Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes
Abstract
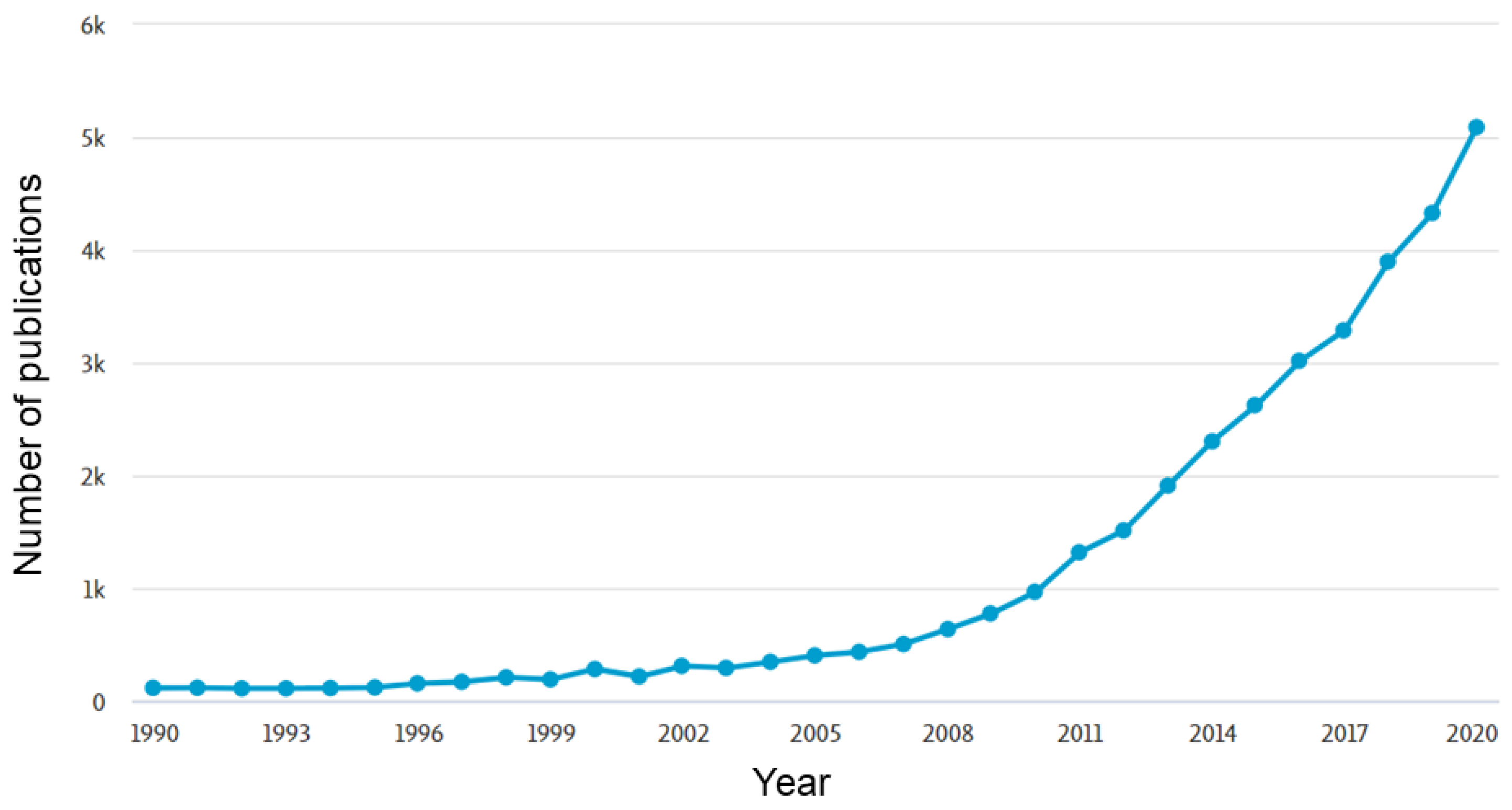
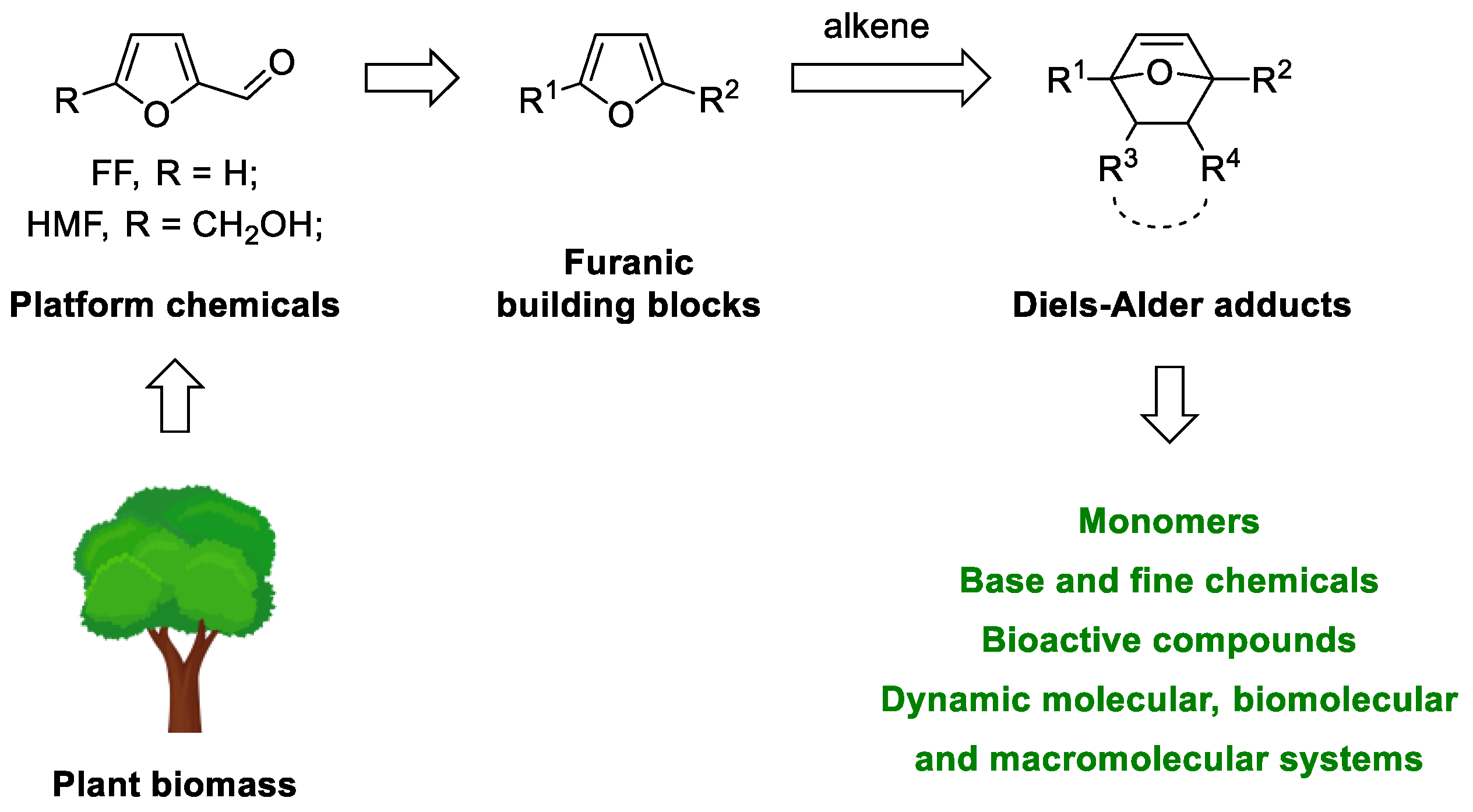
:1. Introduction
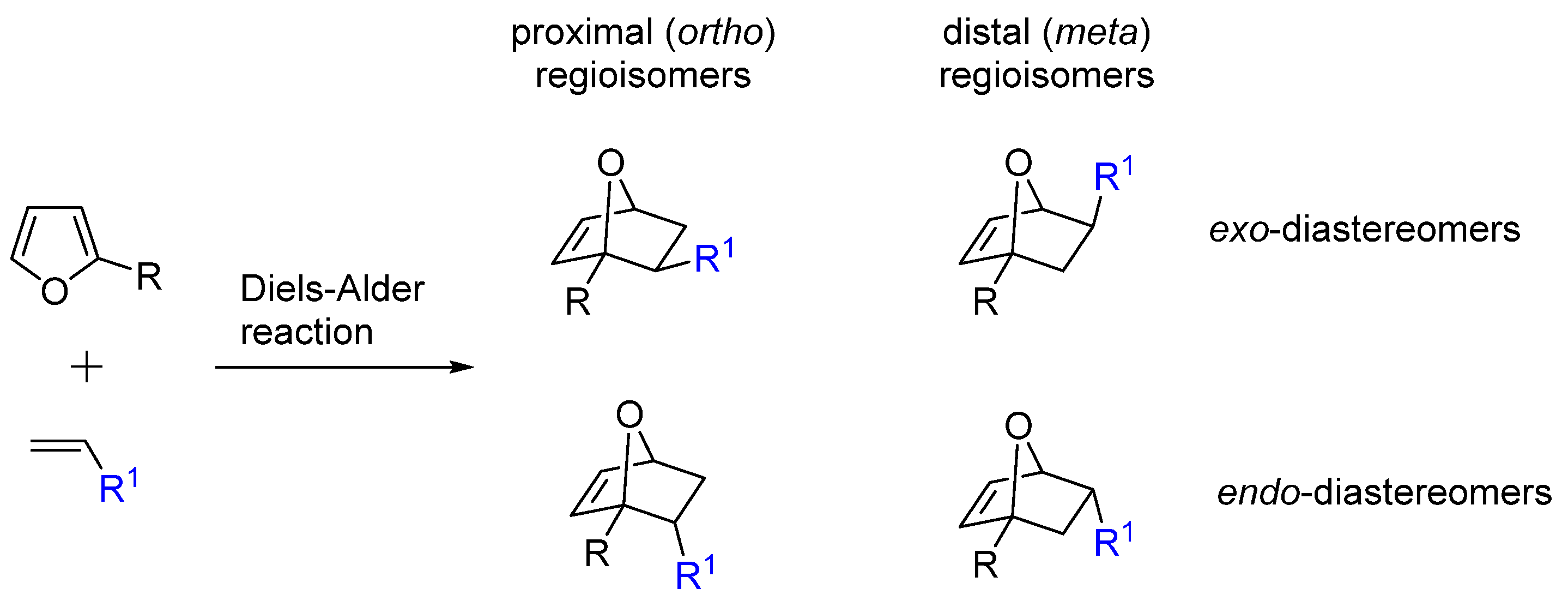
2. Selectivity of Diels-Alder Cycloaddition with Furfural Derivatives as Substrates
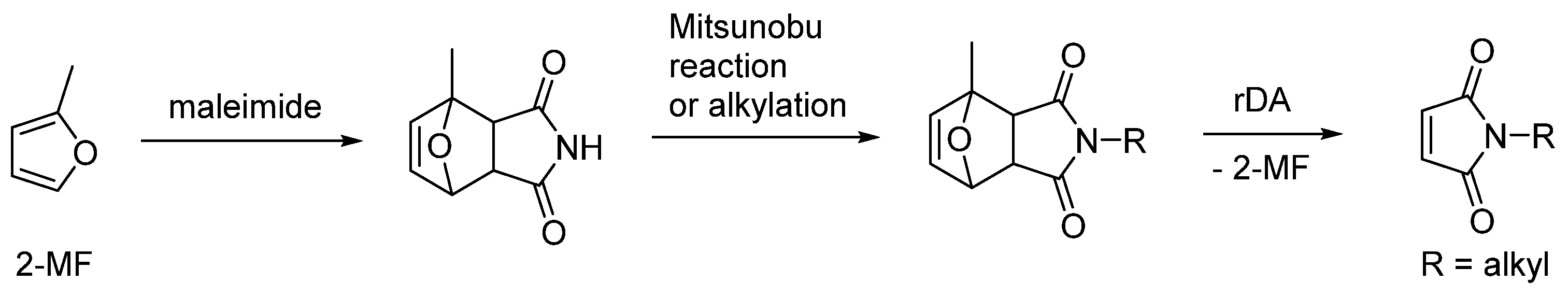
2.1. 2-Methylfuran

| № | Dienophile | Conditions | Selectivity | Yield of Adducts (%), [Ref.] |
|---|---|---|---|---|
| 1 | Dimethyl maleate | HfCl4, CH2Cl2, −30 °C | Endo/exo 84:16 | 94, [76] |
| 2 | Dimethyl maleate | HfCl4, CH2Cl2, −50 °C | Endo/exo > 98:2 | 82, [76] |
| 3 |  | 22 °C |  | 90, [77] |
| 4 | 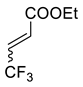 | 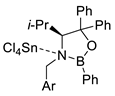 Ar = 1-naphthyl (cat.), CH2Cl2, −78 °C |  99 de, 99 ee | 99, [78] |
| 5 | 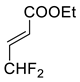 |  Ar = 1-naphthyl (cat.), CH2Cl2, −78 °C | 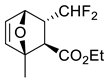 99 de, 99 ee | 74, [78] |
| 6 |  | 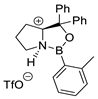 (cat.), CH2Cl2, −78 °C | Ortho (endo/exo 94:6), 98 ee (for endo isomer) | 74, [79] |
| 7 | Benzyl acrylate | HfCl4, CH2Cl2, −30 °C | Endo/exo 28:72 (mixture of regio isomers) | 84, [76] |
| 8 | Benzyl acrylate | HfCl4, CH2Cl2, −50 °C | Endo/exo (31:69) (mixture of regio isomers) | 85, [76] |
| 9 | Acrylonitrile | ZnI2, neat, 50 °C | N.d. | 69, [80] |
| 10 | Acrylonitrile | Neat, 60 °C | Ortho 66 (endo/exo 61:39), meta 34 (endo/exo 56:44) | 69, [31,32] |
| 11 | 1-Cyanovinyl acetate | ZnI2, neat, 0 °C, 8 days | Ortho (endo/exo 1:1) 2 | 52, [81] |
| 12 | 1-Cyanovinyl acetate | ZnI2, neat, 20 °C, 26 h | Ortho endo2 | 17, [81] |
| 13 | 1-Cyanovinyl acetate | ZnI2, neat, RT, 24 h | Ortho (endo/exo 3:1) 2 | 30, [82] |
| 14 | 1-Cyanovinyl acetate | MgI2, neat, RT, 24 h | Ortho (endo/exo 4:1) 2 | 57, [82] |
| 15 | 2-Chloroacrylonitrile | ZnI2, neat, 0 °C | Ortho/meta 10:1 (mixture of endo/exo) | 91 1, [83] |
| 16 | 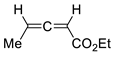 | Benzene, reflux | 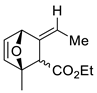 Endo/exo 1,1:1 | 70, [84] |
| 17 |  | Eu(fod), RT | 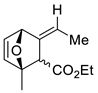 Endo/exo 2,1:1 | 80, [84] |
| 18 | 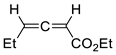 | Eu(fod), RT |  Endo/exo ~2,8:1 | 80, [84] |
| 19 | 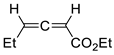 | Benzene, reflux | 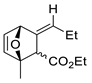 Endo/exo ~1:1 | 73, [84] |
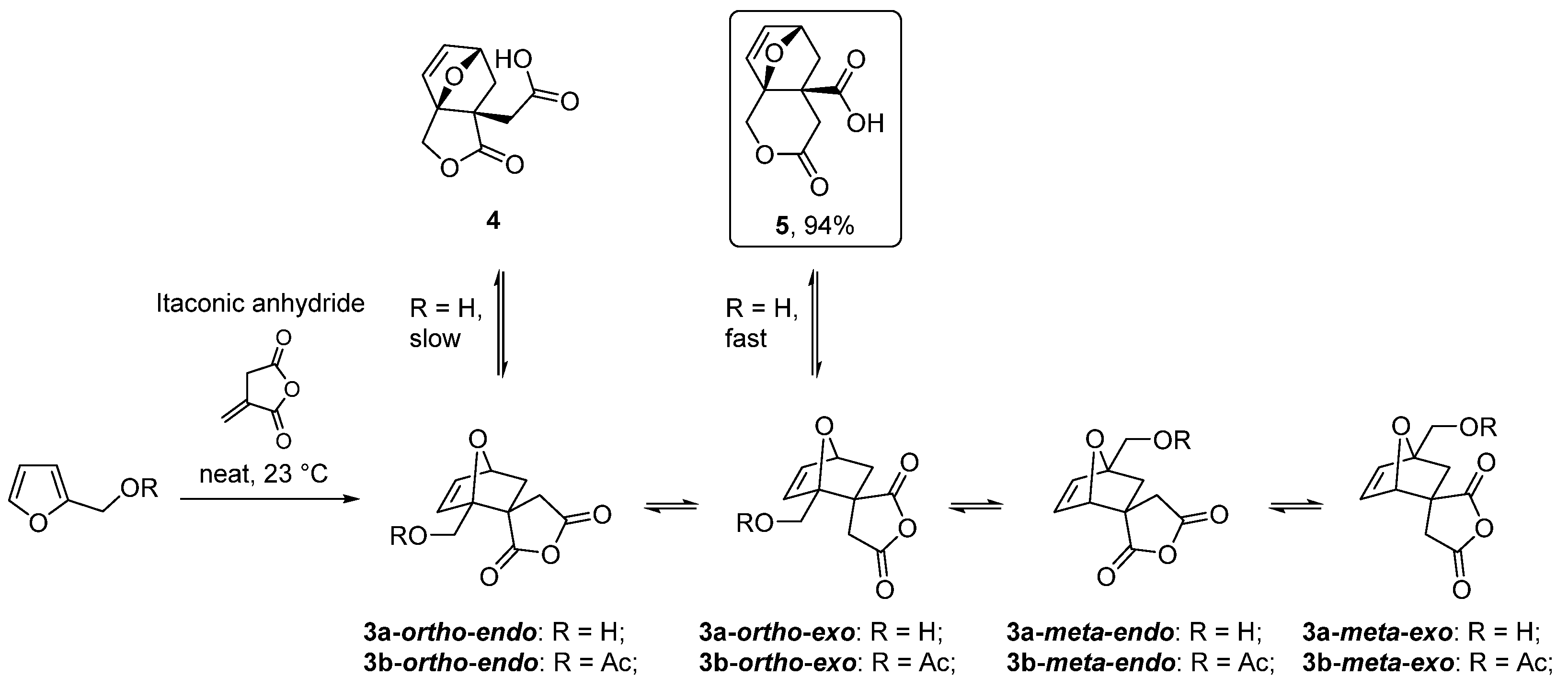
| 20 | Itaconic anhydride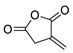 | Neat, 23 °C | Ortho (endo:exo)/meta (endo:exo) 3:1/11:8 3 | 13 4, [85] |
2.2. Furanic Acetals
2.3. Functionalized Furfural Derivatives

| № | R | Dienophile | Conditions | Selectivity | Yield of Adducts (%), [Ref.] |
|---|---|---|---|---|---|
| 1 | Allyl | N-Me-maleimide | Toluene, 50 °C, 24 h | N.d. | 65 (endo), [103] |
| 2 | Allyl | N-Ph-maleimide | Toluene, 50 °C, 24 h | N.d. | 26 (exo), [103] |
| 3 | Bn | Maleic anhydride | Toluene, RT, 3 days | Exo | 43, [91] |
| 4 | Bn | Citraconic anhydride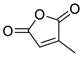 | 15 kbar, CH2Cl2, 60 h | Exo (ortho/meta 5:7) | 31 1, [68] |
| 5 | Vinyl | Maleic anhydride | Et2O, 22‒24 °C, 48 h | Endo | 72, [104] |
| 6 | Vinyl | Maleic anhydride | Et2O, 35 °C, 48 h | Endo/exo 8:1 | 66, [104] |
| 7 | Vinyl | Maleic anhydride | THF, 22‒24 °C, 90 h | Endo/exo 8:1 | 66, [104] |
| 8 | Vinyl | Maleic anhydride | MeCN, 22‒24 °C, 48 h | Endo/exo 4:1 | 68, [104] |
| 9 | Vinyl | Maleic anhydride | Toluene, 22‒24 °C | Endo/exo 12:1 | 64, [104] |
| 10 | Vinyl | Maleic anhydride | Toluene, 80 °C | Endo/exo 4:1 | 66, [104] |
| 11 | Vinyl | N-Ph-maleimide | Et2O, 22‒24 °C | Endo/exo 1:2.8 | 47, [104] |
| 12 | Vinyl | N-Ph-maleimide | Toluene, 80 °C | Endo/exo 4:1 | 66, [104] |
| 13 | Ac | Maleic anhydride | Et2O, 25 °C, 7 days | Exo | 34, [105] |
| 14 | Ac | Maleic anhydride | Toluene, RT, 97 h | Exo | 74, [88] |
| 15 | Ac | Citraconic anhydride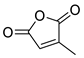 | 15 kbar, CH2Cl2, 60 h | Exo (ortho/meta 6:5) | 59 1, [68] |
| 16 | Ac | N-Me-maleimide | CH2Cl2, 23 °C | Endo/exo 77:23 | N.d., [86] |
| 17 | Ac | N-Dodecylmaleimide | THF, 23 °C | Endo/exo 64:36 | N.d., [86] |
| 18 | Ac | N-Ph-maleimide | CH2Cl2, 23 °C | Endo/exo 65:35 | N.d., [86] |
| 19 | Ac | N-(p-Nitrophenyl)maleimide | CH2Cl2, 23 °C | Endo/exo 55:45 | N.d., [86] |
| 20 | Ac | N-(p-Methoxyphenyl)maleimide | CH2Cl2, 23 °C | Endo/exo 67:33 | N.d., [86] |
| 21 | Ac | N-(Methoxy-2-propyl)maleimide | CH2Cl2, 23 °C | Endo/exo 76:24 | N.d., [86] |
| 22 | Ac | N-(2-Methoxyethyl)maleimide | CH2Cl2, 23 °C | Endo/exo 75:25 | N.d., [86] |
| 23 | Bz | Maleic anhydride | Toluene, 80 °C, 456 h | Exo | 46, [88] |
| 24 2 | Bz | Maleic anhydride | Et2O, 24 °C, 24 h | Endo | 84, [106] |
| 25 | Bz | N-Me-maleimide | CH2Cl2, 23 °C | Endo/exo 70:30 | N.d., [86] |
| 26 | Bz | N-Dodecylmaleimide | THF, 23 °C | Endo/exo 63:37 | N.d., [86] |
| 27 | COiBu | N-Pr-maleimide | CHCl3, 55 °C | Endo/exo 60:40 | N.d., [107] |
| 28 | COiBu | N-iBu-maleimide | CHCl3, 55 °C | Endo/exo 45:55 | N.d., [107] |
| 29 | COiBu | N-tBu-maleimide | CHCl3, 55 °C | Endo/exo 51:49 | N.d., [107] |
| 30 | COiBu | N-Bn-maleimide | CHCl3, 55 °C | Endo/exo 44:56 | N.d., [107] |
| 31 | COiBu | 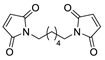 | CHCl3, 55 °C | Endo/exo 26:74 | N.d., [107] |
| 32 | COiBu | N-(2-Methylphenyl)-maleimide | CHCl3, 55 °C | Endo/exo 67:33 | N.d., [107] |
| 33 | COiBu | BMI | CHCl3, 55 °C | Endo/exo 19:81 | N.d., [107] |
| 34 | COtBu | N-Me-maleimide | CH2Cl2, 23 °C | Endo/exo 71:29 | N.d., [86] |
| 35 | COtBu | N-Dodecylmaleimide | THF, 23 °C | Endo/exo 62:38 | N.d., [86] |
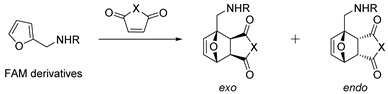
| № | R | Dienophile | Conditions | Selectivity | Yield of Adducts (%), [Ref.] |
|---|---|---|---|---|---|
| 1 | Ac | Maleic anhydride | Et20, 23 °C | Exo | 100, [109] |
| 2 | Ac | Maleimide | H3BO3/PEG-400, 90 °C | Exo | 84, [110] |
| 3 | Ac | N-Ph-maleimide | H3BO3/PEG-400, 90 °C | Exo | 78, [110] |
| 4 | Ac | N-(4-Chlorobenzyl)maleimide | H3BO3/PEG-400, 90 °C | Exo | 92, [110] |
| 5 | Boc 1 | Maleic anhydride | Toluene, 50 °C | Exo | 94, [111] |
| 6 | Boc 1 | Thiomaleic anhydride | Benzene, RT | Exo | 68, [112] |
| 7 | Boc 1 |  | EtOAc, reflux | Endo/exo (1:3.4) | 85, [113] |
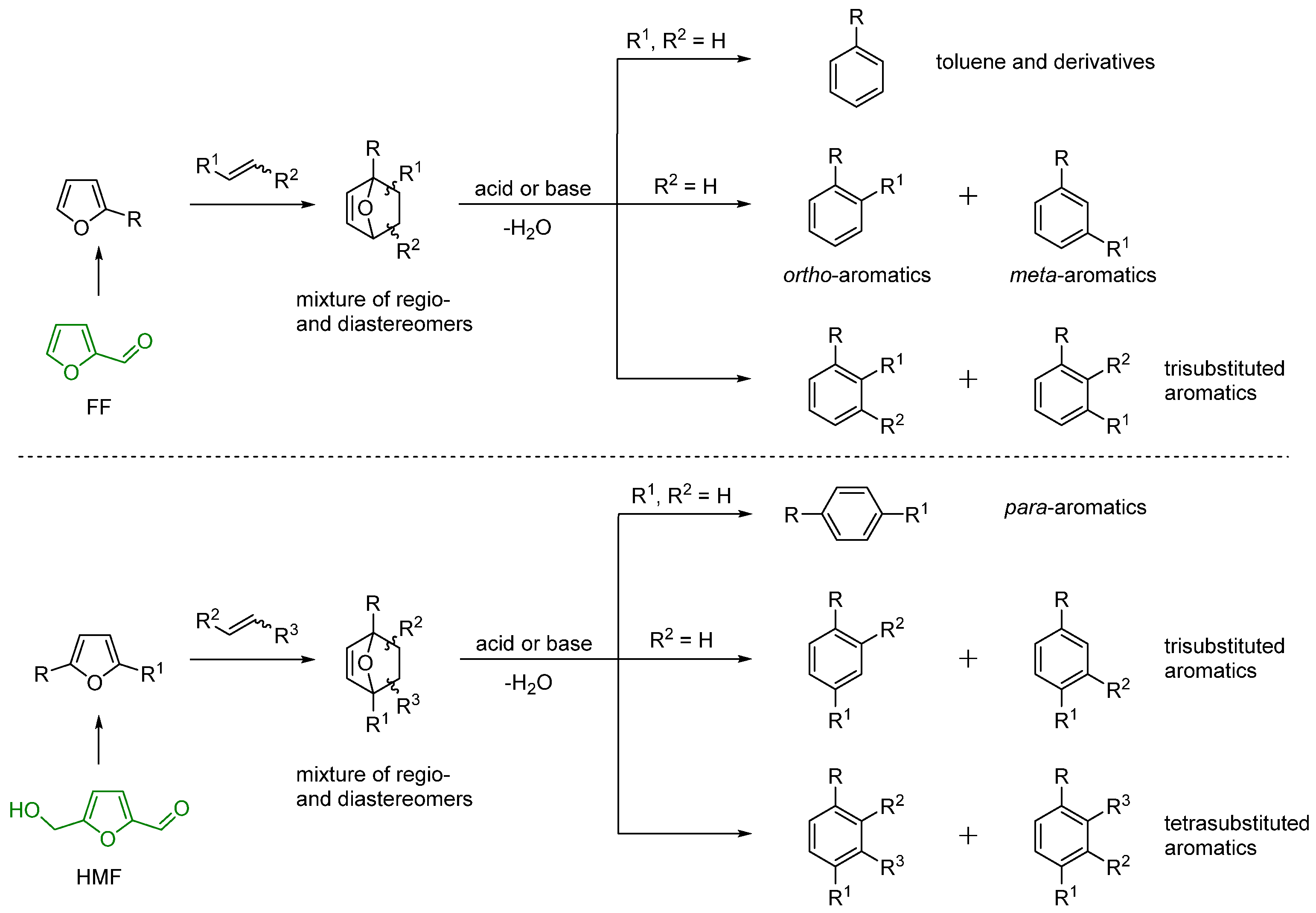
3. Regioselectivity in the Synthesis of Aromatics Using the IMDA Reaction of Furfural Derivatives with Alkenes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-MF | 2-methylfuran |
| Ac | acetate |
| BAMF | 2,5-bis(acetoxymethyl)furan |
| BHMF | 2,5-bis(hydroxymethyl)furan |
| BMI | 4,4’-bis(maleimido)diphenylmethane |
| BOC | tert-butyloxycarbonyl |
| Bn | benzyl |
| Bz | benzoyl |
| DA | Diels–Alder |
| DFT | density functional theory |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| Emim | 1-ethyl-3-methylimidazolium |
| EWG | electron-withdrawing group |
| FAM | furfuryl amine |
| FF | furfural |
| HMF | 5-(hydroxymethyl)furfural |
| HOMO | highest occupied molecular orbital |
| IMDA | intermolecular Diels–Alder |
| LUMO | lowest unoccupied molecular orbital |
| MOF | metal organic framework |
| MPA | 3-methylphthalic anhydride |
| N.d. | not determined |
| NMR | nuclear magnetic resonance |
| PEG | polyethylene glycol |
| rDA | retro-Diels–Alder |
| RT | room temperature |
| Tf | triflate |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
References
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous flow upgrading of selected C2–C6 Platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Khokhlova, E.A.; Kachala, V.V.; Ananikov, V.P. The first molecular level monitoring of carbohydrate conversion to 5-hydroxymethylfurfural in ionic liquids. B2O3-An efficient dual-function metal-free promoter for environmentally benign applications. ChemSusChem 2012, 5, 783–789. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Nemeth, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2017, 118, 505–613. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Bosch, S.V.D.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of sustainable chemistry, awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, materials science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. The increasing value of biomass: Moving from C6 carbohydrates to multifunctionalized building blocks via 5-(hydroxymethyl)furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef]
- Khemthong, P.; Yimsukanan, C.; Narkkun, T.; Srifa, A.; Witoon, T.; Pongchaiphol, S.; Kiatphuengporn, S.; Faungnawakij, K. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy 2021, 148, 106033. [Google Scholar] [CrossRef]
- Shen, G.; Andrioletti, B.; Queneau, Y. Furfural and 5-(hydroxymethyl)furfural: Two pivotal intermediates for bio-based chemistry. Curr. Opin. Green Sustain. Chem. 2020, 26, 100384. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds. Green Chem. 2021, 23, 670–688. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal catalysts for the efficient transformation of biomass-derived HMF and furfural to value added chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Drault, F.; Snoussi, Y.; Paul, S.; Itabaiana, I.; Wojcieszak, R. Recent advances in carboxylation of furoic acid into 2,5-furandicarboxylic acid: Pathways towards bio-based polymers. ChemSusChem 2020, 13, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamoorthy, D.; Weinstock, A.K.; Damodaran, K.; Iwig, D.F.; Mathers, R.T. Diglycerol-based polyesters: Melt polymerization with hydrophobic anhydrides. ChemSusChem 2014, 7, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamoorthy, D.; Lewis, S.P.; Cavazza, M.P.; Hoover, A.M.; Iwig, D.F.; Damodaran, K.; Mathers, R.T. Streamlining the conversion of biomass to polyesters: Bicyclic monomers with continuous flow. Green Chem. 2014, 16, 1774–1783. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels-Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels-Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Koehler, K.C.; Durackova, A.; Kloxin, C.J.; Bowman, C.N. Kinetic and thermodynamic measurements for the facile property prediction of diels-alder-conjugated material behavior. AIChE J. 2012, 58, 3545–3552. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Roman-Leshkóv, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels-Alder catalysis for biomass-derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Scodeller, I.; Mansouri, S.; Morvan, D.; Muller, E.; de Oliveira Vigier, K.; Wischert, R.; Jerome, F. Synthesis of renewable meta-xylylenediamine from biomass-derived furfural. Angew. Chem. Int. Ed. 2018, 57, 10510–10514. [Google Scholar] [CrossRef]
- Scodeller, I.; Vigier, K.D.O.; Muller, E.; Ma, C.; Guégan, F.; Wischert, R.; Jérôme, F. A combined experimental–theoretical study on Diels-Alder reaction with bio-based furfural: Towards renewable aromatics. ChemSusChem 2021, 14, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Galkin, K.I.; Gordeev, E.G.; Ananikov, V.P. Efficient route for the construction of polycyclic systems from bioderived HMF. Green Chem. 2017, 19, 4858–4864. [Google Scholar] [CrossRef] [Green Version]
- Cioc, R.C.; Lutz, M.; Pidko, E.A.; Crockatt, M.; Van Der Waal, J.K.; Bruijnincx, P.C.A. Direct Diels-Alder reactions of furfural derivatives with maleimides. Green Chem. 2021, 23, 367–373. [Google Scholar] [CrossRef]
- Ax, J.; Wenz, G. Thermoreversible networks by Diels-Alder reaction of cellulose furoates with bismaleimides. Macromol. Chem. Phys. 2012, 213, 182–186. [Google Scholar] [CrossRef]
- Boutelle, R.C.; Northrop, B.H. Substituent effects on the reversibility of furan–maleimide cycloadditions. J. Org. Chem. 2011, 76, 7994–8002. [Google Scholar] [CrossRef]
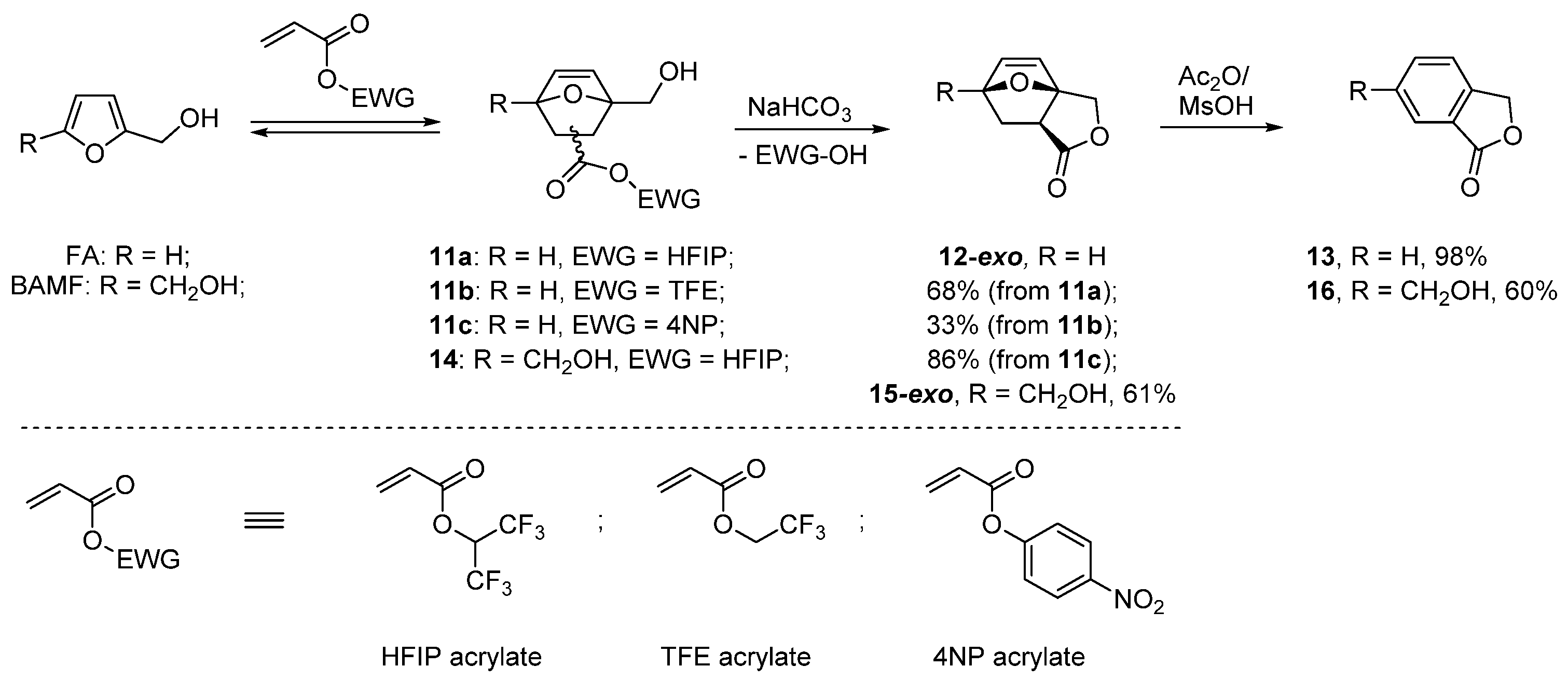
- Lancefield, C.S.; Folker, B.; Cioc, R.C.; Stanciakova, K.; Bulo, R.E.; Lutz, M.; Crockatt, M.; Bruijnincx, P.C.A. Dynamic trapping as a selective route to renewable phthalide from biomass-derived furfuryl alcohol. Angew. Chem. Int. Ed. 2020, 59, 23480–23484. [Google Scholar] [CrossRef]
- Salvati, M.E.; Balog, A.; Wei, D.D.; Pickering, D.; Attar, R.M.; Geng, J.; Rizzo, C.A.; Hunt, J.; Gottardis, M.M.; Weinmann, R.; et al. Identification of a novel class of androgen receptor antagonists based on the bicyclic-1H-isoindole-1,3(2H)-dione nucleus. Bioorganic Med. Chem. Lett. 2005, 15, 389–393. [Google Scholar] [CrossRef]
- Bakhtiari, A.B.; Hsiao, D.; Jin, G.; Gates, B.D.; Branda, N.R. An efficient method based on the photothermal effect for the release of molecules from metal nanoparticle surfaces. Angew. Chem. Int. Ed. 2009, 48, 4166–4169. [Google Scholar] [CrossRef]
- Park, J.; Heo, J.-M.; Seong, S.; Noh, J.; Kim, J.-M. Self-assembly using a retro Diels-Alder reaction. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Pal, S.; Alizadeh, M.; Kong, P.; Kilbinger, A.F.M. Oxanorbornenes: Promising new single addition monomers for the metathesis polymerization. Chem. Sci. 2021, 12, 6705–6711. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Chang, H.; Huber, G.W.; Dumesic, J.A. Chemical-switching strategy for synthesis and controlled release of norcantharimides from a biomass-derived chemical. ChemSusChem 2020, 13, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Toyoda, S.; Ishikawa, H.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Asymmetric Diels-Alder reaction involving dynamic enantioselective crystallization. J. Org. Chem. 2018, 83, 9300–9304. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Arkivoc 2005, 2001, 17. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, B.; Tin, S.; de Vries, J.G. Bio-based building blocks from 5-hydroxymethylfurfural via 1-hydroxyhexane-2,5-dione as intermediate. Chem. Sci. 2019, 10, 6024–6034. [Google Scholar] [CrossRef] [Green Version]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butler, I.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem. 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Fan, W.; Verrier, C.; Queneau, Y.; Popowycz, F. 5-Hydroxymethylfurfural (HMF) in Organic Synthesis: A review of its recent applications towards fine chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar] [CrossRef]
- Hu, L.; Xu, J.; Zhou, S.; He, A.; Tang, X.; Lin, L.; Xu, J.; Zhao, Y. Catalytic advances in the production and application of biomass-derived 2,5-Dihydroxymethylfuran. ACS Catal. 2018, 8, 2959–2980. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef] [Green Version]
- Zang, H.; Wang, K.; Zhang, M.; Xie, R.; Wang, L.; Chen, E.Y.-X. Catalytic coupling of biomass-derived aldehydes into intermediates for biofuels and materials. Catal. Sci. Technol. 2018, 8, 1777–1798. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. One-pot conversion of carbohydrates into furan derivatives via furfural and 5-Hydroxylmethylfurfural as intermediates. ChemSusChem 2016, 9, 2015–2036. [Google Scholar] [CrossRef]
- Pal, P.; Saravanamurugan, S. Recent advances in the development of 5-Hydroxymethylfurfural oxidation with base (Nonprecious)-metal-containing catalysts. ChemSusChem 2019, 12, 145–163. [Google Scholar] [CrossRef]
- Singh, S.K. Heterogeneous bimetallic catalysts for upgrading biomass-derived furans. Asian J. Org. Chem. 2018, 7, 1901–1923. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Kravchenko, O.A.; Ananikov, V.P. Conversion of plant biomass to furan derivatives and sustainable access to the new generation of polymers, functional materials and fuels. Russ. Chem. Rev. 2017, 86, 357–387. [Google Scholar] [CrossRef]
- Mascal, M. 5-(Chloromethyl)furfural (CMF): A platform for transforming cellulose into commercial products. ACS Sustain. Chem. Eng. 2019, 7, 5588–5601. [Google Scholar] [CrossRef]
- Kong, Q.-S.; Li, X.-L.; Xu, H.-J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Sauer, J. Diels-Alder reactions II: The reaction mechanism. Angew. Chem. Int. Ed. 1967, 6, 16–33. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic Aspects of Diels-Alder Reactions: A Critical Survey. Angew. Chem. Int. Ed. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Craig, D. Stereochemical aspects of the intramolecular Diels-Alder reaction. Chem. Soc. Rev. 1987, 16, 187–238. [Google Scholar] [CrossRef]
- Coxon, J.M.; Froese, R.D.J.; Ganguly, B.; Marchand, A.P.; Morokuma, K. On the origins of diastereofacial selectivity in diels-alder cycloadditions. Synlett 1999, 1999, 1681–1703. [Google Scholar] [CrossRef]
- Fernandez, I.; Bickelhaupt, F.M. Deeper insight into the Diels-Alder Reaction through the activation strain model. Chem. Asian J. 2016, 11, 3297–3304. [Google Scholar] [CrossRef]
- Sánchez, A.; Pedroso, E.; Grandas, A. Maleimide-dimethylfuran exo adducts: Effective maleimide protection in the synthesis of oligonucleotide conjugates. Org. Lett. 2011, 13, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, M.; Wu, Y.; Li, L.; Wang, Z.; Wang, R.; Zhou, G. Development of high-molecular-weight fully renewable biopolyesters based on oxabicyclic diacid and 2,5-Furandicarboxylic acid: Promising as packaging and medical materials. ACS Sustain. Chem. Eng. 2021, 9, 6799–6809. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Genuino, H.C.; Sliwa, M.; van der Waal, J.C.; de Jong, E.; van Haveren, J.; Weckhuysen, B.M.; Bruijnincx, P.C.; van Es, D.S. Substituted phthalic anhydrides from biobased furanics: A new approach to renewable aromatics. ChemSusChem 2015, 8, 3052–3056. [Google Scholar] [CrossRef]
- Beusker, P.H.; Aben, R.W.M.; Seerden, J.-P.G.; Smits, J.M.M.; Scheeren, H.W. Exploration of high-pressure cycloadducts of furans and citraconic anhydride as precursors for CD-ring fragments of paclitaxel and its analogues. Eur. J. Org. Chem. 1998, 1998, 2483–2492. [Google Scholar] [CrossRef]
- Lu, Z.; Weber, R.; Twieg, R.J. Improved synthesis of DCDHF fluorophores with maleimide functional groups. Tetrahedron Lett. 2006, 47, 7213–7217. [Google Scholar] [CrossRef] [Green Version]
- Daeffler, C.S.; Miyake, G.M.; Li, J.; Grubbs, R.H. Partial kinetic resolution of oxanorbornenes by ring-opening metathesis polymerization with a chiral ruthenium initiator. ACS Macro Lett. 2014, 3, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Yasir, M.; Kilbinger, A.F.M. Catalytic living ring opening metathesis polymerisation: The importance of ring strain in chain transfer agents. Angew. Chem. Int. Ed. 2019, 58, 15278–15282. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Liu, P.; Markwart, J.C.; Suraeva, O.; Wurm, F.R.; Smart, J.; Lattuada, M.; Kilbinger, A.F.M. One-step ring opening metathesis block-like copolymers and their compositional analysis by a novel retardation technique. Angew. Chem. Int. Ed. 2020, 59, 13597–13601. [Google Scholar] [CrossRef]
- Román, E.; Gil, M.; Luque-Agudo, V.; Serrano, J. Expeditious ‘On-Water’ Cycloaddition between N-substituted maleimides and furans. Synlett 2014, 25, 2179–2183. [Google Scholar] [CrossRef]
- Jarosz, S.; Mach, M.; Szewczyk, K.; Skóra, S.; Ciunik, Z. Synthesis of Sugar-Derived 2′- and 3′-Substituted furans and their application in diels−alder reactions. Eur. J. Org. Chem. 2001, 2001, 2955. [Google Scholar] [CrossRef]
- Jeong, H.; John, J.M.; Schrock, R.R. Formation of Alternating trans-A-alt-B copolymers through ring-opening metathesis polymerization initiated by molybdenum imido alkylidene complexes. Organometallics 2015, 34, 5136–5145. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakamura, M.; Nakao, S.; Inoue, T.; Shoji, M. The HfCl4-Mediated Diels-Alder reaction of furan. Angew. Chem. Int. Ed. 2002, 41, 4079–4082. [Google Scholar] [CrossRef]
- Troelsen, N.S.; Shanina, E.; Gonzalez-Romero, D.; Dankova, D.; Jensen, I.S.A.; Sniady, K.J.; Nami, F.; Zhang, H.; Rademacher, C.; Cuenda, A.; et al. The 3F Library: Fluorinated Fsp(3) -Rich fragments for expeditious (19) F NMR based screening. Angew. Chem. Int. Ed. 2020, 59, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Shibatomi, K.; Kobayashi, F.; Narayama, A.; Fujisawa, I.; Iwasa, S. A Diels-Alder approach to the enantioselective construction of fluoromethylated stereogenic carbon centers. Chem. Commun. 2012, 48, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.H.; Kim, K.H.; Sim, J.Y.; Corey, E.J. Catalytic enantioselective Diels-Alder reactions of furans and 1,1,1-trifluoroethyl acrylate. Tetrahedron Lett. 2007, 48, 5735–5737. [Google Scholar] [CrossRef]
- Morton, C.J.; Gilmour, R.; Smith, D.M.; Lightfoot, P.; Slawin, A.M.; MacLean, E.J. Synthetic studies related to diketopyrrolopyrrole (DPP) pigments. Part 1: The search for alkenyl-DPPs. Unsaturated nitriles in standard DPP syntheses: A novel cyclopenta[c]pyrrolone chromophore. Tetrahedron 2002, 58, 5547–5565. [Google Scholar] [CrossRef]
- Kernen, P.; Vogel, P. The homoconjugated electron-releasing carbonyl group of 1-Methylbicyclo[2.2.1]hept-5-en-2-one. Regioselective syntheses of 5-chloro- and 6-chloro-1-methylbicyclo[2.2.1]hept-5-en-2-one. Helvetica Chim. Acta 1993, 76, 2338–2343. [Google Scholar] [CrossRef]
- Harrity, J.P.A.; Stevenson, N.G.; De Savi, C. Furan Diels-Alder cycloaddition approach to the highly oxygenated core of scyphostatin. Synlett 2006, 2006, 2272–2274. [Google Scholar] [CrossRef]
- Chen, J.-C.; Zheng, G.-J.; Zhang, C.; Fang, L.-J.; Li, Y.-L. Enhancive synthesis of -lβ, 11-Diol-4-en-eudesmol. Chin. J. Chem. 2010, 21, 904–906. [Google Scholar] [CrossRef]
- Ishar, M.P.S.; Wali, A.; Gandhi, R.P. Regio- and stereo-selectivity in uncatalysed and catalysed Diels-Alder reactions of allenic esters with furan and 2-methylfuran. J. Chem. Soc. Perkin Trans. 1 1990, 1, 2185–2192. [Google Scholar] [CrossRef]
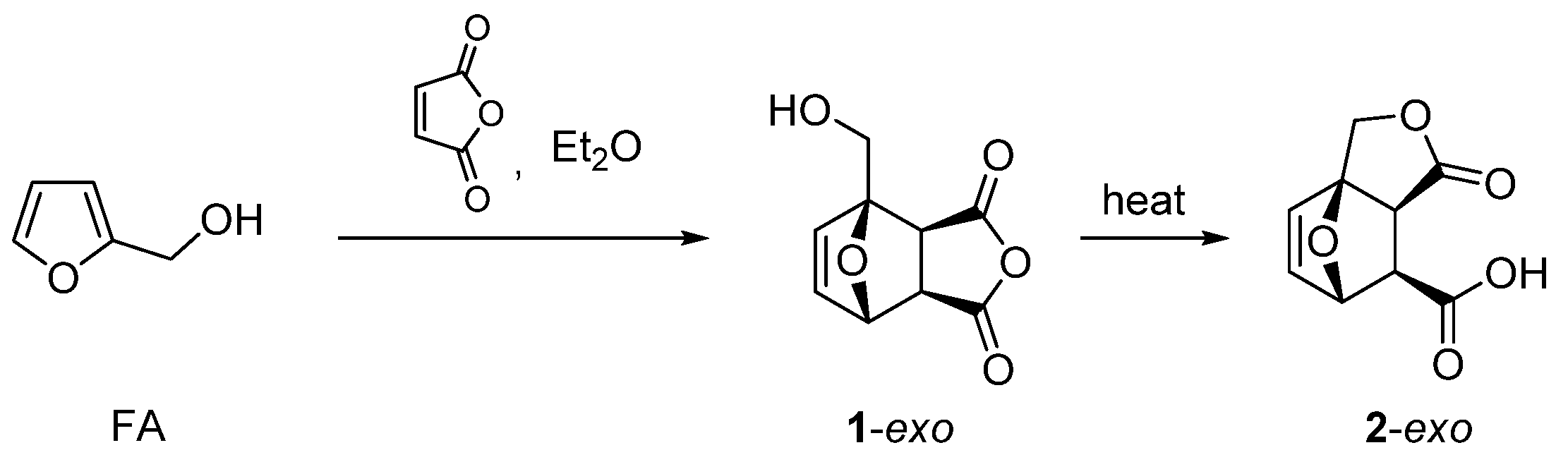
- Pehere, A.D.; Xu, S.; Thompson, S.K.; Hillmyer, M.A.; Hoye, T.R. Diels-Alder reactions of furans with itaconic anhydride: Overcoming unfavorable thermodynamics. Org. Lett. 2016, 18, 2584–2587. [Google Scholar] [CrossRef] [Green Version]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels-Alder and retro-Diels-Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, S.-S.; Kim, H.S.; Lee, K.M. A formal total synthesis of Dysiherbaine and Neodysiherbaine A. Eur. J. Org. Chem. 2012, 2012, 4192–4199. [Google Scholar] [CrossRef]
- Baba, Y.; Hirukawa, N.; Tanohira, A.N.; Sodeoka, M. Structure-based design of a highly selective catalytic site-directed inhibitor of ser/thr protein phosphatase 2B (Calcineurin). J. Am. Chem. Soc. 2003, 125, 9740–9749. [Google Scholar] [CrossRef]
- Zhang, J.; Lawrance, G.A.; Chau, N.; Robinson, P.J.; McCluskey, A. From Spanish fly to room-temperature ionic liquids (RTILs): Synthesis, thermal stability and inhibition of dynamin 1 GTPase by a novel class of RTILs. New J. Chem. 2008, 32, 28–36. [Google Scholar] [CrossRef]
- McCluskey, A.; Ackland, S.P.; Bowyer, M.C.; Baldwin, M.L.; Garner, J.; Walkom, C.C.; Sakoff, J.A. Cantharidin analogues: Synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorganic Chem. 2003, 31, 68–79. [Google Scholar] [CrossRef]
- Ganesan, M.; Muraleedharan, K.M. Oxanorbornane-based amphiphilic systems: Design, synthesis and material properties. RSC Adv. 2012, 2, 4048–4051. [Google Scholar] [CrossRef]
- Ramesh, N.; Ganesan, M.; Sarangi, N.K.; Muraleedharan, K.M.; Patnaik, A. Tailoring strained oxanorbornane headgroups to dimensionally controlled nanostructures through hydrogen bonding. RSC Adv. 2014, 4, 9762–9770. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Zhang, X. Dynamic Diels-Alder reactions of maleimide–furan amphiphiles and their fluorescence ON/OFF behaviours. Org. Biomol. Chem. 2018, 16, 7871–7877. [Google Scholar] [CrossRef]
- Ochi, R.; Nishida, T.; Ikeda, M.; Hamachi, I. Design of peptide-based bolaamphiphiles exhibiting heat-set hydrogelation via retro-Diels-Alder reaction. J. Mater. Chem. B 2014, 2, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ochi, R.; Kurita, Y.-S.; Pochan, D.J.; Hamachi, I. Heat-induced morphological transformation of supramolecular nanostructures by retro-Diels-Alder reaction. Chem. A Eur. J. 2012, 18, 13091–13096. [Google Scholar] [CrossRef]
- Fan, B.; Trant, J.F.; Hemery, G.; Sandre, O.; Gillies, E.R. Thermo-responsive self-immolative nanoassemblies: Direct and indirect triggering. Chem. Commun. 2017, 53, 12068–12071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djidi, D.; Mignard, N.; Taha, M. Thermosensitive polylactic-acid-based networks. Ind. Crop. Prod. 2015, 72, 220–230. [Google Scholar] [CrossRef]
- Heath, W.H.; Palmieri, F.; Adams, J.R.; Long, B.K.; Chute, J.; Holcombe, T.W.; Zieren, S.; Truitt, M.J.; White, J.L.; Willson, C.G. Degradable cross-linkers and strippable imaging materials for step-and-flash imprint lithography. Macromolecules 2008, 41, 719–726. [Google Scholar] [CrossRef]
- Budd, M.; Stephens, R.; Afsar, A.; Salimi, S.; Hayes, W. Exploiting thermally-reversible covalent bonds for the controlled release of microencapsulated isocyanate crosslinkers. React. Funct. Polym. 2019, 135, 23–31. [Google Scholar] [CrossRef]
- Taimoory, S.M.; Sadraei, S.I.; Fayoumi, R.A.; Nasri, S.; Revington, M.; Trant, J.F. Preparation and characterization of a small library of thermally-labile end-caps for variable-temperature triggering of self-immolative polymers. J. Org. Chem. 2018, 83, 4427–4440. [Google Scholar] [CrossRef]
- Czifrak, K.; Lakatos, C.; Karger-Kocsis, J.; Daroczi, L.; Zsuga, M.; Keki, S. One-pot synthesis and characterization of novel shape-memory poly(epsilon-caprolactone) based polyurethane-epoxy co-networks with Diels(-)Alder couplings. Polymers 2018, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Pelter, A.; Singaram, B. The reactions of furfuryl alcohols with maleic anhydride. J. Chem. Soc. Perkin Trans. 1983, 1, 1383–1386. [Google Scholar] [CrossRef]
- Clavier, H.; Broggi, J.; Nolan, S.P. Ring-rearrangement metathesis (RRM) mediated by ruthenium-indenylidene complexes. Eur. J. Org. Chem. 2010, 2010, 937–943. [Google Scholar] [CrossRef]
- Oparina, L.A.; Vysotskaya, O.V.; Stepanov, A.V.; Ushakov, I.A.; Apartsin, K.A.; Gusarova, N.; Trofimov, B.A. Furfuryl vinyl ethers in [4+2]-cycloaddition reactions. Russ. J. Org. Chem. 2017, 53, 203–209. [Google Scholar] [CrossRef]
- Buser, S.; Vasella, A. 7-Oxanorbornane and norbornane mimics of a Distortedβ-D-Mannopyranoside: Synthesis and evaluation asβ-mannosidase inhibitors. Helvetica Chim. Acta 2005, 88, 3151–3173. [Google Scholar] [CrossRef]
- Galkin, K.I.; Kucherov, F.; Markov, O.N.; Egorova, K.S.; Posvyatenko, A.V.; Ananikov, V.P. Facile chemical access to biologically active norcantharidin derivatives from biomass. Molecules 2017, 22, 2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels-Alder crosslink reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Truong, T.T.; Nguyen, H.T.; Phan, M.N.; Nguyen, L.-T.T. Study of Diels-Alder reactions between furan and maleimide model compounds and the preparation of a healable thermo-reversible polyurethane. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1806–1814. [Google Scholar] [CrossRef]
- Hart, M.E.; Chamberlin, A.R.; Walkom, C.; Sakoff, J.A.; McCluskey, A. Modified norcantharidins; synthesis, protein phosphatases 1 and 2A inhibition, and anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 1969–1973. [Google Scholar] [CrossRef]
- Galvis, C.E.P.; Kouznetsov, V.V. An unexpected formation of the novel 7-oxa-2-azabicyclo[2.2.1]hept-5-ene skeleton during the reaction of furfurylamine with maleimides and their bioprospection using a zebrafish embryo model. Org. Biomol. Chem. 2013, 11, 407–411. [Google Scholar] [CrossRef]
- Saroj, S.; Janni, D.S.; Ummadi, C.R.; Manheri, M.K. Functionalizable oxanorbornane-based head-group in the design of new Non-ionic amphiphiles and their drug delivery properties. Mater. Sci. Eng. C 2020, 112, 110857. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Sasaki, K.; Rahaman, M.Y.; Bowers, A.A. One-pot syntheses of dissymmetric diamides based on the chemistry of cyclic monothioanhydrides. Scope and limitations and application to the synthesis of glycodipeptides. J. Org. Chem. 2009, 74, 3886–3893. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Mukherjee, S.; Brooks, W.L.A.; Dai, Y.; Sumerlin, B.S. Doubly-dynamic-covalent polymers composed of oxime and oxanorbornene links. Polym. Chem. 2016, 7, 1971–1978. [Google Scholar] [CrossRef]
- Green, S.K.; Patet, R.E.; Nikbin, N.; Williams, C.L.; Chang, C.-C.; Yu, J.; Gorte, R.J.; Caratzoulas, S.; Fan, W.; Vlachos, D.; et al. Diels-Alder cycloaddition of 2-methylfuran and ethylene for renewable toluene. Appl. Catal. B Environ. 2016, 180, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Kucherov, F.A.; Romashov, L.V.; Averochkin, G.M.; Ananikov, V.P. Biobased C6-furans in organic synthesis and industry: Cycloaddition chemistry as a key approach to aromatic building blocks. ACS Sustain. Chem. Eng. 2021, 9, 3011–3042. [Google Scholar] [CrossRef]
- Ravasco, J.; Gomes, R.F.A. Recent advances on Diels-Alder-driven preparation of bio-based aromatics. ChemSusChem 2021, 14, 3047–3053. [Google Scholar] [CrossRef]
- Patet, R.E.; Koehle, M.; Lobo, R.F.; Caratzoulas, S.; Vlachos, D.G. General acid-type catalysis in the dehydrative aromatization of furans to aromatics in H-[Al]-BEA, H-[Fe]-BEA, H-[Ga]-BEA, and H-[B]-BEA Zeolites. J. Phys. Chem. C 2017, 121, 13666–13679. [Google Scholar] [CrossRef]
- Chang, C.-C.; Green, S.K.; Williams, C.L.; Dauenhauer, P.J.; Fan, W. Ultra-selective cycloaddition of dimethylfuran for renewable p-xylene with H-BEA. Green Chem. 2014, 16, 585–588. [Google Scholar] [CrossRef]
- Wang, D.; Osmundsen, C.M.; Taarning, E.; Dumesic, J.A. Selective production of aromatics from alkylfurans over solid acid catalysts. ChemCatChem 2013, 5, 2044–2050. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Chen, S.S.; Li, S.C.; Chen, C.H.; Shishido, T.; Tsang, D.C.W.; Yamauchi, Y.; Li, Y.P.; Wu, K.C. Diels-Alder conversion of acrylic acid and 2,5-Dimethylfuran to para-Xylene over Heterogeneous Bi-BTC metal-organic framework catalysts under mild conditions. Angew. Chem. Int. Ed. 2021, 60, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Wang, Z.; Gilbert, C.J.; Fan, W.; Huber, G.W. Production of p-xylene from biomass by catalytic fast pyrolysis using ZSM-5 catalysts with reduced pore openings. Angew. Chem. Int. Ed. 2012, 51, 11097–11100. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, Z.; Li, S.; Parvulescu, A.-N.; Müller, U.; Zhang, W. Excellent performances of dealuminated H-Beta Zeolites from organotemplate-free synthesis in conversion of biomass-derived 2,5-Dimethylfuran to renewable p -Xylene. ChemSusChem 2018, 11, 3803–3811. [Google Scholar] [CrossRef]
- Ni, L.; Xin, J.; Dong, H.; Lu, X.; Liu, X.; Zhang, S. A simple and mild approach for the synthesis of p -Xylene from Bio-Based 2,5-Dimethyfuran by using metal triflates. ChemSusChem 2017, 10, 2394–2401. [Google Scholar] [CrossRef]
- Ni, L.; Xin, J.; Jiang, K.; Chen, L.; Yan, D.; Lu, X.; Zhang, S. One-step conversion of biomass-derived furanics into aromatics by brønsted acid ionic liquids at room temperature. ACS Sustain. Chem. Eng. 2018, 6, 2541–2551. [Google Scholar] [CrossRef]
- Potts, K.T.; Walsh, E.B. Furfural dimethylhydrazone: A versatile diene for arene cycloaromatization. J. Org. Chem. 2002, 49, 4099–4101. [Google Scholar] [CrossRef]
- Jacques, V.; Czarnik, A.W.; Judge, T.M.; Van der Ploeg, L.H.T.; DeWitt, S.H. Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl. Acad. Sci. USA 2015, 112, E1471–E1479. [Google Scholar] [CrossRef] [Green Version]
- Karaluka, V.; Murata, K.; Masuda, S.; Shiramatsu, Y.; Kawamoto, T.; Hailes, H.C.; Sheppard, T.D.; Kamimura, A. Development of a microwave-assisted sustainable conversion of furfural hydrazones to functionalised phthalimides in ionic liquids. RSC Adv. 2018, 8, 22617–22624. [Google Scholar] [CrossRef] [Green Version]
- Higson, S.; Subrizi, F.; Sheppard, T.D.; Hailes, H.C. Chemical cascades in water for the synthesis of functionalized aromatics from furfurals. Green Chem. 2016, 18, 1855–1858. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, E.; Watson, D.A.; Lobo, R.F. Renewable production of phthalic anhydride from biomass-derived furan and maleic anhydride. Green Chem. 2014, 16, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Thiyagarajan, S.; Genuino, H.C.; van der Waal, J.C.; de Jong, E.; Weckhuysen, B.M.; van Haveren, J.; Bruijnincx, P.C.; van Es, D.S. A facile solid-phase route to renewable aromatic chemicals from biobased furanics. Angew. Chem. Int. Ed. 2016, 55, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-H.; He, H.-L.; Zhang, Y.-B.; Li, Z. Oxidative aromatization of biobased chemicals to benzene derivatives through tandem catalysis. ACS Sustain. Chem. Eng. 2020, 8, 14322–14329. [Google Scholar] [CrossRef]


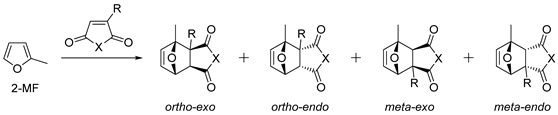
| № | Dienophile | Conditions | Selectivity | Yield of DA Adducts (%), [Ref.] |
|---|---|---|---|---|
| 1 | X = O | Neat, RT, N2, 24 h | Exo | 91, [66] |
| 2 | X = O | Neat, RT, 10–15 °C, 2–3 h | Exo | 96 (crude), [67] |
| 3 | Citraconic anhydride  | CH2Cl2, RT, 15 kbar | Exo (ortho/meta 1:1) | 65 1, [68] |
| 4 | X = NH | Et2O, RT, 3 days | Endo/exo 2 | 21 (for endo), [69] |
| 5 | X = NH | THF, reflux, 4 h | Exo | 94, [70] |
| 6 | X = NMe | Toluene, 90 °C | Exo | 92, [71] |
| 7 | X = NMe | Et2O, 90 °C | Exo | 66, [72] |
| 8 | X = NEt | H2O, 65 °C | Endo/exo 1.4:1 | 100, [73] |
| 9 | X = N(tBu) | H2O, 65 °C | Exo | 100, [73] |
| 10 | X = NPh | H2O, 65 °C | Endo/exo 1.6:1 | 100, [73] |
| 11 | X = NPh | 4:1 Toluene/benzene, RT, 1.1 GPa | Endo/exo 1.66:1 | 85, [74] |
| 12 | X = NPh | CDCl3, 60 °C | Exo with traces of endo | 90, [44] |
| 13 | X = NPh | Hexane or heptane, TFA, glass beads, 80 °C, 5–8 days 3 | (-)-Exo, 86–90 ee | 80, [44] |
| 14 | X = NPhF5 | Neat, reflux | Exo | 50, [75] |
| 15 |  | THF, 65 °C | Exo | 64, [70] |
| 16 | X = NCH2CH2COOH | CHCl3, 38 °C, 5 days | Endo/exo 28:72 | 100, [65] |
| 17 4 | X = NCH2CH2COOH | CHCl3, 38 °C, 5 days | Exo | 100, [65] |
| 18 5 | X = NCH2CH2COOH | CH2Cl2, RT, overnight | Endo/exo 78:22 | 100, [65] |
| 19 5 | X = NCH2CH2COOH | CH3CN, 60 °C, 6 h | Endo/exo 22:78 | 100, [65] |
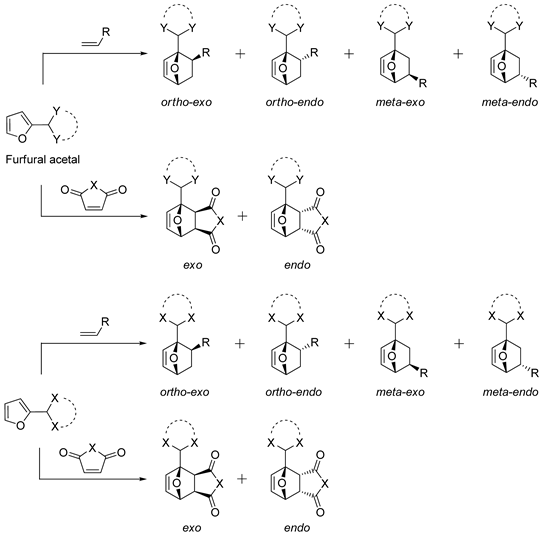
| № | Furfural Acetal | Dienophile | Conditions | Selectivity | Yield of Adducts (%), [Ref.] |
|---|---|---|---|---|---|
| 1 | 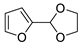 | N-Methylmaleimide | CH2Cl2, 23 °C | Endo/exo 87:13 | N.d., [86] |
| 2 |  | Methyl vinyl ketone | Neat, 60 °C | Ortho 13 (endo/exo 74:26), meta 87 (endo/exo 65:35) | 36, [32] |
| 3 |  | Methyl acrylate | Neat, 60 °C | Ortho 33 (endo/exo 87:13), meta 67 (endo/exo 77:23) | 40, [32] |
| 4 | 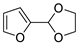 | Acrolein | Neat, 60 °C | Ortho 38 (endo/exo 71:29), meta 62 (endo/exo 43:57) | 28, [32] |
| 5 |  | Acrylonitrile | Neat, 60 °C, 120 h | Ortho 48 (endo/exo 72:28), meta 52 (endo/exo 42:58) | 76, [32] |
| 6 | 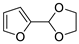 | Acrylonitrile | ZnCl2, neat, 60 °C | Ortho 50 (endo/exo 70:30), meta 50 (endo/exo 56:44) | 75, [32] |
| 7 | 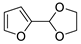 | Acrylonitrile | ZnI2, neat, 60 °C | Ortho 53 (endo/exo 70:30), meta 67 (endo/exo 60:40) | 75, [31] |
| 8 | 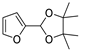 | Acrylonitrile | ZnCl2, neat, 60 °C | Ortho 43 (endo/exo 85:15), meta 57 (endo/exo 56:44) | 68, [32] |
| 9 |  | Acrylonitrile | ZnCl2, neat, 60 °C | Ortho 39 (endo/exo 67:33), meta 61 (endo/exo 54:46) | 67, [32] |
| 10 |  | Acrylonitrile | ZnCl2, neat, 30 °C | Ortho 91 (endo/exo 66:33), meta 9 (endo/exo 53:47) | 73, [32] |
| 11 | 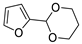 | Acrylonitrile | ZnCl2, neat, 60 °C | Ortho 53 (endo/exo 60:40), meta 47 (endo/exo 54:46) | 81, [32] |
| 12 | 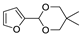 | Acrylonitrile | ZnCl2, neat, 60 °C | Ortho 52 (endo/exo 62:38), meta 48 (endo/exo 56:44) | 85, [32] |
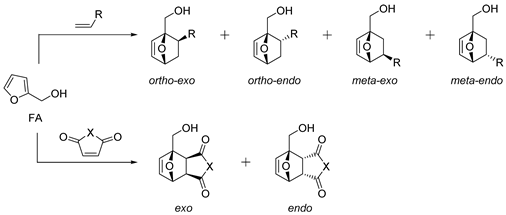
| № | Dienophile | Conditions | Selectivity | Yield of Adducts (%), [Ref.] |
|---|---|---|---|---|
| 1 1 | Maleimide | Ethyl acetate, 24 °C | Endo/exo 96:4 | 87, [33] |
| 2 2 | Maleimide | Ethyl acetate, 24 °C | Endo/exo 97:3 | 42, [33] |
| 3 | N-Me-maleimide | Et2O, 90 °C | Endo/exo 21:79 | 43, [72] |
| 4 | N-Bn-maleimide | CH3CN, 35 °C | Endo/exo 70:30 | 75, [96] |
| 5 | N-Propargylmaleimide | CH3CN, 35 °C | Endo/exo 80:20 | 72, [96] |
| 6 | N-(2-Hydroxymethyl)maleimide | Ethyl acetate, 80 °C | Exo | 76, [97] |
| 7 | N-(2-Hydroxyethyl)maleimide | Benzene, reflux | Exo | 86, [98] |
| 8 | N-(3-Hydroxypropyl)maleimide | Toluene, 80 °C | Endo/exo 30:70 3 | 77, [99] |
| 9 | N-(4-Hydroxyphenyl)maleimide | Acetone, 55 °C | Exo | 71, [40] |
| 10 | N-(4-Hydroxyphenyl)maleimide | Acetonitrile, 35 °C | Endo/exo 80:20 | N.d., [40] |
| 11 | N-(p-Methoxyphenyl)maleimide | CH3CN, 40 °C, 18 h | Mostly exo | 89, [100] |
| 12 | N-(p-Nitrophenyl)maleimide | CH3CN, 60 °C | Endo/exo 70:23 | 52, [100] |
| 13 | BMI 4 | Toluene, 75–80 °C, 2 days | Mostly exo | 92, [101] |
| 14 | Acrylonitrile | Neat, 60 °C | Ortho 56 (endo/exo 69:31), meta 44 (endo/exo 56:44) | 81, [32] |
| 15 |  | Neat, RT, 96 h | N.d. | 66, [37] |
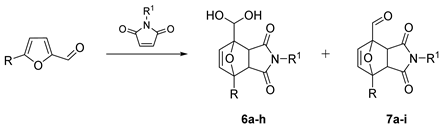
| № | Furanic Substrate | R1 | Products, Selectivity 1 |
|---|---|---|---|
| 1 | R = H | Me | 6a (endo/exo 18:40), 7a (endo/exo 1:3) |
| 2 | R = H | H | 6b (endo/exo 8:30), 7b (endo/exo 0:0) |
| 3 | R = H | Et | 6c (endo/exo 8:28, 7c (endo/exo 1:6) |
| 4 | R = H | nPr | 6d (endo/exo 1:7), 7d (endo/exo 1:11) |
| 5 | R = H | Ph | 6e (endo/exo 0:1), 7e (endo/exo 1:5) |
| 6 | R = Me | Me | 6f (endo/exo 3:8), 7f (endo/exo 0:3) |
| 7 | R = CH2OH | Me | 6g (endo/exo 37:13), 7g (endo/exo 0:0) |
| 8 | R = CH2OMe | Me | 6h (endo/exo 7:5), 7h (endo/exo 3:3) |
| 9 | 2-Acetylfuran | Me | 7i (endo/exo traces:32) |

| № | R | Conditions | Products Yield (%), [Ref.] |
|---|---|---|---|
| 1 | H | H-BEA zeolite, heptane, 62 bar, 250 °C | Toluene (46%), [119] |
| 2 | H | H-Beta-22 zeolite, 300 °C, 20 h | Toluene (50%), [123] |
| 3 | COOH | Bi-BTC, 160 °C, 24 h | Toluene (65%), 2-methyl benzoic acid (23%), [121] |
| 4 | COOH | [Emim]NTf2, Sc(OTf)3, 15 °C, 0.5 h | Toluene (12%), 2-methyl benzoic acid (2%), 3-methyl benzoic acid (9%), [124] |
| 5 | COOH | [BSO3HMIm]HSO4, 100 °C, 2h | Toluene (12%), 2-methyl benzoic acid (30%), 3-methyl benzoic acid (3%), [125] |

| № | Substrates | Conditions | Yield of Aromatic Product, [Ref.] |
|---|---|---|---|
| 5 | 2-Furaldehyde dimethylhydrazone, maleic anhydride | CHCl3, RT | 94, [126] |
| 6 | 2-Furaldehyde dimethylhydrazone, N-Et-maleimide | CHCl3, RT | 90, [126] |
| 7 | 2-Furaldehyde, N,N-dimethylhydrazine, N-Et-maleimide | H2O, 50 °C | 97, [129] |
| 8 | 2-Furaldehyde, N,N-dimethylhydrazine, maleimide | H2O, 50 °C | 86, [129] |
| 9 | 2-Furaldehyde, N,N-dimethylhydrazine, N-cyclopropylmaleimide | H2O, 50 °C | 80, [129] |
| 10 | 2-Furaldehyde, N,N-dimethylhydrazine, N-Ph-maleimide | H2O, 50 °C | 73, [129] |
| 11 | 2-Furaldehyde, N,N-dimethylhydrazine, N-(4-Methylbenzyl)maleimide | H2O, 50 °C | 68, [129] |
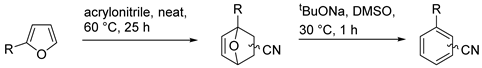
| № | Oxanorbornene | Yield of DA Adducts 1 | Yield of Aromatic Products 1 |
|---|---|---|---|
| 1 | R = dioxolane acetal | 76 (ortho/meta ~1:1) 2 | 84 (ortho/meta ~1:1.5) |
| 2 | R = dioxolane acetal | 76 (ortho/meta ~1:1) | 86 (ortho/meta ~1:1.8) 3 |
| 3 | R = Me | 53 (ortho), 13 (meta) | 97 (ortho), 62 (meta) 4 |
| 4 | R = CH2OEt | 36 (ortho), 18 (meta) | 94 (ortho), 100 (meta) 4 |
| 5 | R = CH2OH | 47 (ortho), 26 (meta) | 21 (ortho), 42 (meta) 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkin, K.I.; Ananikov, V.P. Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes. Int. J. Mol. Sci. 2021, 22, 11856. https://doi.org/10.3390/ijms222111856
Galkin KI, Ananikov VP. Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes. International Journal of Molecular Sciences. 2021; 22(21):11856. https://doi.org/10.3390/ijms222111856
Chicago/Turabian StyleGalkin, Konstantin I., and Valentine P. Ananikov. 2021. "Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes" International Journal of Molecular Sciences 22, no. 21: 11856. https://doi.org/10.3390/ijms222111856







