mirrors the decrease of the de novo lipogenesis in the liver. Metabolomics 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pregel, P.; Bollo, E.; Cannizzo, F.T.; Rampazzo, A.; Appino, S.; Biolatti, B. Effect of anabolics on bovine granulosa-luteal cell primary cultures. Folia Histochem. Cytobiol. 2007, 45, 265–271. [Google Scholar]
- Groot, M.J.; Biolatti, B. Histopathological effects of boldenone in cattle. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Brännvall, K.; Bogdanovic, N.; Korhonen, L.; Lindholm, D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur. J. Neurosci. 2005, 21, 871–878. [Google Scholar] [CrossRef]
- Cops, E.J.; Bianco-Miotto, T.; Moore, N.L.; Clarke, C.L.; Birrell, S.N.; Butler, L.M.; Tilley, W.D. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J. Steroid Biochem. Mol. Biol. 2008, 110, 236–243. [Google Scholar] [CrossRef]
- Pomara, C.; Barone, R.; Marino Gammazza, A.; Sangiorgi, C.; Barone, F.; Pitruzzella, A.; Locorotondo, N.; Di Gaudio, F.; Salerno, M.; Maglietta, F.; et al. Effects of nandrolone stimulation on testosterone biosynthesis in leydig cells. J. Cell Physiol. 2016, 231, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Chimento, A.; Sirianni, R.; Zolea, F.; De Luca, A.; Lanzino, M.; Catalano, S.; Andò, S.; Pezzi, V. Nandrolone and stanozolol induce Leydig cell tumor proliferation through an estrogen-dependent mechanism involving IGF-I system. J. Cell Physiol. 2012, 227, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Giannitrapani, L.; Soresi, M.; La Spada, E.; Cervello, M.; D’alessandro, N.; Montalto, G. Sex hormones and risk of liver tumor. Ann. N. Y. Acad. Sci. 2006, 1089, 228–236. [Google Scholar] [CrossRef]
- Schwingel, P.A.; Cotrim, H.P.; Salles, B.R.; Almeida, C.E.; dos Santos, C.R., Jr.; Nachef, B.; Andrade, A.R.; Zoppi, C.C. Anabolic-androgenic steroids: A possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011, 31, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Jiang, X.; Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J. Gastroenterol. 2014, 20, 9229–9236. [Google Scholar]
- Huang, C.K.; Luo, J.; Lee, S.O.; Chang, C. Concise review: Androgen receptor differential roles in stem/progenitor cells including prostate, embryonic, stromal, and hematopoietic lineages. Stem Cells 2014, 32, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Shan, J.; Shen, J.; Wang, Y.; Yan, P.; Liu, L.; Zhao, W.; Xu, Y.; Zhu, W.; Su, L.; et al. Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma. Oncotarget 2016, 7, 36814–36828. [Google Scholar] [CrossRef] [Green Version]
- Olempska, M.; Eisenach, P.A.; Ammerpohl, O.; Ungefroren, H.; Fandrich, F.; Kalthoff, H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 92–97. [Google Scholar]
- Yin, S.; Li, J.; Hu, C.; Chen, X.; Yao, M.; Yan, M.; Jiang, G.; Ge, C.; Xie, H.; Wan, D.; et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer 2007, 120, 1444–1450. [Google Scholar] [CrossRef]
- Monzani, E.; Facchetti, F.; Galmozzi, E.; Corsini, E.; Benetti, A.; Cavazzin, C.; Gritti, A.; Piccinini, A.; Porro, D.; Santinami, M.; et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur. J. Cancer 2007, 43, 935–946. [Google Scholar] [CrossRef]
- Baba, T.; Convery, P.A.; Matsumura, N.; Whitaker, R.S.; Kondoh, E.; Perry, T.; Huang, Z.; Bentley, R.C.; Mori, S.; Fujii, S.; et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009, 28, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Chen, A.; Song, H.; Tao, J.; Yang, H.; Zuo, M. Prognostic value of cancer stem cell marker CD133 in ovarian cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3080–3088. [Google Scholar]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the metabolic plasticity of cancer: Mitochondrial reprogramming and hybrid metabolic states. Cells 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wartalski, K.; Tabarowski, Z.; Duda, M. Magnetic isolation and characterization of porcine ovarian putative stem cells (PSCs): An in vitro study. JFIV Reprod. Med. Genet. 2016, 4, 191. [Google Scholar]
- Gorczyca, G.; Wartalski, K.; Tabarowski, Z.; Duda, M. Effects of vinclozolin exposure on the expression and activity of SIRT1 and SIRT6 in the porcine ovary. J. Physiol. Pharmacol. 2019, 70, 153–165. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-MC (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.B.; Van Houten, B. Bioenergetic analysis of intact mammalian cells using the Seahorse XF24 Extracellular Flux analyzer and a luciferase ATP assay. Methods Mol. Biol. 2014, 1105, 589–602. [Google Scholar]
- Scrima, R.; Menga, M.; Pacelli, C.; Agriesti, F.; Cela, O.; Piccoli, C.; Cotoia, A.; De Gregorio, A.; Gefter, J.V.; Cinnella, G.; et al. Para-hydroxyphenylpyruvate inhibits the pro-inflammatory stimulation of macrophage preventing LPS-mediated nitro-oxidative unbalance and immunometabolic shift. PLoS ONE 2017, 12, e0188683. [Google Scholar]
- Muller, B.; Lewis, N.; Adeniyi, T.; Leese, H.J.; Brison, D.R.; Sturmey, R.G. Application of extracellular flux analysis for determining mitochondrial function in mammalian oocytes and early embryos. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorczyca, G.; Wartalski, K.; Wiater, J.; Samiec, M.; Tabarowski, Z.; Duda, M. Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation. Int. J. Mol. Sci. 2021, 22, 11800. https://doi.org/10.3390/ijms222111800
Gorczyca G, Wartalski K, Wiater J, Samiec M, Tabarowski Z, Duda M. Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation. International Journal of Molecular Sciences. 2021; 22(21):11800. https://doi.org/10.3390/ijms222111800
Chicago/Turabian StyleGorczyca, Gabriela, Kamil Wartalski, Jerzy Wiater, Marcin Samiec, Zbigniew Tabarowski, and Małgorzata Duda. 2021. "Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation" International Journal of Molecular Sciences 22, no. 21: 11800. https://doi.org/10.3390/ijms222111800
APA StyleGorczyca, G., Wartalski, K., Wiater, J., Samiec, M., Tabarowski, Z., & Duda, M. (2021). Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation. International Journal of Molecular Sciences, 22(21), 11800. https://doi.org/10.3390/ijms222111800





