Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants
Abstract
1. Introduction
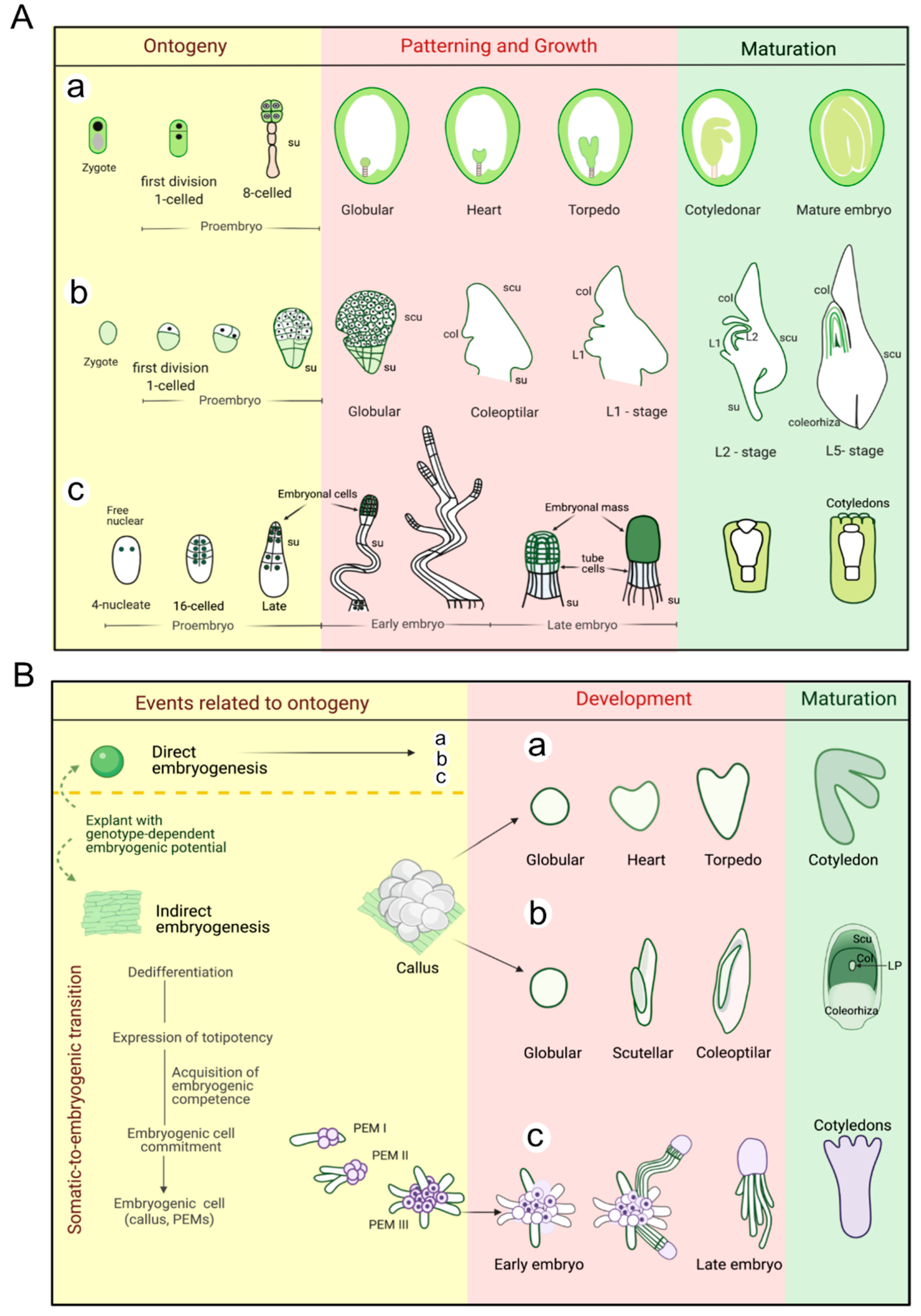
2. Early Events in Plant Embryogenesis
2.1. Ontogenetic Events during Zygotic Embryogenesis (ZE)
2.2. Induction of Somatic Embryogenesis (SE)
2.2.1. Dicots
2.2.2. Monocots
2.2.3. Gymnosperms
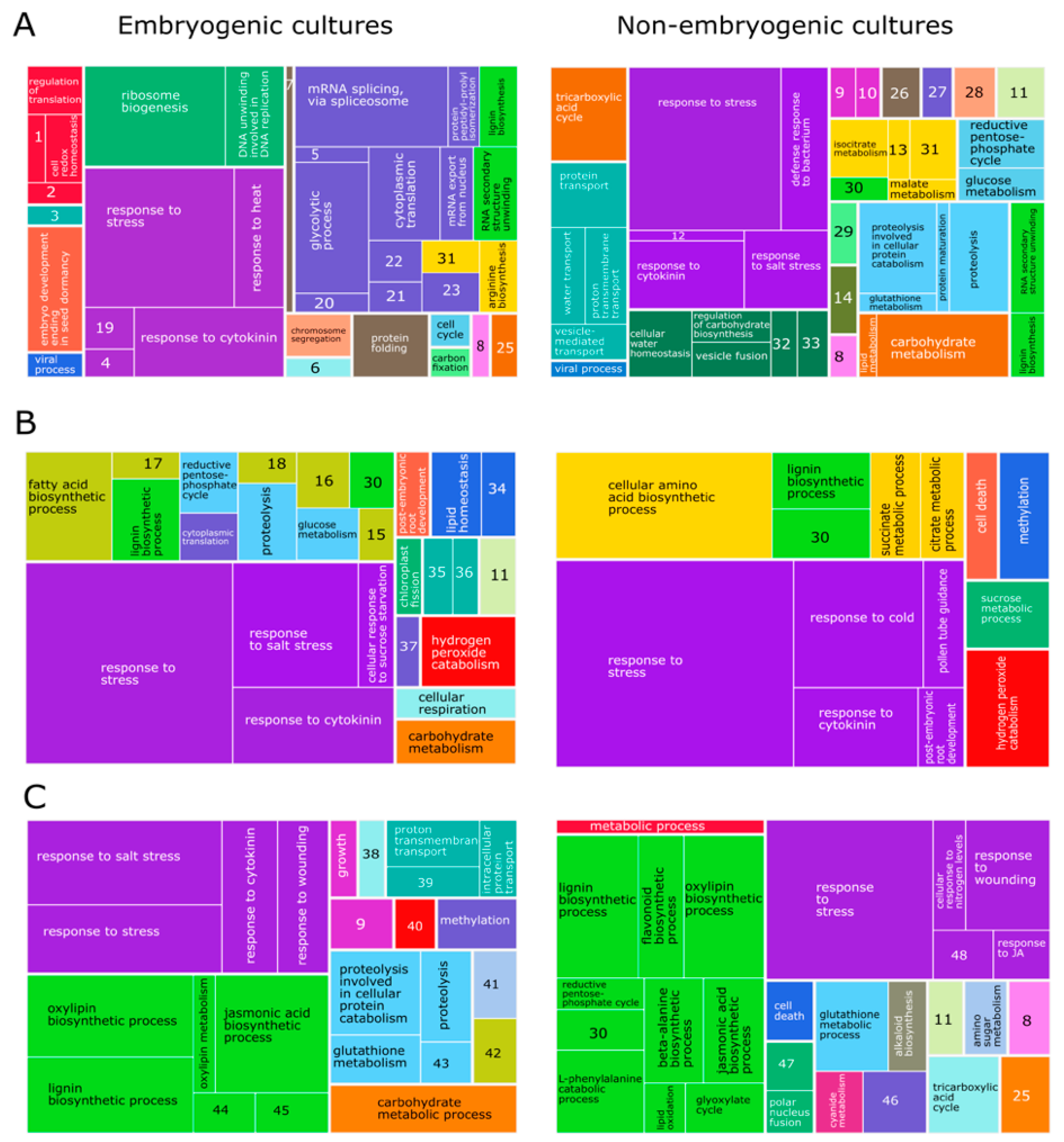
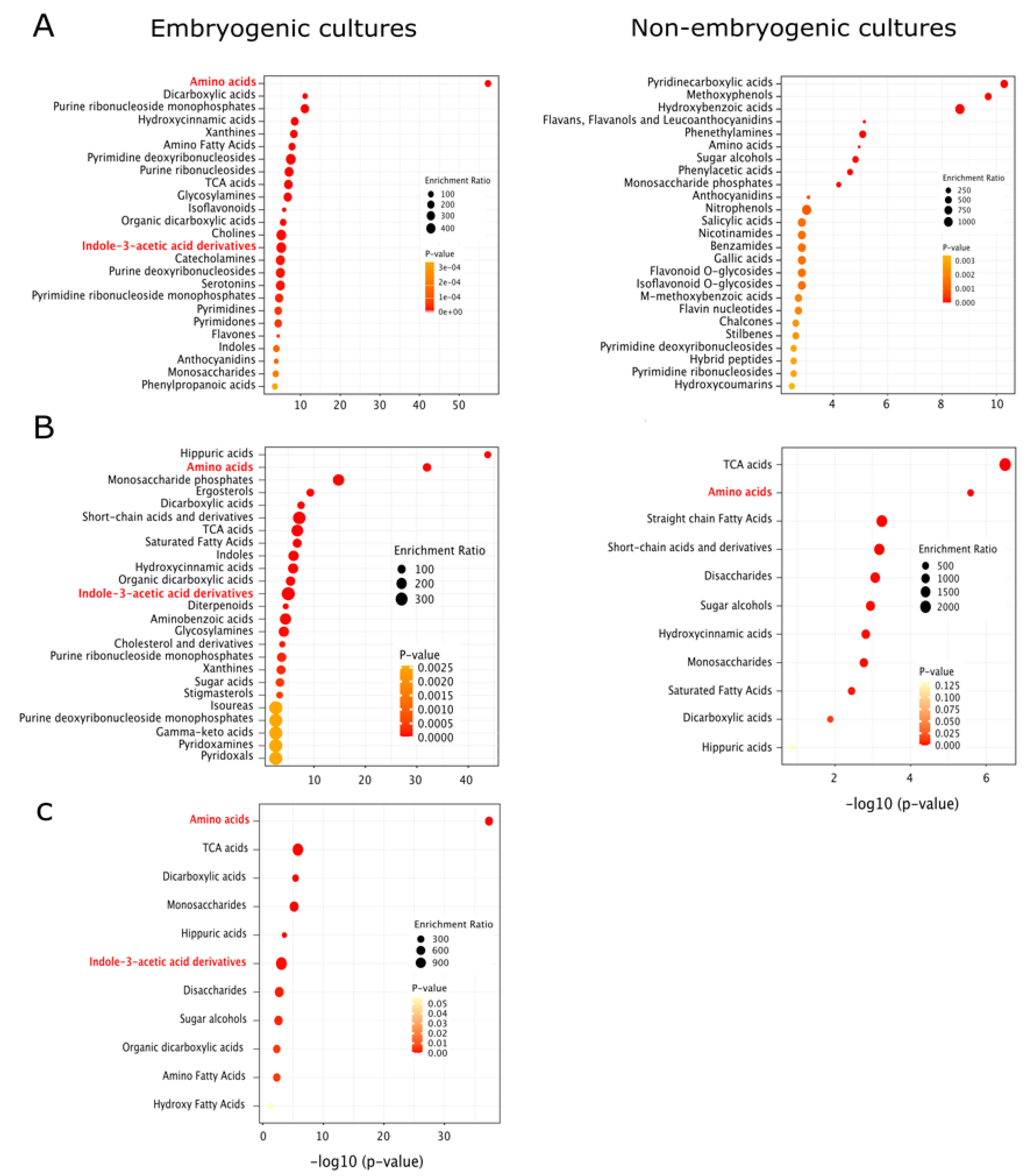
2.2.4. General Analysis
3. Zygotic and Somatic Embryo Development
3.1. Dicots
3.2. Monocots
3.3. Gymnosperms
3.4. General Analysis
4. Zygotic and Somatic Embryo Maturation
4.1. Dicots
4.2. Monocots
4.3. Gymnosperms
4.4. General Analysis
5. Perspectives: What We Know about ZE and SE
5.1. Induction
5.2. Development
5.3. Maturation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| 2DE | Two-dimensional electrophoresis |
| EC | Embryogenic callus |
| EMSN | Extracellular matrix surface Network |
| EP | Embryogenic potency |
| GST | Glutathione-s-transferases |
| HSPs | Heat shock proteins |
| JA | Jasmonic acid |
| LEAs | Late embryogenesis-abundant proteins |
| MS | Mass spectrometry |
| NEC | Non-embryogenic cultures |
| PGRs | Plant growth regulators |
| PCA | Principal component analysis |
| POX | Peroxidase |
| PP | Phenylpropanoid pathway |
| PA | Polyamine |
| ROS | Reactive oxygen species |
| SAM | S-adenosylmethionine |
| SE | Somatic embryogenesis |
| SOD | Superoxide dismutase |
| TMT | Tandem mass tags |
| ZE | Zygotic embryogenesis. |
References
- Armenta-Medina, A.; Gillmor, C.S. Genetic, molecular and parent-of-origin regulation of early embryogenesis in flowering plants. Curr. Top. Dev. Biol. 2019, 131, 497–543. [Google Scholar]
- Bayer, M.; Slane, D.; Jürgens, G. Early plant embryogenesis—Dark ages or dark matter? Curr. Opin. Plant Biol. 2017, 35, 30–36. [Google Scholar] [CrossRef]
- Moller, B.; Weijers, D. Auxin control of embryo patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar] [CrossRef] [PubMed]
- Dodeman, V.L.; Ducreux, G.; Kreis, M. Zygotic Embryogenesis versus somatic embryogenesis. J. Exp. Bot. 1997, 48, 1493–1509. [Google Scholar] [CrossRef]
- Mayer, U.; Buttner, G.; Jurgens, G. Apical-basal pattern formation in the Arabidopsis embryo: Studies on the role of the gnom gene. Development 1993, 117, 149–162. [Google Scholar] [CrossRef]
- Raghavan, V.; Sharma, K.K. Zygotic Embryogenesis in gymnosperms and angiosperms. In In Vitro Embryogenesis in Plants. Current Plant Science and Biotechnology in Agriculture; Thorpe, T.A., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 73–115. [Google Scholar] [CrossRef]
- Webber, J.M. Polyembryony. Bot. Rev. 1940, 6, 575–598. [Google Scholar] [CrossRef]
- Mordhorst, A.P.; Toonen, M.A.J.; de Vries, S.C.; Meinke, D. Plant embryogenesis. CRC Crit. Rev. Plant Sci. 1997, 16, 535–576. [Google Scholar] [CrossRef]
- Fehér, A.; Bernula, D.; Gémes, K. The Many ways of somatic embryo initiation. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer International Publishing: Cham, Switzerland, 2016; pp. 23–37. [Google Scholar]
- Radoeva, T.; Weijers, D. A roadmap to embryo identity in plants. Trends Plant Sci. 2014, 19, 709–716. [Google Scholar] [CrossRef]
- Heringer, A.S.; Santa-Catarina, C.; Silveira, V. Insights from proteomic studies into plant somatic embryogenesis. Proteomics 2018, 18, 1700265. [Google Scholar] [CrossRef]
- Winkelmann, T. What can we learn from seeds? somatic versus zygotic embryogenesis. Acta Hort. 2017, 1187, 1–12. [Google Scholar] [CrossRef]
- Solís-Ramos, L.Y.; Andrade-Torres, A.; Sáenz-Carbonell, L.A.; Oropeza-Salín, C.M.; Castaño de la Serna, E. Somatic Embryogenesis in Recalcitrant Plants; Andrade-Torres, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 597–618. [Google Scholar] [CrossRef]
- Lelu-Walter, M.-A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Pâques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, bbenefits, challenges and future direction. Tree Genet. Genomes 2013, 9, 883–899. [Google Scholar] [CrossRef]
- Goldberg, R.B.; de Paiva, G.; Yadegari, R. Plant Embryogenesis: Zygote to seed. Science 1994, 266, 605. [Google Scholar] [CrossRef] [PubMed]
- Khanday, I.; Sundaresan, V. Plant zygote development: Recent insights and applications to clonal seeds. Curr. Opin. Plant Biol. 2021, 59, 101993. [Google Scholar] [CrossRef]
- Attree, S.M.; Fowke, L.C. Embryogeny of gymnosperms: Advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult. 1993, 35, 1–35. [Google Scholar] [CrossRef]
- Hudec, L.; Konrádová, H.; Hašková, A.; Lipavská, H. Norway spruce embryogenesis: Changes in carbohydrate profile, structural development and response to polyethylene glycol. Tree Physiol. 2016, 36, 548–561. [Google Scholar] [CrossRef]
- Aburaya, S.; Aoki, W.; Kuroda, K.; Minakuchi, H.; Ueda, M. Temporal proteome dynamics of Clostridium cellulovorans cultured with major plant cell wall polysaccharides. BMC Microbiol. 2019, 19, 118. [Google Scholar] [CrossRef]
- Chandramouli, K.; Qian, P.-Y. Proteomics: Challenges, techniques and possibilities to overcome biological sample complexity. Hum. Gen. Proteom 2009, 2009, 239204. [Google Scholar] [CrossRef]
- Jorrín-Novo, J.V.; Maldonado, A.M.; Echevarría-Zomeño, S.; Valledor, L.; Castillejo, M.A.; Curto, M.; Valero, J.; Sghaier, B.; Donoso, G.; Redondo, I. Plant proteomics update (2007–2008): Second-generation proteomic techniques, an appropriate experimental design, and data analysis to fulfill MIAPE standards, increase plant proteome coverage and expand biological knowledge. J. Proteomics 2009, 72, 285–314. [Google Scholar] [CrossRef]
- Ge, F.; Hu, H.; Huang, X.; Zhang, Y.; Wang, Y.; Li, Z.; Zou, C.; Peng, H.; Li, L.; Gao, S.; et al. Metabolomic and proteomic analysis of maize embryonic callus induced from immature embryo. Sci. Rep. 2017, 7, 1004. [Google Scholar] [CrossRef] [PubMed]
- Elhiti, M.; Stasolla, C.; Wang, A. Molecular regulation of plant somatic embryogenesis. In Vitro Cell Dev. Biol. 2013, 49, 631–642. [Google Scholar] [CrossRef]
- Osorio-Montalvo, P.; Sáenz-Carbonell, L.; De-la-Peña, C. 5-Azacytidine: A Promoter of epigenetic changes in the quest to improve plant somatic embryogenesis. Int. J. Mol. Sci. 2018, 19, 3182. [Google Scholar] [CrossRef] [PubMed]
- Staszak, A.; Pawłowski, T. Proteomic analysis of embryogenesis and the acquisition of seed dormancy in Norway maple (Acer platanoides L.). Int. J. Mol. Sci. 2014, 15, 10868–10891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, X.; Guo, K.; Deng, J.; Xu, J.; Gao, W.; Lindsey, K.; Zhang, X. ROS Homeostasis regulates somatic embryogenesis via the regulation of auxin signaling in cotton. Mol. Cell Proteomics 2016, 15, 2108–2124. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Tang, Z.; Yu, X.; Wu, J.; Zeng, F. Metabolome and transcriptome association analysis reveals dynamic regulation of purine metabolism and flavonoid synthesis in transdifferentiation during somatic embryogenesis in cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Fan, Y.; Fan, Y.; Tang, Z.; Zeng, F. Dynamic TMT-based quantitative proteomics analysis of critical initiation process of totipotency during cotton somatic embryogenesis transdifferentiation. Int. J. Mol. Sci. 2019, 20, 1691. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Parreira, J.R.; Santos, R.; Duque, A.S.; Francisco, R.; Tomé, D.F.A.; Ricardo, C.P.; Coelho, A.V.; Fevereiro, P. A proteomics study of the induction of somatic embryogenesis in Medicago truncatula using 2DE and MALDI-TOF/TOF. Physiol. Plant 2012, 146, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Igielski, R.; Kępczyńska, E. Gene expression and metabolite profiling of gibberellin biosynthesis during induction of somatic embryogenesis in Medicago runcatula Gaertn. PLoS ONE 2017, 12, e0182055. [Google Scholar] [CrossRef]
- Isah, T. proteome study of somatic embryogenesis in Nothapodytes nimmoniana (J. Graham) Mabberly. 3 Biotech 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Isah, T.; Umar, S. Proteome study of embryogenic competence acquisition in the callus cultures of Nothapodytes nimmoniana (J. Graham) Mabberly. Acta Physiol. Plant 2019, 41, 96. [Google Scholar] [CrossRef]
- Olivares-García, C.A.; Mata-Rosas, M.; Peña-Montes, C.; Quiroz-Figueroa, F.; Segura-Cabrera, A.; Shannon, L.M.; Loyola-Vargas, V.M.; Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Ibarra-Laclette, E.; et al. Phenylpropanoids are connected to cell wall fortification and stress tolerance in avocado somatic embryogenesis. Int. J. Mol. Sci. 2020, 21, 5679. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbasi, B.H.; Ali, H.; Ali, M.; Adil, M.; Hussain, I. Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Slybum Marianum L. Plant Cell Tissue Organ Cult. 2015, 120, 127–139. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Y.; Zou, H.; Su, S.; Li, S.; Shan, X.; Xi, J.; Yuan, Y. Comparative proteomic analysis of the H99 inbred maize (Zea mays L.) Line in embryogenic and non-embryogenic callus during somatic embryogenesis. Plant Cell Tissue Organ Cult. 2013, 113, 103–119. [Google Scholar] [CrossRef]
- Varhaníková, M.; Uvackova, L.; Skultety, L.; Pretova, A.; Obert, B.; Hajduch, M. Comparative quantitative proteomic analysis of embryogenic and non-embryogenic calli in maize suggests the role of oxylipins in plant totipotency. J. Proteomics 2014, 104, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shan, X.; Wu, Y.; Su, S.; Li, S.; Liu, H.; Han, J.; Yuan, Y. ITRAQ-based quantitative proteomic analysis of embryogenic and non-embryogenic calli derived from a maize (Zea Mays L.) inbred line Y423. Int. J. Mol. Sci. 2018, 19, 4004. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.M.; Karim, R.; Tan, Y.S.; Teh, H.F.; Danial, A.D.; Ho, L.S.; Khalid, N.; Appleton, D.R.; Harikrishna, J.A. Amino acid and secondary metabolite production in embryogenic and non-embryogenic callus of fingerroot ginger (Boesenbergia rotunda). PLoS ONE 2016, 11, e0156714. [Google Scholar] [CrossRef]
- Mamedes-Rodrigues, T.C.; Batista, D.S.; Vieira, N.M.; Matos, E.M.; Fernandes, D.; Nunes-Nesi, A.; Cruz, C.D.; Viccini, L.F.; Nogueira, F.T.S.; Otoni, W.C. Regenerative potential, metabolic profile, and genetic stability of Brachypodium distachyon embryogenic calli as affected by successive subcultures. Protoplasma 2018, 255, 655–667. [Google Scholar] [CrossRef]
- Kumaravel, M.; Uma, S.; Backiyarani, S.; Saraswathi, M.S.; Vaganan, M.M.; Muthusamy, M.; Sajith, K.P. Differential proteome analysis during early somatic embryogenesis in Musa Spp. AAA Cv. Grand naine. Plant Cell Rep. 2017, 36, 163–178. [Google Scholar] [CrossRef]
- Marimuthu, K.; Subbaraya, U.; Suthanthiram, B.; Marimuthu, S.S. Molecular analysis of somatic embryogenesis through proteomic approach and optimization of protocol in recalcitrant Musa spp. Physiol. Plant 2019, 167, 282–301. [Google Scholar] [CrossRef]
- De Carvalho Silva, R.; Carmo, L.S.T.; Luis, Z.G.; Silva, L.P.; Scherwinski-Pereira, J.E.; Mehta, A. Proteomic identification of differentially expressed proteins during the acquisition of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). J. Proteomics 2014, 104, 112–127. [Google Scholar] [CrossRef]
- Ribeiro, D.G.; de Almeida, R.F.; Fontes, W.; de Souza Castro, M.; de Sousa, M.V.; Ricart, C.A.O.; da Cunha, R.N.V.; Lopes, R.; Scherwinski-Pereira, J.E.; Mehta, A. Stress and cell cycle regulation during somatic embryogenesis plays a key role in oil palm callus development. J. Proteomics 2019, 192, 137–146. [Google Scholar] [CrossRef]
- Aroonluk, S.; Roytrakul, S.; Jantasuriyarat, C. Identification and characterization of phosphoproteins in somatic embryogenesis acquisition during oil palm tissue culture. Plants 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.P.F.; Vieira, L.N.; Heringer, A.S.; Puttkammer, C.C.; Silveira, V.; Guerra, M.P. DNA methylation and proteome profiles of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures as affected by plant growth regulators supplementation. Plant Cell Tissue Organ Cult. 2016, 125, 353–374. [Google Scholar] [CrossRef]
- Douétts-Peres, J.C.; Aragão, V.P.M.; Cruz, M.A.L.; Reis, R.S.; Elbl, P.; dos Santos, A.L.W.; Floh, E.I.S.; Silveira, V.; Santa-Catarina, C. AaMps1 protein inhibition regulates the protein profile, nitric oxide, carbohydrate and polyamine contents in embryogenic suspension cultures of Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae). Plant Cell Tissue Organ Cult. 2019, 138, 273–286. [Google Scholar] [CrossRef]
- Gautier, F.; Eliášová, K.; Leplé, J.-C.; Vondráková, Z.; Lomenech, A.-M.; le Metté, C.; Label, P.; Costa, G.; Trontin, J.-F.; Teyssier, C.; et al. Repetitive somatic embryogenesis induced cytological and proteomic changes in embryogenic lines of Pseudotsuga menziesii [Mirb.]. BMC Plant Biol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Gautier, F.; Label, P.; Eliášová, K.; Leplé, J.-C.; Motyka, V.; Boizot, N.; Vondráková, Z.; Malbeck, J.; Trávníčková, A.; le Metté, C.; et al. Cytological, Biochemical and molecular events of the embryogenic state in Douglas-fir (Pseudotsuga menziesii [Mirb.]). Front. Plant Sci. 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Businge, E.; Brackmann, K.; Moritz, T.; Egertsdotter, U. Metabolite profiling reveals clear metabolic changes during somatic embryo development of Norway spruce (Picea abies). Tree Physiol. 2012, 32, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Klubicová, K.; Uvácková, L.; Danchenko, M.; Nemecek, P.; Skultéty, L.; Salaj, J.; Salaj, T. Insights into the early stage of Pinus nigra Arn. somatic embryogenesis using discovery proteomics. J. Proteomics 2017, 169, 99–111. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, J.H.; Pulkkinen, P.; Kong, L.S. Changes in the metabolome of Picea balfouriana embryogenic tissues that were linked to different levels of 6-BAP by gas chromatography-mass spectrometry approach. PLoS ONE 2015, 10, e0141841. [Google Scholar] [CrossRef][Green Version]
- Winkelmann, T.; Heintz, D.; Van Dorsselaer, A.; Serek, M.; Braun, H.P. Proteomic analyses of somatic and zygotic embryos of Cyclamen persicum Mill. reveal new insights into seed and germination physiology. Planta 2006, 224, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Rode, C.; Gallien, S.; Heintz, D.; van Dorsselaer, A.; Braun, H.-P.; Winkelmann, T. Enolases: Storage compounds in seeds? evidence from a proteomic comparison of zygotic and somatic embryos of Cyclamen persicum Mill. Plant Mol. Biol. 2011, 75, 305–319. [Google Scholar] [CrossRef]
- Rode, C.; Lindhorst, K.; Braun, H.-P.; Winkelmann, T. From callus to embryo: A proteomic view on the development and maturation of somatic embryos in Cyclamen Persicum. Planta 2012, 235, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.W.; Rode, C.; Colditz, F.; Haase, C.; Braun, H.-P.; Winkelmann, T. Proteomic and histological analyses of endosperm development in Cyclamen persicum as a basis for optimization of somatic embryogenesis. Plant Sci. 2013, 201, 52–65. [Google Scholar] [CrossRef]
- Winkelmann, T.; Ratjens, S.; Bartsch, M.; Rode, C.; Niehaus, K.; Bednarz, H. Metabolite profiling of somatic embryos of Cyclamen Persicum in comparison to zygotic embryos, endosperm, and testa. Front. Plant Sci. 2015, 6, 597. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, C.; Wang, Q.; Yang, Z.; Wang, Y.; Zhang, X.; Wu, Z.; Hou, Y.; Wu, J.; Li, F. ITRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J. Proteome Res. 2015, 14, 268–278. [Google Scholar] [CrossRef]
- Noah, A.M.; Niemenak, N.; Sunderhaus, S.; Haase, C.; Omokolo, D.N.; Winkelmann, T.; Braun, H.-P. Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J. Proteomics 2013, 78, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Zhang, J.; Wang, Q.; Zhou, B.; Zhong, J.; Zhang, C.; Qiu, X.; Wen, B.; Zhang, S.; Fu, X.; et al. Stress responsive proteins are actively regulated during rice (Oryza sativa) embryogenesis as indicated by quantitative proteomics analysis. PLoS ONE 2013, 8, e74229. [Google Scholar] [CrossRef]
- Silveira, V.; Santa-Catarina, C.; Balbuena, T.S.; Moraes, F.M.S.; Ricart, C.A.O.; Sousa, M.V.; Guerra, M.P.; Handro, W.; Floh, E.I.S. Endogenous abscisic acid and protein contents during seed development of Araucaria angustifolia. Biol. Plant 2008, 52, 101–104. [Google Scholar] [CrossRef]
- Balbuena, T.S.; Silveira, V.; Junqueira, M.; Dias, L.L.C.; Santa-Catarina, C.; Shevchenko, A.; Floh, E.I.S. Changes in the 2-DE protein profile during zygotic embryogenesis in the brazilian pine (Araucaria angustifolia). J. Proteomics 2009, 72, 337–352. [Google Scholar] [CrossRef]
- Shi, J.; Zhen, Y.; Zheng, R.-H. proteome profiling of early seed development in Cunninghamia lanceolata (Lamb.) Hook. J. Exp. Bot. 2010, 61, 2367–2381. [Google Scholar] [CrossRef]
- Zhen, Y.; Ji, S.S.; Zhao, Z.Z.; Xu, S.P.; Wang, Z.J.; Zheng, R.H. Proteomic analysis of seed development in chinese Fir (Cunninghamia lanceolata). Russ. J. Plant Physiol. 2010, 57, 560–567. [Google Scholar] [CrossRef]
- Filonova, L.H.; Bozhkov, P.V.; Brukhin, V.B.; Daniel, G.; Zhivotovsky, B.; von Arnold, S. Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J. Cell Sci. 2000, 24, 4399–4411. [Google Scholar] [CrossRef]
- Morel, A.; Teyssier, C.; Trontin, J.-F.; Eliášová, K.; Pešek, B.; Beaufour, M.; Morabito, D.; Boizot, N.; le Metté, C.; Belal-Bessai, L.; et al. Early molecular events involved in Pinus pinaster Ait. somatic embryo development under reduced water availability: Transcriptomic and proteomic analyses. Physiol. Plant 2014, 152, 184–201. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic embryogenesis: A model for early development in higher plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef]
- Gray, D.J.; Purohit, A. Quiescence and dormancy in somatic embryos. In High-Tech and Micropropagation I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 382–394. [Google Scholar]
- Tsogtbaatar, E.; Cocuron, J.-C.; Sonera, M.C.; Alonso, A.P. Metabolite fingerprinting of pennycress (Thlaspi arvense L.) embryos to assess active pathways during oil synthesis. J. Exp. Bot. 2015, 66, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Komatsu, S.; He, M.; Liu, G.; Shen, S. Proteomic analysis of the seed development in Jatropha curcas: From carbon flux to the lipid accumulation. J. Proteomics 2013, 91, 23–40. [Google Scholar] [CrossRef]
- Niemenak, N.; Kaiser, E.; Maximova, S.N.; Laremore, T.; Guiltinan, M.J. Proteome analysis during pod, Zygotic and somatic embryo maturation of Theobroma cacao. J. Plant Physiol. 2015, 180, 49–60. [Google Scholar] [CrossRef]
- Almeida, F.A.; Vale, E.M.; Reis, R.S.; Santa-Catarina, C.; Silveira, V. LED lamps enhance somatic embryo maturation in association with the differential accumulation of proteins in the Carica papaya L. ‘Golden’ embryogenic callus. Plant Physiol. Biochem. 2019, 143, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sghaier-Hammami, B.; Drira, N.; Jorrín-Novo, J.V. Comparative 2-DE proteomic analysis of Date palm (Phoenix dactylifera L.) somatic and zygotic embryos. J. Proteomics 2009, 73, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Sghaier-Hammami, B.; Valledor, L.; Drira, N.; Jorrin-Novo, J.V. Proteomic analysis of the development and germination of Date palm (Phoenix dactylifera L.) zygotic embryos. Proteomics 2009, 9, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Sghaier-Hammami, B.; Jorrín-Novo, J.V.; Gargouri-Bouzid, R.; Drira, N. Abscisic acid and sucrose increase the protein content in Date palm somatic embryos, causing changes in 2-DE profile. Phytochemistry 2010, 71, 1223–1236. [Google Scholar] [CrossRef]
- Heringer, A.S.; Barroso, T.; Macedo, A.F.; Santa-Catarina, C.; Souza, G.H.M.F.; Floh, E.I.S.; de Souza-Filho, G.A.; Silveira, V. Label-free quantitative proteomics of embryogenic and non-embryogenic callus during sugarcane somatic embryogenesis. PLoS ONE 2015, 10, e0127803. [Google Scholar] [CrossRef]
- Reis, R.S.; de Moura Vale, E.; Heringer, A.S.; Santa-Catarina, C.; Silveira, V. Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J. Proteomics 2016, 130, 170–179. [Google Scholar] [CrossRef]
- Cao, H.; He, M.; Zhu, C.; Yuan, L.; Dong, L.; Bian, Y.; Zhang, W.; Yan, Y. Distinct metabolic changes between wheat embryo and endosperm during grain development revealed by 2D-DIGE-based integrative proteome analysis. Proteomics 2016, 16, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, T.S.; Jo, L.; Pieruzzi, F.P.; Dias, L.L.C.; Silveira, V.; Santa-Catarina, C.; Junqueira, M.; Thelen, J.J.; Shevchenko, A.; Floh, E.I.S. Differential proteome analysis of mature and germinated embryos of Araucaria angustifolia. Phytochemistry 2011, 72, 302–311. [Google Scholar] [CrossRef]
- Jo, L.; dos Santos, A.L.W.; Bueno, C.A.; Barbosa, H.R.; Floh, E.I.S. Proteomic analysis and polyamines, ethylene and reactive oxygen species levels of Araucaria angustifolia (Brazilian pine) embryogenic cultures with different embryogenic potential. Tree Physiol. 2014, 34, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Trontin, J.F.; Corbineau, F.; Lomenech, A.M.; Beaufour, M.; Reymond, I.; Le Metté, C.; Ader, K.; Harvengt, L.; Cadene, M.; et al. Cotyledonary somatic embryos of Pinus pinaster Ait. Most closely resemble fresh, maturing cotyledonary zygotic embryos: Biological, carbohydrate and proteomic analyses. Planta 2014, 240, 1075–1095. [Google Scholar] [CrossRef]
- Businge, E.; Bygdell, J.; Wingsle, G.; Moritz, T.; Egertsdotter, U. The effect of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol. Plant 2013, 149, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Zhang, J.; Xia, Y.; Kong, L.; OuYang, F.; Zhang, S.; Zhang, H.; Wang, J. Proteomic analysis of stress-related proteins and metabolic pathways in Picea asperata somatic embryos during partial desiccation. Plant Biotechnol. J. 2017, 15, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Castander-Olarieta, A.; Pereira, C.; Montalbán, I.A.; Mendes, V.M.; Correia, S.; Suárez-Álvarez, S.; Manadas, B.; Canhoto, J.; Moncaleán, P. Proteome-wide analysis of heat-stress in Pinus radiata somatic embryos reveals a combined response of sugar metabolism and translational regulation mechanisms. Front. Plant Sci. 2021, 12, 631239. [Google Scholar] [CrossRef]
- Dowlatabadi, R.; Weljie, A.M.; Thorpe, T.A.; Yeung, E.C.; Vogel, H.J. Metabolic footprinting study of white spruce somatic embryogenesis using NMR spectroscopy. Plant Physiol. Biochem. 2009, 47, 343–350. [Google Scholar] [CrossRef]
- Okamoto, T.; Higuchi, K.; Shinkawa, T.; Isobe, T.; Lörz, H.; Koshiba, T.; Kranz, E. Identification of major proteins in maize egg cells. Plant Cell Physiol. 2004, 45, 1406–1412. [Google Scholar] [CrossRef]
- Abiko, M.; Furuta, K.; Yamauchi, Y.; Fujita, C.; Taoka, M.; Isobe, T.; Okamoto, T. Identification of proteins enriched in rice egg or sperm cells by single-cell proteomics. PLoS ONE 2013, 8, e69578. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Pingault, L.; Zogli, P.; Schiefelbein, J. Plant systems biology at the single-cell level. Trends Plant Sci. 2017, 22, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, V.; Loyola-Vargas, V.M. Advanced proteomic approaches to elucidate somatic embryogenesis. Front. Plant Sci. 2018, 9, 1658. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez-Escobar, J.; Bojórquez-Velázquez, E.; Elizalde-Contreras, J.M.; Guerrero-Analco, J.A.; Loyola-Vargas, V.M.; Mata-Rosas, M.; Ruiz-May, E. Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants. Int. J. Mol. Sci. 2021, 22, 11807. https://doi.org/10.3390/ijms222111807
Juarez-Escobar J, Bojórquez-Velázquez E, Elizalde-Contreras JM, Guerrero-Analco JA, Loyola-Vargas VM, Mata-Rosas M, Ruiz-May E. Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants. International Journal of Molecular Sciences. 2021; 22(21):11807. https://doi.org/10.3390/ijms222111807
Chicago/Turabian StyleJuarez-Escobar, Janet, Esaú Bojórquez-Velázquez, Jose M. Elizalde-Contreras, José A. Guerrero-Analco, Víctor M. Loyola-Vargas, Martín Mata-Rosas, and Eliel Ruiz-May. 2021. "Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants" International Journal of Molecular Sciences 22, no. 21: 11807. https://doi.org/10.3390/ijms222111807
APA StyleJuarez-Escobar, J., Bojórquez-Velázquez, E., Elizalde-Contreras, J. M., Guerrero-Analco, J. A., Loyola-Vargas, V. M., Mata-Rosas, M., & Ruiz-May, E. (2021). Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants. International Journal of Molecular Sciences, 22(21), 11807. https://doi.org/10.3390/ijms222111807







