Wnt Signaling in Inner Blood–Retinal Barrier Maintenance
Abstract
:1. Introduction
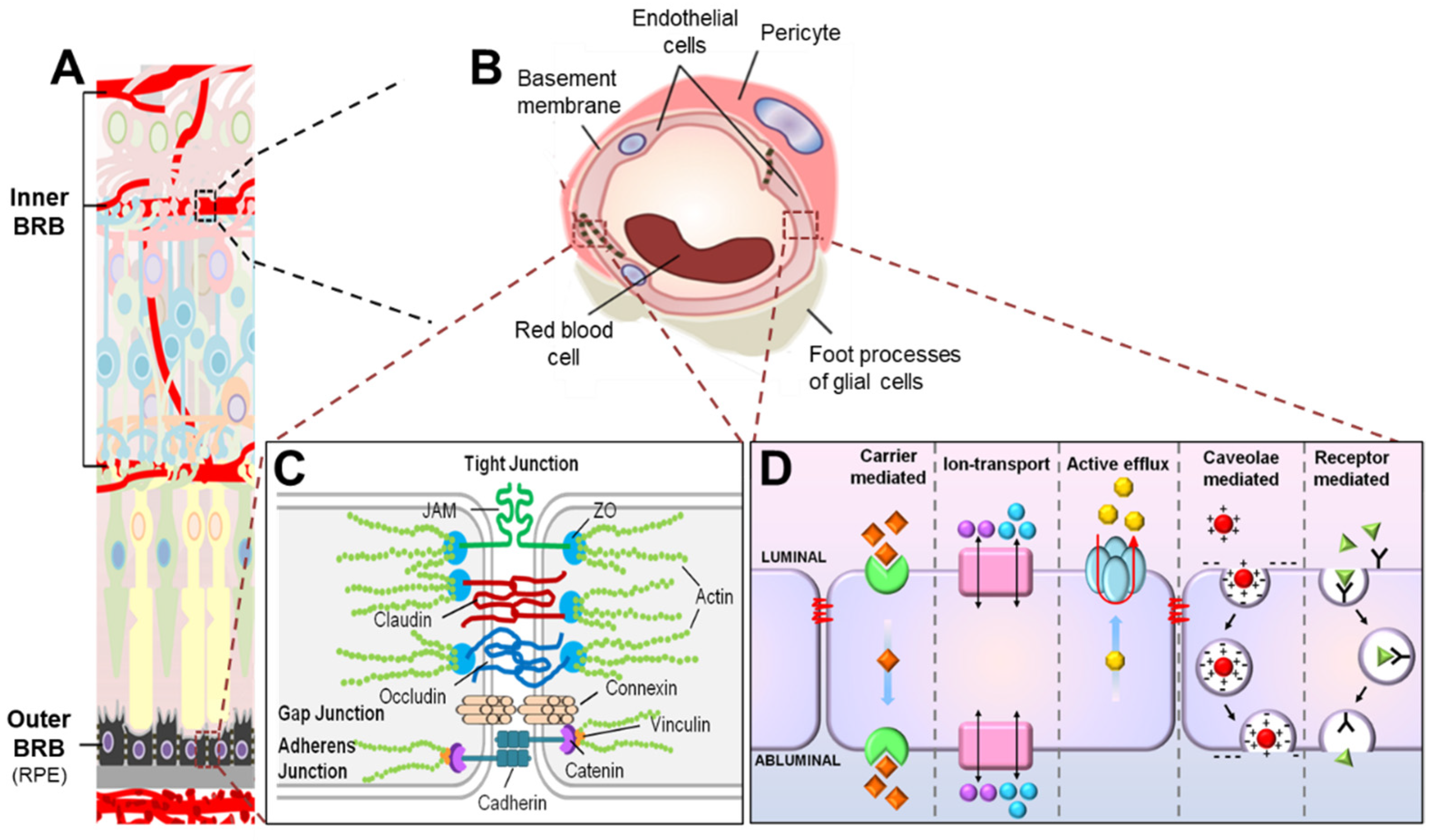
2. Molecular Components of the iBRB
2.1. Retinal Vascular Endothelium Is the Cellular Site of iBRB
2.2. Endothelial Junctions Are Key Components of Paracellular Transport across the iBRB
2.2.1. Tight Junctions Are Mainly Composed of ZO, Occludin, and Claudins
2.2.2. Formation of Tight Junction Is Facilitated by Adherens Junctions and Gap Junctions
2.3. Transcytosis Is a Main Route of Transcellular Transport across the Inner BRB
2.3.1. Caveolin1 Is Essential for Caveolar-Mediated Endothelial Transcytosis
2.3.2. Normal Mature RMECs Have Low Rate of Caveolar-Mediated Transcytosis
2.3.3. Protein Markers of High and Low EC Transcytosis: PLVAP and MFSD2A
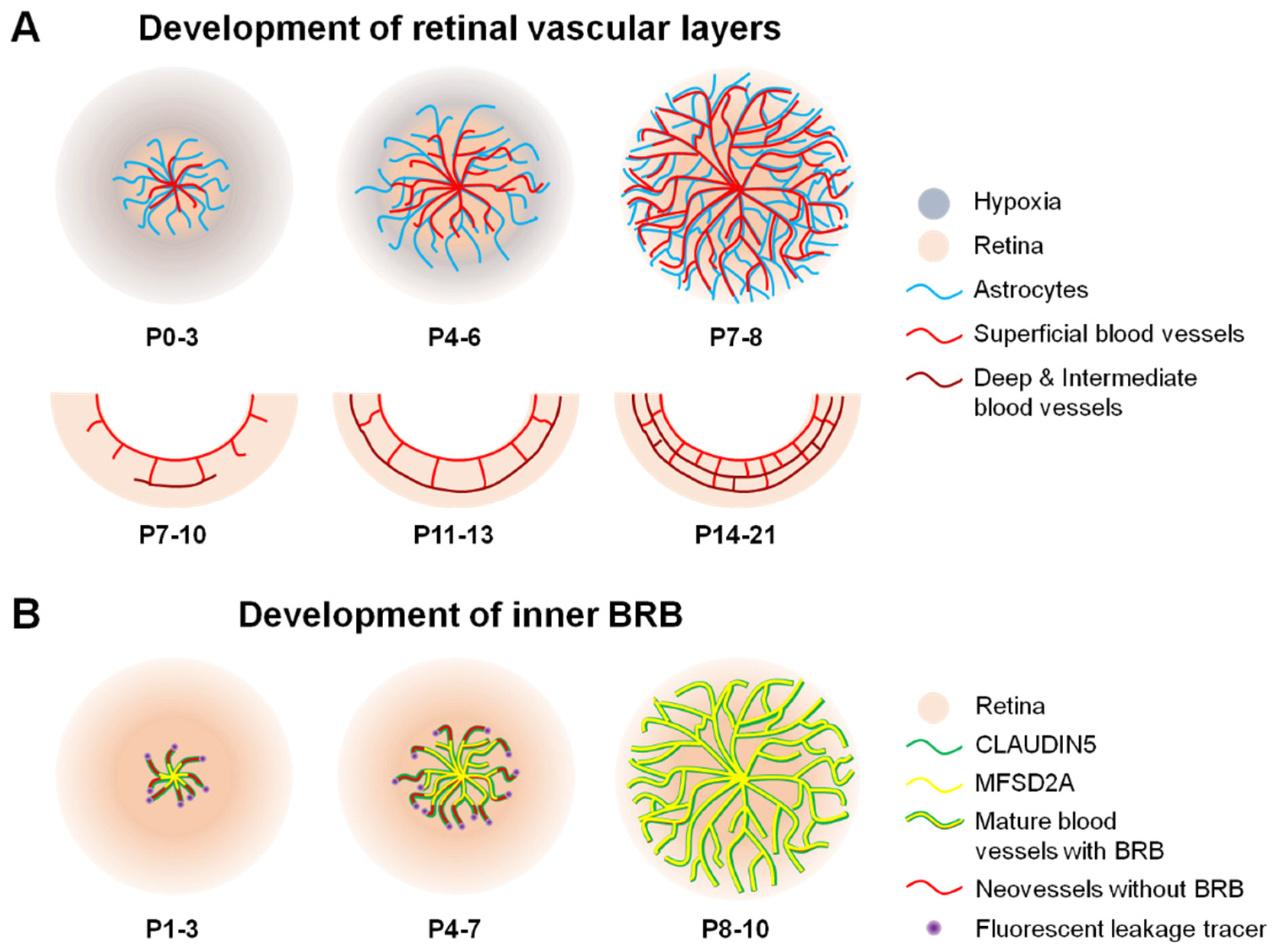
3. Development of the Inner BRB
3.1. Development of Retinal Vasculature
3.2. Formation of iBRB
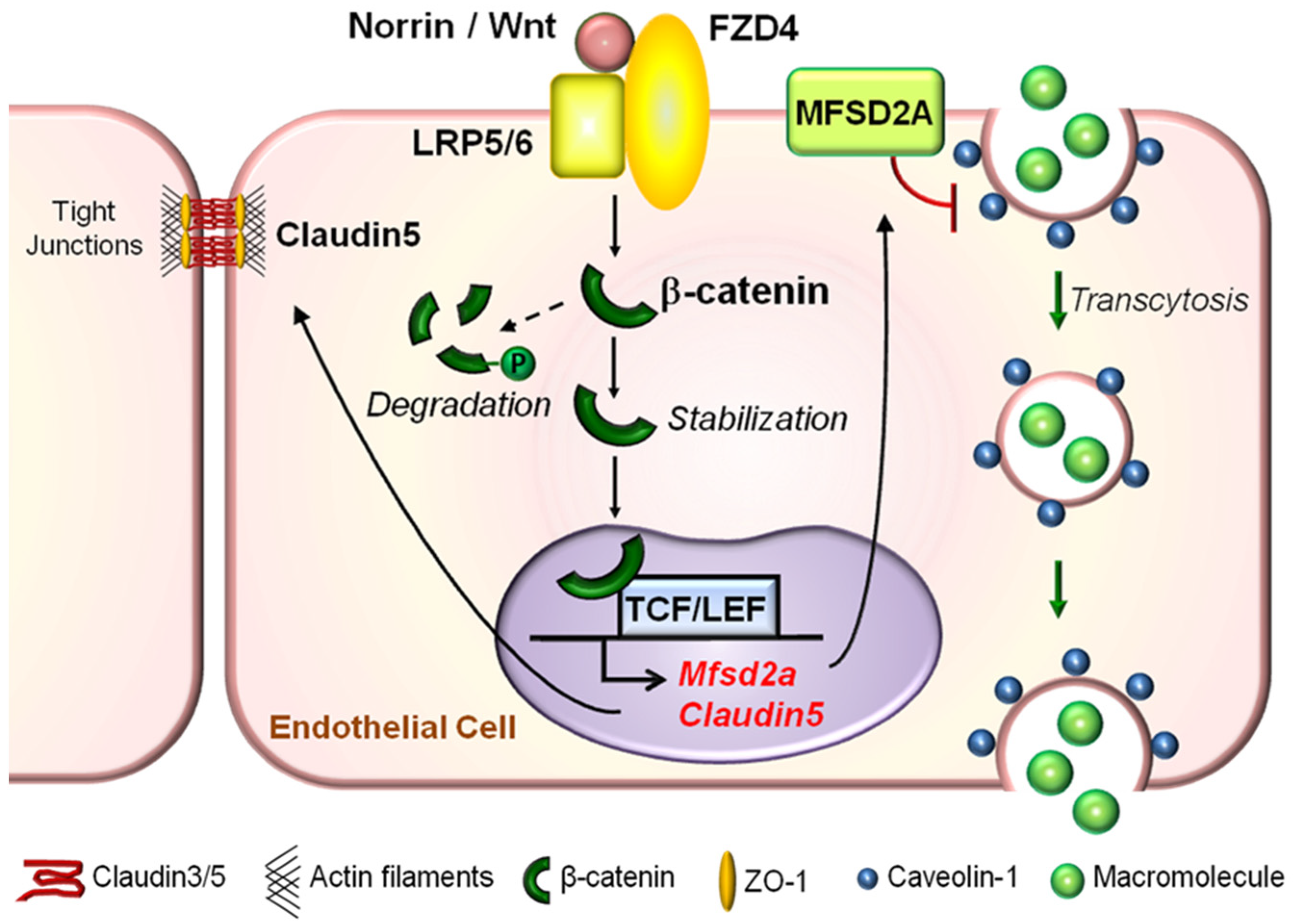
4. Wnt Signaling and iBRB Maintenance
4.1. Molecular Components of the Wnt Signaling Pathway
4.2. Wnt Signaling Pathway Restricts Paracellular Transport in iBRB
4.3. Wnt Signaling Pathway Limits Transcellular Transport in Vascular Endothelium to Maintain Physiological iBRB
5. Interplay of Wnt/β-Catenin Signaling with Other Mechanisms Underpinning iBRB Maintenance and Breakdown in Eye Diseases
5.1. VEGF Is a Main Culprit in DR and DME
5.2. Contribution of Other Non-Endothelial Cells in Regulation of iBRB
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnaudigel, O. Die vitale Färbung mit Trypanblau am Auge. Albrecht Von Graefes Arch. Ophthalmol. 1913, 86, 93–105. [Google Scholar] [CrossRef]
- Palm, E. On the occurrence in the retina of conditions corresponding to the blood-brain barrier. Acta Ophthalmol. 1947, 25, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G.; Shakib, M.; Ashton, N. Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br. J. Ophthalmol. 1966, 50, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, A.; Liebner, S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. S6), 3–9. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, W.; Zhu, X.; Mao, Y.; Liu, X.; Chen, X.; Huang, J.; Tang, S.; Rizzolo, L.J. Differential Expression of Claudins in Retinas during Normal Development and the Angiogenesis of Oxygen-Induced Retinopathy. Investig. Opthalmol. Vis. Sci. 2011, 52, 7556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Sapieha, P.; Hua, J.; Hatton, C.J.; Juan, A.M.; Aderman, C.M.; et al. Wnt Signaling Mediates Pathological Vascular Growth in Proliferative Retinopathy. Circulation 2011, 124, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Strong, S.; Liew, G.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.K.; Kim, J.H.; Kim, W.J.; Lee, H.Y.; Park, J.A.; Lee, S.W.; Yoon, D.K.; Kim, H.H.; Chung, H.; Yu, Y.S.; et al. AKAP12 Regulates Human Blood-Retinal Barrier Formation by Downregulation of Hypoxia-Inducible Factor-1. J. Neurosci. 2007, 27, 4472–4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, J.; Macdonald, M.L.E.; Kaykas, A.; Sheldahl, L.C.; Zeisler, J.; Dubé, M.-P.; Zhang, L.-H.; Singaraja, R.R.; Guernsey, D.L.; Zheng, B.; et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 2002, 32, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Schuback, D.E.; Chen, Z.Y.; Craig, I.W.; Breakefield, X.O.; Sims, K.B. Mutations in the Norrie disease gene. Hum. Mutat. 1995, 5, 285–292. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Nathans, J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010, 16, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, C.-H.; Huang, S.; Chen, J. Wnt Signaling in vascular eye diseases. Prog. Retin. Eye Res. 2019, 70, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.-H.; Huang, S.; Fu, Z.; Tomita, Y.; Britton, W.R.; Cho, S.S.; Chen, C.T.; Sun, Y.; Ma, J.-X.; et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv. 2020, 6, eaba7457. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Rattner, A.; Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Norrin/Frizzled4 Signaling in Retinal Vascular Development and Blood Brain Barrier Plasticity. Cell 2012, 151, 1332–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Lai, M.B.; Khandan, L.; Lee, L.A.; Chen, Z.; Junge, H.J. Norrin-induced Frizzled4 endocytosis and endo-lysosomal trafficking control retinal angiogenesis and barrier function. Nat. Commun. 2017, 8, 16050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raviola, G. The structural basis of the blood-ocular barriers. Exp. Eye Res. 1977, 25, 27–63. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Kim, D.H.; Kim, K.-W. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J. Neurosci. Res. 2009, 87, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, J.R.; Renkin, E.M.; Borrero, L.M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am. J. Physiol. 1951, 167, 13–46. [Google Scholar] [CrossRef]
- Diaz-Coranguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.J.; Bacallao, R.; Zampighi, G. Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 1993, 361, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E. Endothelial cell–cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskins, J.; Gu, L.; Wittchen, E.S.; Hibbard, J.; Stevenson, B.R. ZO-3, a Novel Member of the MAGUK Protein Family Found at the Tight Junction, Interacts with ZO-1 and Occludin. J. Cell Biol. 1998, 141, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Jesaitis, L.; Goodenough, D. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994, 124, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood–brain barrier. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A Single Gene Product, Claudin-1 or -2, Reconstitutes Tight Junction Strands and Recruits Occludin in Fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The Mouse Blood-Brain Barrier Transcriptome: A New Resource for Understanding the Development and Function of Brain Endothelial Cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, I.; Hughes, J.M.; Vogels, I.M.C.; Schalkwijk, C.G.; Van Noorden, C.J.F.; Schlingemann, R.O. Altered expression of genes related to blood–retina barrier disruption in streptozotocin-induced diabetes. Exp. Eye Res. 2009, 89, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Ebnet, K.; Suzuki, A.; Ohno, S.; Vestweber, D. Junctional adhesion molecules (JAMs): More molecules with dual functions? J. Cell Sci. 2004, 117, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.; Fleming, T. Specific mRNA detection in single lineage-marked blastomeres from preimplantation embryos. Trends Genet. 1995, 11, 5–7. [Google Scholar] [CrossRef]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef]
- Dejana, E.; Giampietro, C. Vascular endothelial-cadherin and vascular stability. Curr. Opin. Hematol. 2012, 19, 218–223. [Google Scholar] [CrossRef]
- Kojima, T.; Spray, D.C.; Kokai, Y.; Chiba, H.; Mochizuki, Y.; Sawada, N. Cx32 Formation and/or Cx32-Mediated Intercellular Communication Induces Expression and Function of Tight Junctions in Hepatocytic Cell Line. Exp. Cell Res. 2002, 276, 40–51. [Google Scholar] [CrossRef]
- Slavi, N.; Toychiev, A.H.; Kosmidis, S.; Ackert, J.; Bloomfield, S.A.; Wulff, H.; Viswanathan, S.; Lampe, P.D.; Srinivas, M. Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy. Proc. Natl. Acad. Sci. 2018, 115, E5934–E5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh-Meyer, H.V.; Zhang, J.; Acosta, M.L.; Rupenthal, I.D.; Green, C.R. Connexin43 in retinal injury and disease. Prog. Retin. Eye Res. 2016, 51, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; O’Kane, R.L.; Simpson, I.A.; Viña, J.R. Structure of the Blood–Brain Barrier and Its Role in the Transport of Amino Acids. J. Nutr. 2006, 136, 218S–226S. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Johanson, C.E. REVIEW: Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Terasaki, T. Contribution of Carrier-Mediated Transport Systems to the Blood–Brain Barrier as a Supporting and Protecting Interface for the Brain; Importance for CNS Drug Discovery and Development. Pharm. Res. 2007, 24, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Vorbrodt, A.W. Ultrastructural cytochemistry of blood-brain barrier endothelia. Prog. Histochem. Cytochem. 1988, 18, III–V, VII–VIII, 1–96. [Google Scholar] [CrossRef]
- O’Donnell, M.E.; Lam, T.I.; Tran, L.Q.; Foroutan, S.; Anderson, S.E. Estradiol Reduces Activity of the Blood–Brain Barrier Na–K–Cl Cotransporter and Decreases Edema Formation in Permanent Middle Cerebral Artery Occlusion. J. Cereb. Blood Flow Metab. 2006, 26, 1234–1249. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.J.; Nicola, P.A.; Wang, S.; Barrand, M.A.; Hladky, S.B. Transporters involved in regulation of intracellular pH in primary cultured rat brain endothelial cells. J. Physiol. 2006, 576, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Bassetti, C.L. Implications of ATP-binding cassette transporters for brain pharmacotherapies. Trends Pharmacol. Sci. 2007, 28, 128–134. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Hyman, S.; McComb, J.G.; Lipovac, M.N.; Tang, G.; Davson, H. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim. Biophys. Acta (BBA)-Biomembr. 1990, 1025, 191–198. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Cerebrovascular permeability to peptides: Manipulations of transport systems at the blood-brain barrier. Pharm. Res. 1995, 12, 1395–1406. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Mackic, J.B.; Djuricic, B.; Davson, H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J. Neurochem. 1989, 53, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Deane, R. IgG-Assisted Age-Dependent Clearance of Alzheimer’s Amyloid Peptide by the Blood-Brain Barrier Neonatal Fc Receptor. J. Neurosci. 2005, 25, 11495–11503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Méresse, S.; Delbart, C.; Fruchart, J.C.; Cecchelli, R. Low-density lipoprotein receptor on endothelium of brain capillaries. J. Neurochem. 1989, 53, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [Green Version]
- Palade, G.E. The fine structure of blood capillaries. J. Appl. Phys. 1953, 24, 1424. [Google Scholar]
- Simionescu, N. The microvascular endothelium segmental differentiations; transcytosis; selective distribution of anionic sites. Adv. Inflamm. Res. 1979, 1, 61–70. [Google Scholar]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E. The Fine Structure of the Gall Bladder Epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.G.; Nichols, B.J. Exploring the caves: Cavins, caveolins and caveolae. Trends Cell Biol. 2010, 20, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae: Structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Shvets, E.; Ludwig, A.; Nichols, B.J. News from the caves: Update on the structure and function of caveolae. Curr. Opin. Cell Biol. 2014, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, G.; Zhang, X.; Minshall, R.D. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ. Res. 2009, 105, 676–685. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Fliesler, S.J.; Zhao, Y.-Y.; Stallcup, W.B.; Cohen, A.W.; Elliott, M.H. Loss of Caveolin-1 Causes Blood–Retinal Barrier Breakdown, Venous Enlargement, and Mural Cell Alteration. Am. J. Pathol. 2014, 184, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gu, X.; Boyce, T.M.; Zheng, M.; Reagan, A.M.; Qi, H.; Mandal, N.; Cohen, A.W.; Callegan, M.C.; Carr, D.J.; et al. Caveolin-1 increases proinflammatory chemoattractants and blood-retinal barrier breakdown but decreases leukocyte recruitment in inflammation. Investig. Ophthalmol. Vis. Sci 2014, 55, 6224–6234. [Google Scholar] [CrossRef]
- Anderson, R.G.W. Transendothelial movement and caveolae. Nat. Biotechnol. 2008, 26, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Predescu, D.; Vogel, S.M.; Malik, A.B. Functional and morphological studies of protein transcytosis in continuous endothelia. Am. J. Physiol Lung Cell Mol. Physiol 2004, 287, L895–L901. [Google Scholar] [CrossRef]
- Hofman, P.; Hoyng, P.; vanderWerf, F.; Vrensen, G.F.J.M.; Schlingemann, R.O. Lack of Blood–Brain Barrier Properties in Microvessels of the Prelaminar Optic Nerve Head. Investig. Ophthalmol. Vis. Sci. 2001, 42, 895–901. [Google Scholar]
- Song, L.; Ge, S.; Pachter, J.S. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 2007, 109, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.-B.; Ma, T.; Wang, P.; Xue, Y.-X. Effect of caveolin-1 on the expression of tight junction-associated proteins in rat glioma-derived microvascular endothelial cells. Int. J. Clin. Exp. Pathol. 2015, 8, 13067. [Google Scholar]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333.e3. [Google Scholar] [CrossRef] [Green Version]
- Schlingemann, R.O.; Dingjan, G.M.; Emeis, J.J.; Blok, J.; Warnaar, S.O.; Ruiter, D.J. Monoclonal antibody PAL-E specific for endothelium. Lab. Investig. 1985, 52, 71–76. [Google Scholar]
- Wisniewska-Kruk, J.; Klaassen, I.; Vogels, I.M.; Magno, A.L.; Lai, C.M.; Van Noorden, C.J.; Schlingemann, R.O.; Rakoczy, E.P. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp. Eye Res. 2014, 122, 123–131. [Google Scholar] [CrossRef]
- Wisniewska-Kruk, J.; Van Der Wijk, A.-E.; Van Veen, H.A.; Gorgels, T.G.M.F.; Vogels, I.M.C.; Versteeg, D.; Van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Plasmalemma Vesicle–Associated Protein Has a Key Role in Blood-Retinal Barrier Loss. Am. J. Pathol. 2016, 186, 1044–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.A.P.; Zhang, J.; Aydin, D.; Xu, Y.; Andreone, B.J.; Langen, U.H.; Dror, R.O.; Gu, C.; Feng, L. Structure and mechanism of blood–brain-barrier lipid transporter MFSD2A. Nature 2021, 596, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Z.; Xiao, N.; Zhang, Y.Z.; Zhao, C.X.; Guo, X.H.; Lu, L.M. Mfsd2a-based pharmacological strategies for drug delivery across the blood-brain barrier. Pharm. Res. 2016, 104, 124–131. [Google Scholar] [CrossRef] [PubMed]
- van der Wijk, A.E.; Vogels, I.M.C.; van Veen, H.A.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Spatial and temporal recruitment of the neurovascular unit during development of the mouse blood-retinal barrier. Tissue Cell 2018, 52, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.-H.; Huang, S.; Chen, J. Assessment and Characterization of Hyaloid Vessels in Mice. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.-H.; Sapieha, P. Retinal vascular development. In Anti-Angiogenic Therapy in Ophthalmology; Essentials in Ophthalmology; Stahl, A., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–19. [Google Scholar] [CrossRef]
- Wacker, A.; Gerhardt, H. Endothelial development taking shape. Curr. Opin. Cell Biol. 2011, 23, 676–685. [Google Scholar] [CrossRef]
- Liu, C.H.; Wang, Z.; Sun, Y.; Chen, J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017, 31, 4665–4681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubala, E.; Mrsny, R.; Paneghetti, L.; Shima, D. Development of the Rodent Inner Blood-Retinal-Barrier. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5144. [Google Scholar]
- Yao, H.; Wang, T.; Deng, J.; Liu, D.; Li, X.; Deng, J. The development of blood-retinal barrier during the interaction of astrocytes with vascular wall cells. Neural Regen. Res. 2014, 9, 1047–1054. [Google Scholar] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.A.; Tuor, U.I. Blood-eye barriers in the rat: Correlation of ultrastructure with function. J. Comp. Neurol. 1994, 340, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Weiner, G.A.; Shah, S.H.; Angelopoulos, C.M.; Bartakova, A.B.; Pulido, R.S.; Murphy, A.; Nudleman, E.; Daneman, R.; Goldberg, J.L. Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun. 2019, 10, 2477. [Google Scholar] [CrossRef]
- Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina. PLoS ONE 2015, 10, e0143650. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, C.-H.; Sun, Y.; Gong, Y.; Favazza, T.L.; Morss, P.C.; Saba, N.J.; Fredrick, T.W.; He, X.; Akula, J.D.; et al. Pharmacologic Activation of Wnt Signaling by Lithium Normalizes Retinal Vasculature in a Murine Model of Familial Exudative Vitreoretinopathy. Am. J. Pathol. 2016, 186, 2588–2600. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Joyal, J.-S.; Juan, A.M.; Hatton, C.J.; Aderman, C.M.; Dennison, R.J.; Willett, K.L.; et al. Retinal Expression of Wnt-Pathway Mediated Genes in Low-Density Lipoprotein Receptor-Related Protein 5 (Lrp5) Knockout Mice. PLoS ONE 2012, 7, e30203. [Google Scholar] [CrossRef]
- Lobov, I.B.; Rao, S.; Carroll, T.J.; Vallance, J.E.; Ito, M.; Ondr, J.K.; Kurup, S.; Glass, D.A.; Patel, M.S.; Shu, W.; et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 2005, 437, 417–421. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular Development in the Retina and Inner Ear. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Dejana, E. The Role of Wnt Signaling in Physiological and Pathological Angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M.; Melia, M.; Wells, J.A. Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment. JAMA Ophthalmol. 2018, 136, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Macdonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, C.A.; Jones, M.L.; Bernabeu, M.O.; Vion, A.-C.; Barbacena, P.; Fan, J.; Mathivet, T.; Fonseca, C.G.; Ragab, A.; Yamaguchi, T.P.; et al. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. eLife 2016, 5, e07727. [Google Scholar] [CrossRef] [PubMed]
- Stefater Iii, J.A.; Lewkowich, I.; Rao, S.; Mariggi, G.; Carpenter, A.C.; Burr, A.R.; Fan, J.; Ajima, R.; Molkentin, J.D.; Williams, B.O.; et al. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature 2011, 474, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Maye, P.; Zheng, J.; Li, L.; Wu, D. Multiple Mechanisms for Wnt11-mediated Repression of the Canonical Wnt Signaling Pathway. J. Biol. Chem. 2004, 279, 24659–24665. [Google Scholar] [CrossRef] [Green Version]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits β-Catenin–TCF Signaling Depending on Receptor Context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Cahill, H.; Yu, M.; Badea, T.C.; Smallwood, P.M.; Peachey, N.S.; Nathans, J. Norrin, Frizzled-4, and Lrp5 Signaling in Endothelial Cells Controls a Genetic Program for Retinal Vascularization. Cell 2009, 139, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Jo, D.H.; Lee, H.; Kim, J.H.; Kim, K.-W.; Kim, J.H. Nerve growth factor-mediated vascular endothelial growth factor expression of astrocyte in retinal vascular development. Biochem. Biophys. Res. Commun. 2013, 431, 740–745. [Google Scholar] [CrossRef]
- Zhou, Y.; Nathans, J. Gpr124 Controls CNS Angiogenesis and Blood-Brain Barrier Integrity by Promoting Ligand-Specific Canonical Wnt Signaling. Dev. Cell 2014, 31, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Mancuso, M.R.; Maier, C.; Liang, X.; Yuki, K.; Yang, L.; Kwong, J.W.; Wang, J.; Rao, V.; Vallon, M.; et al. Gpr124 is essential for blood–brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhollebeke, B.; Stone, O.A.; Bostaille, N.; Cho, C.; Zhou, Y.; Maquet, E.; Gauquier, A.; Cabochette, P.; Fukuhara, S.; Mochizuki, N.; et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 2015, 4, e06489. [Google Scholar] [CrossRef]
- Junge, H.J.; Yang, S.; Burton, J.B.; Paes, K.; Shu, X.; French, D.M.; Costa, M.; Rice, D.S.; Ye, W. TSPAN12 Regulates Retinal Vascular Development by Promoting Norrin- but Not Wnt-Induced FZD4/β-Catenin Signaling. Cell 2009, 139, 299–311. [Google Scholar] [CrossRef] [Green Version]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.F.; Johnstone, S.E.; Newman, J.J.; Kagey, M.H.; Young, R.A. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008, 22, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Van Raay, T.J.; Moore, K.B.; Iordanova, I.; Steele, M.; Jamrich, M.; Harris, W.A.; Vetter, M.L. Frizzled 5 Signaling Governs the Neural Potential of Progenitors in the Developing Xenopus Retina. Neuron 2005, 46, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetsu, O.; McCormick, F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gaspard, J.P.; Chung, D.C. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001, 61, 6050–6054. [Google Scholar] [PubMed]
- Martowicz, A.; Trusohamn, M.; Jensen, N.; Wisniewska-Kruk, J.; Corada, M.; Ning, F.C.; Kele, J.; Dejana, E.; Nyqvist, D. Endothelial β-Catenin Signaling Supports Postnatal Brain and Retinal Angiogenesis by Promoting Sprouting, Tip Cell Formation, and VEGFR (Vascular Endothelial Growth Factor Receptor) 2 Expression. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2273–2288. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, J.; Cheng, R.; Chen, Y.; Lee, K.; Hu, Y.; Yi, J.; Liu, Z.; Ma, J.X. Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid. Redox Signal. 2013, 18, 1141–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, C.H.; Sun, Y.; Gong, Y.; Fu, Z.; Evans, L.P.; Tian, K.T.; Juan, A.M.; Hurst, C.G.; Mammoto, A.; et al. Endothelial TWIST1 Promotes Pathological Ocular Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8267–8277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodilla, V.; Villanueva, A.; Obrador-Hevia, A.; Robert-Moreno, A.; Fernandez-Majada, V.; Grilli, A.; Lopez-Bigas, N.; Bellora, N.; Alba, M.M.; Torres, F.; et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 6315–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavard, J.; Gutkind, J.S. VE-cadherin and claudin-5: It takes two to tango. Nat. Cell Biol. 2008, 10, 883–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Lai, M.B.; Pedler, M.G.; Johnson, V.; Adams, R.H.; Petrash, J.M.; Chen, Z.; Junge, H.J. Endothelial Cell–Specific Inactivation of TSPAN12 (Tetraspanin 12) Reveals Pathological Consequences of Barrier Defects in an Otherwise Intact Vasculature. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2691–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sabbagh, M.F.; Gu, X.; Rattner, A.; Williams, J.; Nathans, J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife 2019, 8, e43257. [Google Scholar] [CrossRef] [PubMed]
- Scha¨Fer, N.F.; Luhmann, U.F.O.; Feil, S.; Berger, W. Differential Gene Expression in Ndph-Knockout Mice in Retinal Development. Investig. Opthalmol. Vis. Sci. 2009, 50, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.; Wang, Y.; Smallwood, P.M.; Williams, J.; Nathans, J. Dlg1 activates beta-catenin signaling to regulate retinal angiogenesis and the blood-retina and blood-brain barriers. eLife 2019, 8, e45542. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yamamoto, H.; Mohn, L.; Ambühl, L.; Kanai, K.; Schmidt, I.; Kim, K.-P.; Fraccaroli, A.; Feil, S.; Junge, H.J.; et al. Integrin-linked kinase controls retinal angiogenesis and is linked to Wnt signaling and exudative vitreoretinopathy. Nat. Commun. 2019, 10, 5243. [Google Scholar] [CrossRef] [PubMed]
- Roudnicky, F.; Zhang, J.D.; Kim, B.K.; Pandya, N.J.; Lan, Y.; Sach-Peltason, L.; Ragelle, H.; Strassburger, P.; Gruener, S.; Lazendic, M.; et al. Inducers of the endothelial cell barrier identified through chemogenomic screening in genome-edited hPSC-endothelial cells. Proc. Natl. Acad. Sci. USA 2020, 117, 19854–19865. [Google Scholar] [CrossRef] [PubMed]
- Zarkada, G.; Howard, J.P.; Xiao, X.; Park, H.; Bizou, M.; Leclerc, S.; Kunzel, S.E.; Boisseau, B.; Li, J.; Cagnone, G.; et al. Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation. Dev. Cell 2021, 56, 2237–2251.e6. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, J.; Smith, J.R.; Shahriar, S.; Cutforth, T.; Ceja, B.; Agalliu, D. The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron 2017, 96, 1055–1069.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidiac, R.; Abedin, M.; Macleod, G.; Yang, A.; Thibeault, P.E.; Blazer, L.L.; Adams, J.J.; Zhang, L.; Roehrich, H.; Jo, H.N.; et al. A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy. EMBO Mol. Med. 2021, 13, e13977. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Lin, C.-M.; Liebner, S.; Antonetti, D.A. Norrin restores blood-retinal barrier properties after vascular endothelial growth factor–induced permeability. J. Biol. Chem. 2020, 295, 4647–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, A.; Tauhid, L.; Davenport, I.; Huckaba, T.; Graves, R.; Mandal, T.; Muniruzzaman, S.; Ahmed, S.A.; Bhattacharjee, P.S. LRP-1 Pathway Targeted Inhibition of Vascular Abnormalities in the Retina of Diabetic Mice. Curr. Eye Res. 2017, 42, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog. Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.A.; Yang, G.; Goltsov, A.; Song, K.D.; Ren, C.; Wang, J.; Chang, W.; Thompson, T.C. Caveolin-1-LRP6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Res. 2013, 73, 1900–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hassan, G.S.; Williams, T.M.; Minetti, C.; Pestell, R.G.; Tanowitz, H.B.; Frank, P.G.; Sotgia, F.; Lisanti, M.P. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle 2005, 4, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Sotgia, F.; Williams, T.M.; Cohen, A.W.; Minetti, C.; Pestell, R.G.; Lisanti, M.P. Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell Cycle 2005, 4, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, S.; Wang, L.; Li, Q.; Li, J.; Li, Y.; Thannickal, V.J.; Cui, Z. Caveolin-1 regulates dorsoventral patterning through direct interaction with beta-catenin in zebrafish. Dev. Biol. 2010, 344, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Wang, H.; Gu, Y.; Li, X.; Tao, L.; Lu, P. Melatonin exerts protective effects on diabetic retinopathy via inhibition of Wnt/β-catenin pathway as revealed by quantitative proteomics. Exp. Eye Res. 2021, 205, 108521. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Bursell, S.-E.; Clermont, A.; Duh, E.; Ishii, H.; Takagi, C.; Mori, F.; Ciulla, T.A.; Ways, K.; Jirousek, M. Vascular endothelial growth factor–induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform–selective inhibitor. Diabetes 1997, 46, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Morello, C.M. Etiology and natural history of diabetic retinopathy: An overview. Am. J. Health-Syst. Pharm. 2007, 64 (Suppl. 17), S3–S7. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, A.; Mohamed, R.; Kira, D.; Alhusban, S.; Al-Shabrawey, M. N-Methyl-D-aspartate receptor activation, novel mechanism of homocysteine-induced blood–retinal barrier dysfunction. J. Mol. Med. 2020, 99, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, A.; Kajihara, N.; Kukidome, D.; Motoshima, H.; Matsumura, T.; Nishikawa, T.; Araki, E. Hypoglycemia Induces Mitochondrial Reactive Oxygen Species Production through Increased Fatty Acid Oxidation and Promotes Retinal Vascular Permeability in Diabetic Mice. Antioxid. Redox Signal. 2021, 34, 1245–1259. [Google Scholar] [CrossRef]
- Cheung, A.K.H.; Fung, M.K.L.; Lo, A.C.Y.; Lam, T.T.L.; So, K.F.; Chung, S.S.M.; Chung, S.K. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes 2005, 54, 3119–3125. [Google Scholar] [CrossRef] [Green Version]
- Obrosova, I.G.; Kador, P.F. Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.M.; Llorián-Salvador, M.; Tang, M.; Margariti, A.; Chen, M.; Xu, H. IL-17A Damages the Blood–Retinal Barrier through Activating the Janus Kinase 1 Pathway. Biomedicines 2021, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.; Castilho, Á.; Baptista, F.; Simões, N.; Fernandes, C.; Leal, E.; Ambrósio, A.F. High glucose and interleukin-1β downregulate interleukin-1 type I receptor (IL-1RI) in retinal endothelial cells by enhancing its degradation by a lysosome-dependent mechanism. Cytokine 2010, 49, 279–286. [Google Scholar] [CrossRef]
- Yang, J.; Duh, E.J.; Caldwell, R.B.; Behzadian, M.A. Antipermeability Function of PEDF Involves Blockade of the MAP Kinase/GSK/β-Catenin Signaling Pathway and uPAR Expression. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3273. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Franklin, T.; Ghaley, N.; Yang, J.; Brands, M.W.; Caldwell, R.B.; Behzadian, M.A. Diabetes-Induced Superoxide Anion and Breakdown of the Blood-Retinal Barrier: Role of the VEGF/uPAR Pathway. PLoS ONE 2013, 8, e71868. [Google Scholar] [CrossRef] [Green Version]
- Devi, T.S.; Singh, L.P.; Hosoya, K.; Terasaki, T. GSK-3beta/CREB axis mediates IGF-1-induced ECM/adhesion molecule expression, cell cycle progression and monolayer permeability in retinal capillary endothelial cells: Implications for diabetic retinopathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1080–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood–retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic Degradation of VE-Cadherin Alters the Blood-Retinal Barrier in Diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drankowska, J.; Kos, M.; Kościuk, A.; Marzęda, P.; Boguszewska-Czubara, A.; Tylus, M.; Święch-Zubilewicz, A. MMP targeting in the battle for vision: Recent developments and future prospects in the treatment of diabetic retinopathy. Life Sci. 2019, 229, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Vandooren, J.; Siddiquei, M.M.; Martens, E.; Abu El-Asrar, A.M.; Opdenakker, G. Functional links between gelatinase B/matrix metalloproteinase-9 and prominin-1/CD133 in diabetic retinal vasculopathy and neuropathy. Prog. Retin. Eye Res. 2014, 43, 76–91. [Google Scholar] [CrossRef]
- Subramanian, M.L.; Stein, T.D.; Siegel, N.; Ness, S.; Fiorello, M.G.; Kim, D.; Roy, S. Upregulation of Lysyl Oxidase Expression in Vitreous of Diabetic Subjects: Implications for Diabetic Retinopathy. Cells 2019, 8, 1122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, K.K.; Ma, J.X. Inhibition of Connective Tissue Growth Factor Overexpression in Diabetic Retinopathy by SERPINA3K via Blocking the WNT/-Catenin Pathway. Diabetes 2010, 59, 1809–1816. [Google Scholar] [CrossRef] [Green Version]
- Hussein, K.A.; Choksi, K.; Akeel, S.; Ahmad, S.; Megyerdi, S.; El-Sherbiny, M.; Nawaz, M.; Abu El-Asrar, A.; Al-Shabrawey, M. Bone morphogenetic protein 2: A potential new player in the pathogenesis of diabetic retinopathy. Exp. Eye Res. 2014, 125, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulaki, V.; Joussen, A.M.; Mitsiades, N.; Mitsiades, C.S.; Iliaki, E.F.; Adamis, A.P. Insulin-Like Growth Factor-I Plays a Pathogenetic Role in Diabetic Retinopathy. Am. J. Pathol. 2004, 165, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; He, J.; Johnson, D.K.; Wei, Y.; Liu, Y.; Wang, S.; Lutty, G.A.; Duh, E.J.; Carmeliet, P.; Semba, R.D. Deletion of Placental Growth Factor Prevents Diabetic Retinopathy and Is Associated With Akt Activation and HIF1α-VEGF Pathway Inhibition. Diabetes 2015, 64, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yao, Y.; Wang, K.; Li, J.; Chu, T.; Shen, H. MicroRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol. 2020, 57, 1435–1443. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Kern, T.S.; Antonetti, D.A.; Smith, L.E. Pathophysiology of Diabetic Retinopathy: Contribution and Limitations of Laboratory Research. Ophthalmic Res. 2019, 62, 196–202. [Google Scholar] [CrossRef]
- Bolinger, M.; Antonetti, D. Moving Past Anti-VEGF: Novel Therapies for Treating Diabetic Retinopathy. Int. J. Mol. Sci. 2016, 17, 1498. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Korobelnik, J.F.; Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Midena, E.; Boyer, D.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 2016, 123, 2376–2385. [Google Scholar] [CrossRef]
- Park, W.; Kim, J.; Choi, S.; Kim, T.; Park, M.; Kim, S.; You, J.-C.; Kim, J.H.; Ha, K.-S.; Lee, J.-H.; et al. Human plasminogen-derived N-acetyl-Arg-Leu-Tyr-Glu antagonizes VEGFR-2 to prevent blood-retinal barrier breakdown in diabetic mice. Biomed. Pharmacother. 2021, 134, 111110. [Google Scholar] [CrossRef]
- Lupo, G.; Cambria, M.T.; Olivieri, M.; Rocco, C.; Caporarello, N.; Longo, A.; Zanghì, G.; Salmeri, M.; Foti, M.C.; Anfuso, C.D. Anti-angiogenic effect of quercetin and its 8-methyl pentamethyl ether derivative in human microvascular endothelial cells. J. Cell. Mol. Med. 2019, 23, 6565–6577. [Google Scholar] [CrossRef] [Green Version]
- El-Remessy, A.B.; Behzadian, M.A.; Abou-Mohamed, G.; Franklin, T.; Caldwell, R.W.; Caldwell, R.B. Experimental Diabetes Causes Breakdown of the Blood-Retina Barrier by a Mechanism Involving Tyrosine Nitration and Increases in Expression of Vascular Endothelial Growth Factor and Urokinase Plasminogen Activator Receptor. Am. J. Pathol. 2003, 162, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Marsili, S.; Lista, L.; De Rosa, M.; Pavone, V.; Rusciano, D.; Bagnoli, P. Diabetic Retinopathy in the Spontaneously Diabetic Torii Rat: Pathogenetic Mechanisms and Preventive Efficacy of Inhibiting the Urokinase-Type Plasminogen Activator Receptor System. J. Diabetes Res. 2017, 2017, 2904150. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Xu, H.; Zhang, C.; Xie, H.; Yang, Q.; Li, W.; Tian, H.; Lu, L.; Xu, J.-Y.; Xu, G.; et al. Erythropoietin maintains VE-cadherin expression and barrier function in experimental diabetic retinopathy via inhibiting VEGF/VEGFR2/Src signaling pathway. Life Sci. 2020, 259, 118273. [Google Scholar] [CrossRef]
- Murakami, T.; Frey, T.; Lin, C.; Antonetti, D.A. Protein Kinase C Phosphorylates Occludin Regulating Tight Junction Trafficking in Vascular Endothelial Growth Factor-Induced Permeability In Vivo. Diabetes 2012, 61, 1573–1583. [Google Scholar] [CrossRef] [Green Version]
- Harhaj, N.S.; Felinski, E.A.; Wolpert, E.B.; Sundstrom, J.M.; Gardner, T.W.; Antonetti, D.A. VEGF Activation of Protein Kinase C Stimulates Occludin Phosphorylation and Contributes to Endothelial Permeability. Investig. Opthalmol. Vis. Sci. 2006, 47, 5106. [Google Scholar] [CrossRef]
- Arima, M.; Nakao, S.; Yamaguchi, M.; Feng, H.; Fujii, Y.; Shibata, K.; Wada, I.; Kaizu, Y.; Ahmadieh, H.; Ishibashi, T.; et al. Claudin-5 Redistribution Induced by Inflammation Leads to Anti-VEGF–Resistant Diabetic Macular Edema. Diabetes 2020, 69, 981–999. [Google Scholar] [CrossRef]
- Rangasamy, S.; Monickaraj, F.; Legendre, C.; Cabrera, A.P.; Llaci, L.; Bilagody, C.; McGuire, P.; Das, A. Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy. Exp. Eye Res. 2020, 195, 108043. [Google Scholar] [CrossRef]
- Tsukikawa, M.; Stacey, A.W. A Review of Hypertensive Retinopathy and Chorioretinopathy. Clin. Optom. 2020, 12, 67–73. [Google Scholar] [CrossRef]
- Yang, J.M.; Park, C.S.; Kim, S.H.; Noh, T.W.; Kim, J.-H.; Park, S.; Lee, J.; Park, J.R.; Yoo, D.; Jung, H.H.; et al. Dll4 Suppresses Transcytosis for Arterial Blood-Retinal Barrier Homeostasis. Circ. Res. 2020, 126, 767–783. [Google Scholar] [CrossRef]
- Miloudi, K.; Oubaha, M.; Ménard, C.; Dejda, A.; Guber, V.; Cagnone, G.; Wilson, A.M.; Tétreault, N.; Mawambo, G.; Binet, F.; et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 4538–4547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Dong, X.; Lu, S.; Gao, Y.; Sun, G.; Sun, X. Gypenoside XVII alleviates early diabetic retinopathy by regulating Müller cell apoptosis and autophagy in db/db mice. Eur. J. Pharmacol. 2021, 895, 173893. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Xu, H.; Takeuchi, M.; Ito, M.; Chen, M. Microglia increase tight-junction permeability in coordination with Müller cells under hypoxic condition in an in vitro model of inner blood-retinal barrier. Exp. Eye Res. 2021, 205, 108490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-H.; Liu, B.-Q.; Hao, M.-J.; Fan, Y.-X.; Qian, C.; Teng, P.; Zhou, X.-W.; Hu, L.; Liu, W.-T.; Yuan, Z.-L.; et al. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-κB Pathway Through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation 2017, 40, 1475–1486. [Google Scholar] [CrossRef]
- Rao, R.; Honavar, S.G. Retinoblastoma. Indian J. Pediatr. 2017, 84, 937–944. [Google Scholar] [CrossRef]
- Seo, J.H.; Maki, T.; Miyamoto, N.; Choi, Y.K.; Chung, K.K.; Hamanaka, G.; Park, J.H.; Mandeville, E.T.; Takase, H.; Hayakawa, K.; et al. AKAP12 Supports Blood-Brain Barrier Integrity against Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 9078. [Google Scholar] [CrossRef]
- Yemanyi, F.; Vranka, J.; Raghunathan, V.K. Crosslinked Extracellular Matrix Stiffens Human Trabecular Meshwork Cells Via Dysregulating β-catenin and YAP/TAZ Signaling Pathways. Investig. Opthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Campbell, M.; Tachibana, K.; Okada, Y.; Kondoh, M. Claudin-5: A Pharmacological Target to Modify the Permeability of the Blood–Brain Barrier. Biol. Pharm. Bull. 2021, 44, 1380–1390. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemanyi, F.; Bora, K.; Blomfield, A.K.; Wang, Z.; Chen, J. Wnt Signaling in Inner Blood–Retinal Barrier Maintenance. Int. J. Mol. Sci. 2021, 22, 11877. https://doi.org/10.3390/ijms222111877
Yemanyi F, Bora K, Blomfield AK, Wang Z, Chen J. Wnt Signaling in Inner Blood–Retinal Barrier Maintenance. International Journal of Molecular Sciences. 2021; 22(21):11877. https://doi.org/10.3390/ijms222111877
Chicago/Turabian StyleYemanyi, Felix, Kiran Bora, Alexandra K. Blomfield, Zhongxiao Wang, and Jing Chen. 2021. "Wnt Signaling in Inner Blood–Retinal Barrier Maintenance" International Journal of Molecular Sciences 22, no. 21: 11877. https://doi.org/10.3390/ijms222111877
APA StyleYemanyi, F., Bora, K., Blomfield, A. K., Wang, Z., & Chen, J. (2021). Wnt Signaling in Inner Blood–Retinal Barrier Maintenance. International Journal of Molecular Sciences, 22(21), 11877. https://doi.org/10.3390/ijms222111877






