New Insight into Molecular and Hormonal Connection in Andrology
Abstract
1. Introduction
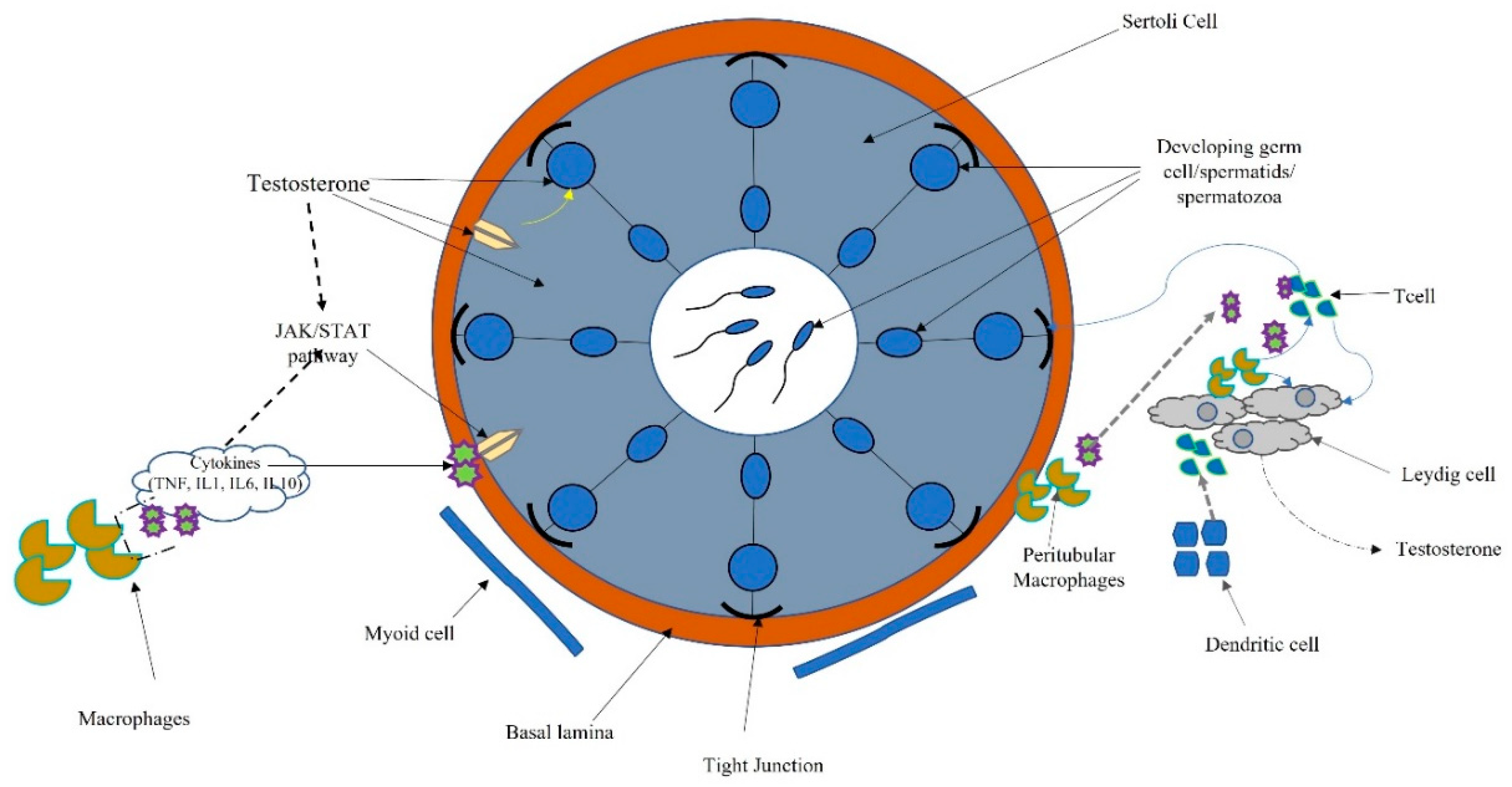
2. The Testis
3. Role of Cytokines
4. Role of miRNAs and Androgens
5. Role of Endothelial Progenitor Cells (EPCS)
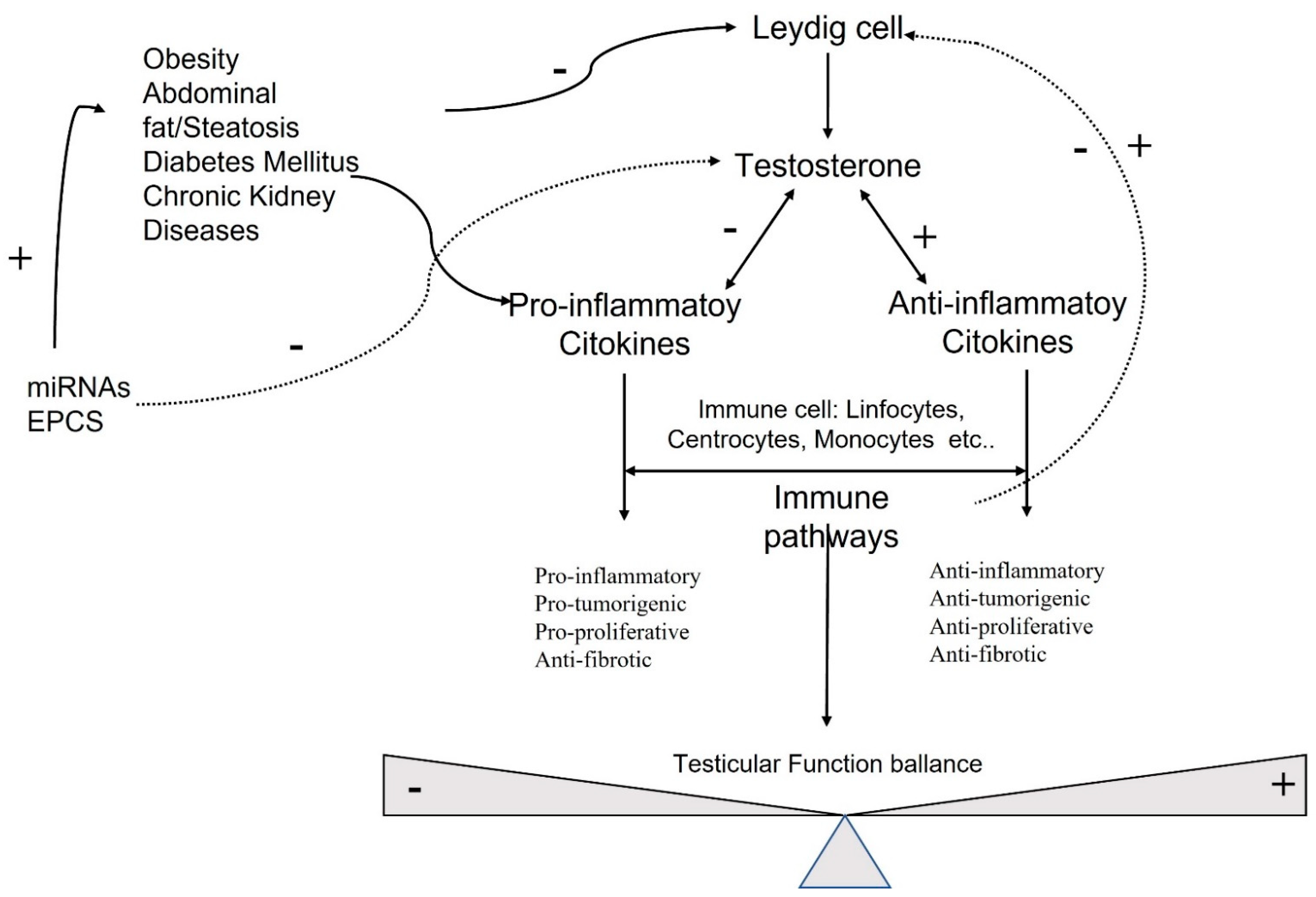
6. Molecular and Hormonal Correlation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanatsu-Shinohara, M.; Shinohara, T. Spermatogonial stem cell self-renewaland development. Annu. Rev. Cell Dev. Biol. 2013, 29, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Meinhardt, A. Cytokines and the immune-testicular axis. J. Reprod. Immunol. 2003, 58, 1–26. [Google Scholar] [CrossRef]
- Ruey-Sheng, W.; Shuyuan, Y.; Chii-Ruey, T.; Chawnshang, C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009, 30, 119–132. [Google Scholar]
- Yao, C.; Liu, Y.; Sun, M.; Niu, M.; Yuan, Q.; Hai, Y.; Guo, Y.; Chen, Z.; Hou, J.; Liu, Y.; et al. MicroRNAs andDNA methylation as epigenetic regulators of mitosis, meiosis andspermiogenesis. Reproduction 2015, 150, R25–R34. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef]
- Haidl, G.; Allam, J.P.; Schuppe, H.-C. Chronic epididymitis: Impact on semen parameters and therapeutic options. Andrologia 2008, 40, 92–96. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Jörgensen, N.; Pöllänen, P.; Giwercman, A.; Skakkebaek, N.E. Incidence of testicular mononuclear cell infiltrates in normal human males and in patients with germ cell neoplasia. Int. J. Androl. 1995, 18, 313–320. [Google Scholar] [CrossRef]
- Morrow, C.M.K.; Mruk, D.; Cheng, C.Y.; Hess, R.A. Claudin and occludin expression and function in the seminiferous epithelium. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1679–1696. [Google Scholar] [CrossRef]
- Gilleron, J.; Carette, D.; Durand, P.; Pointis, G.; Segretain, D. Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int. J. Biochem. Cell Biol. 2009, 41, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Pelletier, R.-M.; Cyr, D.G.; Smith, C.E.; Pelletier, R. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: Intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc. Res. Tech. 2009, 73, 409–494. [Google Scholar] [CrossRef]
- Smith, B.E.; Braun, R.E. Germ cell migration across sertoli cell tight junctions. Science 2012, 338, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Sluka, P.; O′Donnell, L.; Bartles, J.R.; Stanton, P. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J. Endocrinol. 2006, 189, 381–395. [Google Scholar] [CrossRef]
- Sarkar, O.; Mathur, P.P.; Cheng, C.Y.; Mruk, D.D. Interleukin 1 Alpha (IL1A) is a novel regulator of the blood-testis barrier in the Rat1. Biol. Reprod. 2008, 78, 445–454. [Google Scholar] [CrossRef]
- Catizone, A.; Ricci, G.; Caruso, M.; Ferranti, F.; Canipari, R.; Galdieri, M. Hepatocyte growth factor (HGF) regulates blood–testis barrier (BTB) in adult rats. Mol. Cell. Endocrinol. 2012, 348, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Gustafsson, K.; Söder, O.; Pöllänen, P.; Ritzén, E. Isolation and partial characterization of an interleukin-1-like factor from rat testis interstitial fluid. J. Reprod. Immunol. 1988, 14, 139–150. [Google Scholar] [CrossRef]
- Ware, C.F. The TNF receptor superfamily in immune regulation. Immunol. Rev. 2011, 244, 5–8. [Google Scholar] [CrossRef]
- Hvarness, T.; Nielsen, J.E.; Almstrup, K.; Skakkebaek, N.E.; Meyts, E.R.-D.; Claesson, M.H. Phenotypic characterisation of immune cell infiltrates in testicular germ cell neoplasia. J. Reprod. Immunol. 2013, 100, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, J.; Liu, C.; Zhou, J.; Zhu, C.; Zhu, Y.; Li, M.; Fan, L.; Duan, Y.; Li, X. Immature CD11c+ myeloid dendritic cells with inflammatory and regulatory cytokine profile in human seminoma. Int. J. Clin. Exp. Pathol. 2016, 9, 2803–2819. [Google Scholar]
- Head, J.R.; Neaves, W.B.; Billingham, R.E. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation 1983, 36, 423–430. [Google Scholar] [CrossRef]
- Shutao, Z.; Weiwei, Z.; Shepu, X.; Daishu, H. Testicular defense systems: Immune privilege and innate immunity. Cell Mol. Immunol. 2014, 11, 428–437. [Google Scholar]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Yuo, K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar]
- Lee, N.P.Y.; Cheng, C.Y. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Oxid. Med. Cell. Longev. 2008, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.P.; Cheng, C.Y. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 172–185. [Google Scholar]
- Fijak, M.; Damm, L.-J.; Wenzel, J.-P.; Aslani, F.; Walecki, M.; Wahle, E.; Eisel, F.; Bhushan, S.; Hackstein, H.; Baal, N.; et al. Influence of testosterone on inflammatory response in testicular cells and expression of transcription factor Foxp3 in T cells. Am. J. Reprod. Immunol. 2015, 74, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Griffith, T.S. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol. Rev. 2006, 213, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleu-kin-10 in vitro. J. Leukoc. Biol. 2011, 90, 133–143. [Google Scholar] [CrossRef]
- Hedger, M. Immunology of the testis and male reproductive tract. Compr. Toxicol. 2010, 189–230. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environ-mental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Giannoulatou, E.; Maher, G.J.; Ding, Z.; Gillis, A.J.; Dorssers, L.C.; Hoischen, A.; Rajpert-De Meyts, E.; WGS500 Consortium; McVean, G.; Wilkie, A.O.; et al. Whole-genome sequencing of spermatocytic tumors provides insights into the mutational processes operating in the male germline. PLoS ONE 2017, 12, e0178169. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.C.; Chandler, G.L.; Damoulis, V.A.; Fustino, N.J.; Lillard, K.; Looijenga, L.; Margraf, L.; Rakheja, D.; Amatruda, J.F. Mutation in the type IB bone morphogenetic protein receptor Alk6b impairs germ-cell differentiation and causes germ-cell tumors in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 13153–13158. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Graubard, B.I.; Chanock, S.J.; Rubertone, M.V.; Erickson, R.L.; McGlynn, K.A. Genetic variation in the inhibin pathway and risk of testicular germ cell tumors. Cancer Res. 2008, 68, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Kubiczkova, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. TGF-β—An excellent servant but a bad master. J. Transl. Med. 2012, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Qiu, S.D.; Wang, H.X.; Tian, H.; Wang, L.R.; Huo, Y.W. Androgen receptor: A new player associated with apoptosis and proliferation of pancreatic beta-cellin type 1 diabetes mellitus. Apoptosis 2008, 13, 959–971. [Google Scholar] [CrossRef]
- Sacks, D.B.; Bruns, D.E.; Goldstein, D.E.; Maclaren, N.K.; McDonald, J.M.; Parrott, M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011, 34, e61–e99. [Google Scholar] [CrossRef]
- Kim, J.W.; Oh, M.M.; Yoon, C.Y.; Bae, J.H.; Kim, J.J.; Moon, D.G. The effect of diet-induced insulin resistance on DNA methylation of the androgen receptor promoterin the penile cavernosal smooth muscle of mice. Asian J. Androl. 2013, 15, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Kumano, K.; Darden, C.M.; Rahman, I.; Lawrence, M.C.; Naziruddin, B. MicroRNA signatures as future biomarkers for diagnosis of diabetes states. Cells 2019, 8, 1533. [Google Scholar] [CrossRef]
- Massard, C.; Fizazi, K. Targeting continued androgen receptor signaling in prostate cancer. Clin. Cancer Res. 2011, 17, 3876–3883. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef]
- Caporali, A.; Meloni, M.; Völlenkle, C.; Bonci, D.; Sala-Newby, G.B.; Addis, R.; Spinetti, G.; Losa, S.; Masson, R.; Baker, A.H.; et al. Deregulation of mi-croRNA-503 contributes to diabetes mellitus-induced impairmentof endothelial function and reparative angiogenesis after limb ischemia. Circulation 2011, 123, 282–291. [Google Scholar] [CrossRef]
- Hagman, Z.; Haflidadottir, B.S.; Ceder, J.A.; Larne, O.; Bjartell, A.; Lilja, H.; Edsjö, A.; Ceder, Y. miR-205 negatively regulates the androgen receptor and is associated with adverse out-come of prostate cancer patients. Br. J. Cancer 2013, 108, 1668–1676. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targetingZEB1andSIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. ThemiR-200 family determines the epithelial phenotype of cancer cells by targeting the E-c adherin repressors ZEB1andZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef]
- Cui, K.; Li, R.; Chen, R.; Li, M.; Wang, T.; Yang, J.; Chen, Z.; Wang, S.; Liu, J.; Rao, K. Androgen deficiency impairs erectilefunction in rats through promotion of corporal fibrosis. Andrologia 2018, 50, 12797. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.; Kim, N. Original research—Endocrinology: The physiological role of androgens in penile erection: Regulation of corpus cavernosum structure and function. J. Sex. Med. 2005, 2, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Tang, C.; Zhang, Y.; Wu, J.; Bao, J.; Zheng, H.; Xu, C.; Yan, W. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol. Open 2015, 4, 212–223. [Google Scholar] [CrossRef]
- Tong, M.H.; Mitchell, D.A.; McGowan, S.D.; Evanoff, R.; Griswold, M.D. Two miRNAClusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol. Reprod. 2012, 86, 72. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Rentsch, M.; Sasaki, K.-I.; Ellwart, J.W.; Fändrich, F.; Siebert, R.; Cooke, J.; Dimmeler, S.; Heeschen, C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007, 100, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Agostini, C.; Sartore, S.; Avogaro, A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef] [PubMed]
- Katabami, T.; Kato, H.; Asahina, T.; Hinohara, S.; Shin, T.; Kawata, T.; Ohta, A.; Iwamoto, T.; Tanaka, Y. Serum free testosterone and metabolic syndrome in Japanese men. Endocr. J. 2010, 57, 533–539. [Google Scholar] [CrossRef]
- Ferreras, C.; Cole, C.L.; Urban, K.; Jayson, G.; Avizienyte, E. Segregation of late outgrowth endothelial cells into functional endothelial CD34− and progenitor-like CD34+ cell populations. Angiogenesis 2014, 18, 47–68. [Google Scholar] [CrossRef]
- Popa, E.R.; Harmsen, M.; Tio, R.A.; Van Der Strate, B.W.; Brouwer, L.A.; Schipper, M.; Koerts, J.; De Jongste, M.J.; Hazenberg, A.; Hendriks, M. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J. Mol. Cell. Cardiol. 2006, 41, 86–96. [Google Scholar] [CrossRef]
- Dierick, F.; Solinc, J.; Bignard, J.; Soubrier, F.; Nadaud, S. Progenitor/stem cells in vascular remodeling during pulmonary arterial hypertension. Cells 2021, 10, 1338. [Google Scholar] [CrossRef]
- Gu¨ven, H.; Shepherd, R.M.; Bach, R.G.; Capoccia, B.J.; Link, D.C. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J. Am. Coll. Cardiol. 2006, 48, 1579–1587. [Google Scholar] [CrossRef]
- Shet, A. Characterizing blood microparticles: Technical aspects and challenges. Vasc. Heal. Risk Manag. 2008, 4, 769–774. [Google Scholar] [CrossRef][Green Version]
- Zitzmann, M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Ciotola, M.; Giugliano, F.; Schisano, B.; Improta, L.; Improta, M.R.; Beneduce, F.; Rispoli, M.; De Sio, M.; Giugliano, D. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int. J. Impot. Res. 2006, 19, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Cannarella, R.; Condorelli, R.A.; Cimino, L.; Ridolfo, F.; Giurato, G.; Romano, C.; La Vignera, S.; Calogero, A.E. Evidence for long noncoding RNA GAS5 up-regulationin patients with Klinefelter syndrome. BMC Med. Genet. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. Int. J. Lab. Med. 2013, 50, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.A.M.; Neves, K.B.; Pestana, C.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef]
- Rwik, S.; Christopher, B. Do transgenerational epigenetic inheritance and immune system development share common epigenetic processes? J. Dev. Biol. 2021, 9, 20. [Google Scholar]
- Foresta, C.; Zuccarello, D.; Biagioli, A.; De Toni, L.; Prana, E.; Nicoletti, V.; Ambrosini, G.; Ferlin, A. Oestrogen stimulates endothelial progenitor cells via oestrogen receptor-α clin. Endocrinology 2007, 67, 520–525. [Google Scholar]
- Foresta, C.; Caretta, N.; Lana, A.; De Toni, L.; Biagioli, A.; Ferlin, A.; Garolla, A. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J. Clin. Endocrinol. Metab. 2006, 91, 4599–4602. [Google Scholar] [CrossRef]
- Fadini, G.P.; Albiero, M.; Cignarella, A.; Bolego, C.; Pinna, C.; Boscaro, E.; Pagnin, E.; De Toni, R.; De Kreutzenberg, S.; Agostini, C.; et al. Effects of androgens on endothelial progenitor cells in vitro and in vivo. Clin. Sci. 2009, 117, 355–364. [Google Scholar] [CrossRef]
- Heiber, J.F.; Geiger, T.L. Context and location dependence of adaptive Foxp3+ regulatory T cell formation during immunopathological conditions. Cell. Immunol. 2012, 279, 60–65. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francomano, D.; Sanguigni, V.; Capogrosso, P.; Deho, F.; Antonini, G. New Insight into Molecular and Hormonal Connection in Andrology. Int. J. Mol. Sci. 2021, 22, 11908. https://doi.org/10.3390/ijms222111908
Francomano D, Sanguigni V, Capogrosso P, Deho F, Antonini G. New Insight into Molecular and Hormonal Connection in Andrology. International Journal of Molecular Sciences. 2021; 22(21):11908. https://doi.org/10.3390/ijms222111908
Chicago/Turabian StyleFrancomano, Davide, Valerio Sanguigni, Paolo Capogrosso, Federico Deho, and Gabriele Antonini. 2021. "New Insight into Molecular and Hormonal Connection in Andrology" International Journal of Molecular Sciences 22, no. 21: 11908. https://doi.org/10.3390/ijms222111908
APA StyleFrancomano, D., Sanguigni, V., Capogrosso, P., Deho, F., & Antonini, G. (2021). New Insight into Molecular and Hormonal Connection in Andrology. International Journal of Molecular Sciences, 22(21), 11908. https://doi.org/10.3390/ijms222111908





