Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating
Abstract
:1. Introduction
2. Results and Discussion
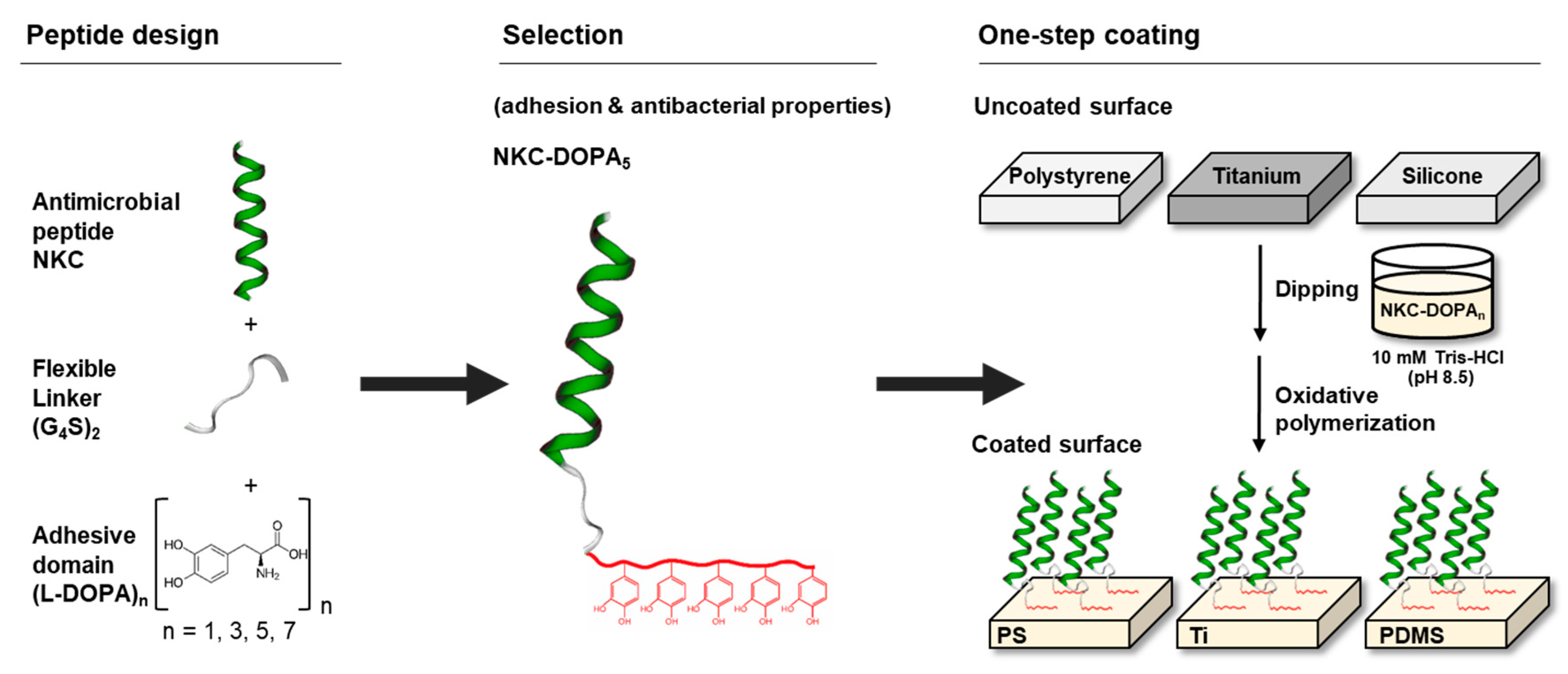
2.1. Design of Adhesive AMPs
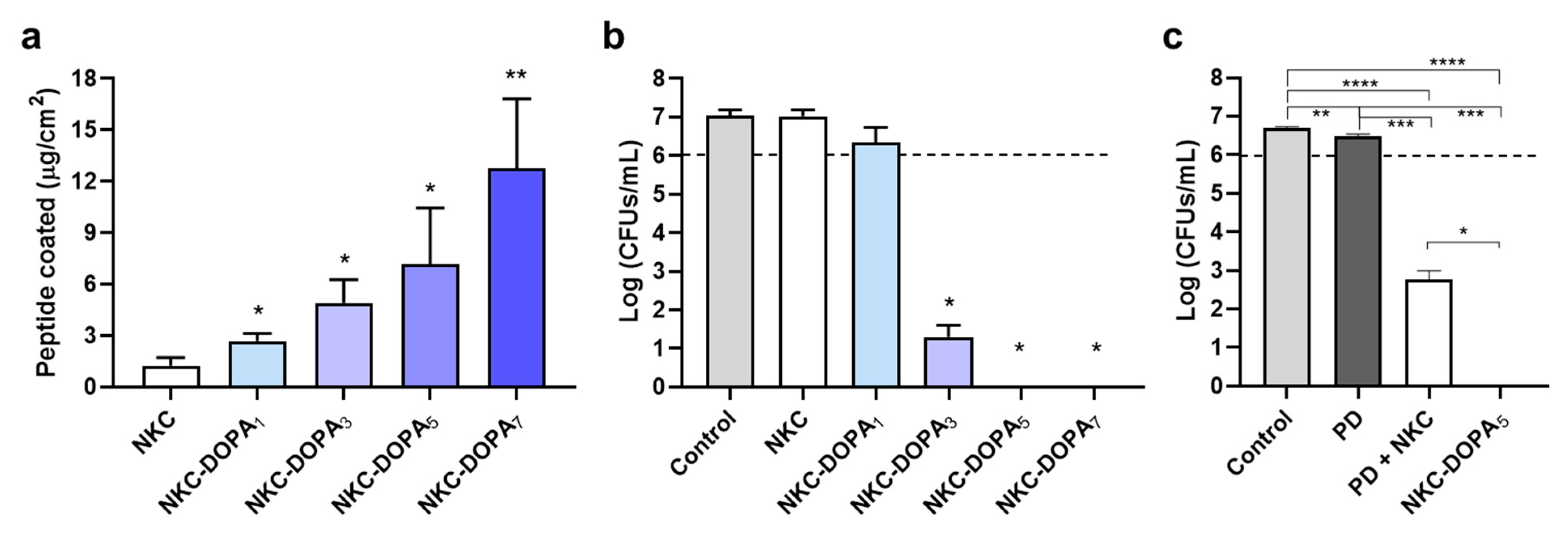
2.2. Adhesion Capacities of the Designed Peptides
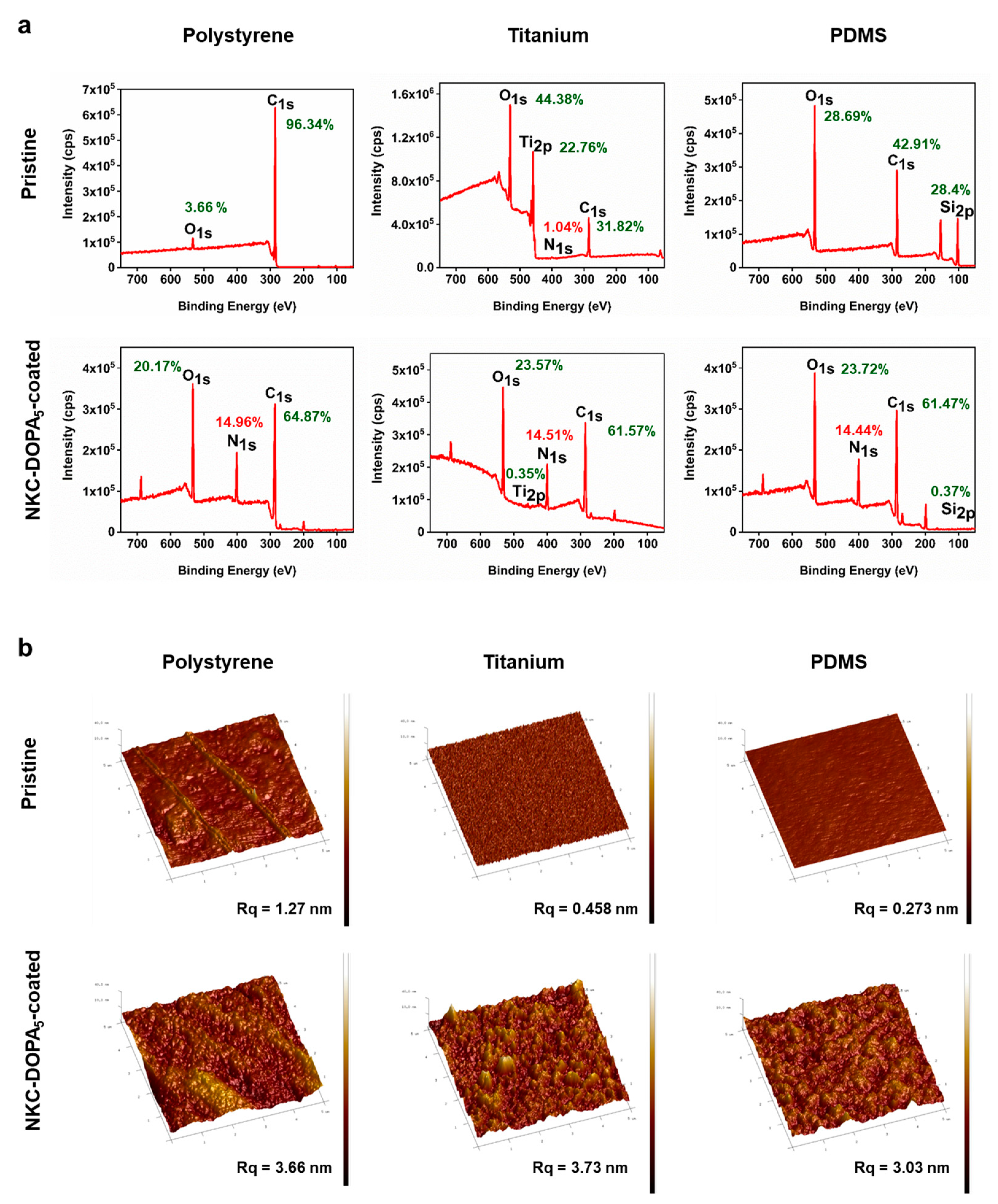
2.3. Characterization of AMP-Coated Surfaces
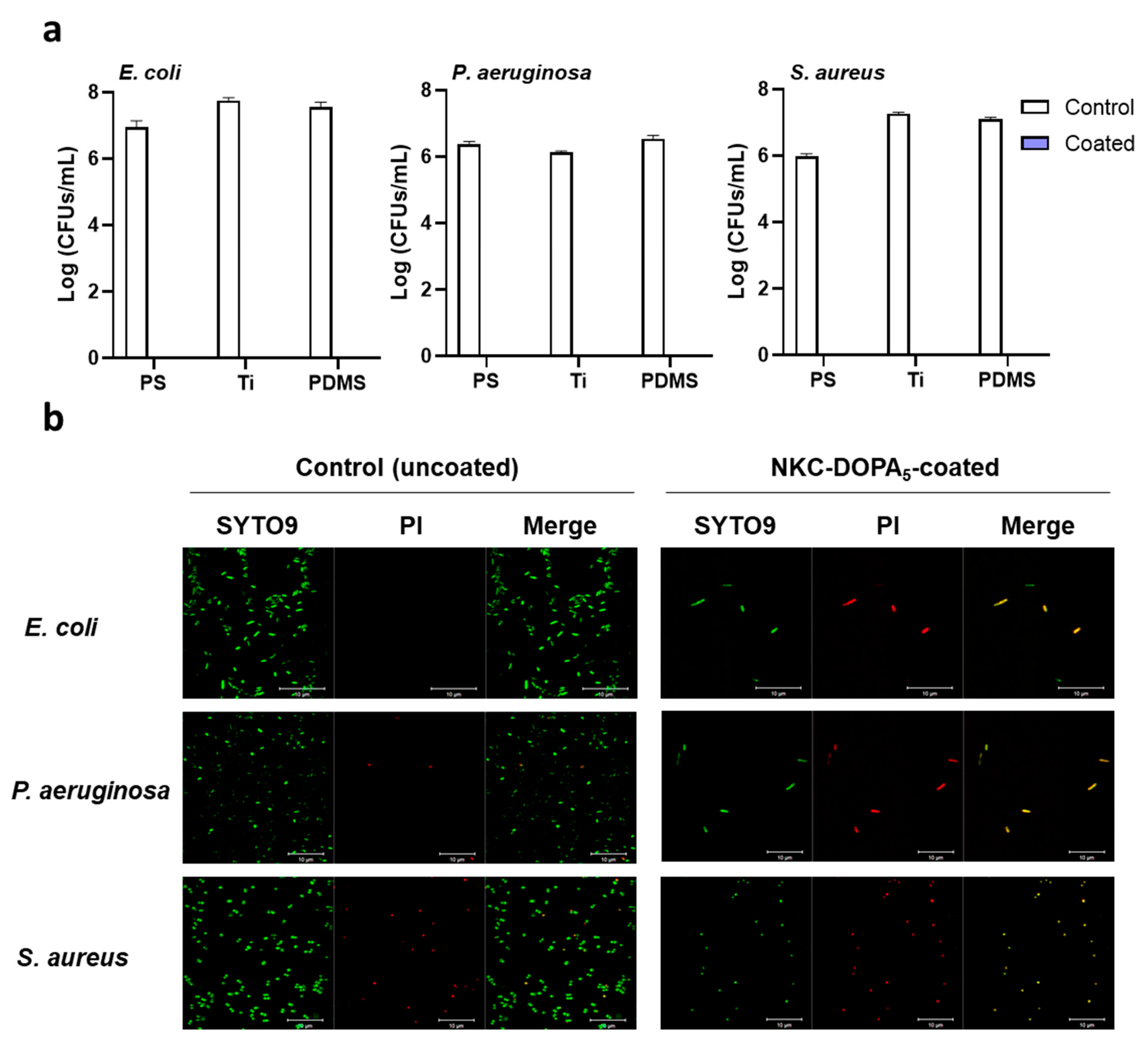
2.4. Antimicrobial and Antifouling Efficacies of NKC-DOPA5
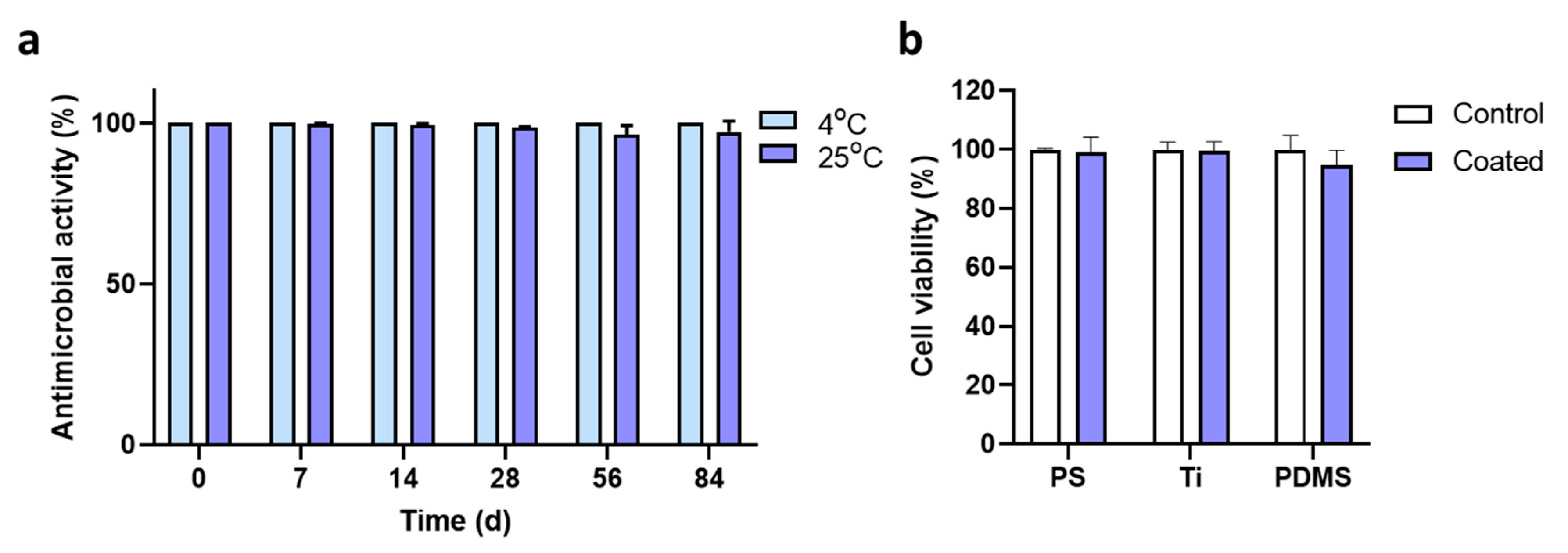
2.5. Characteristics of the Coated Peptide
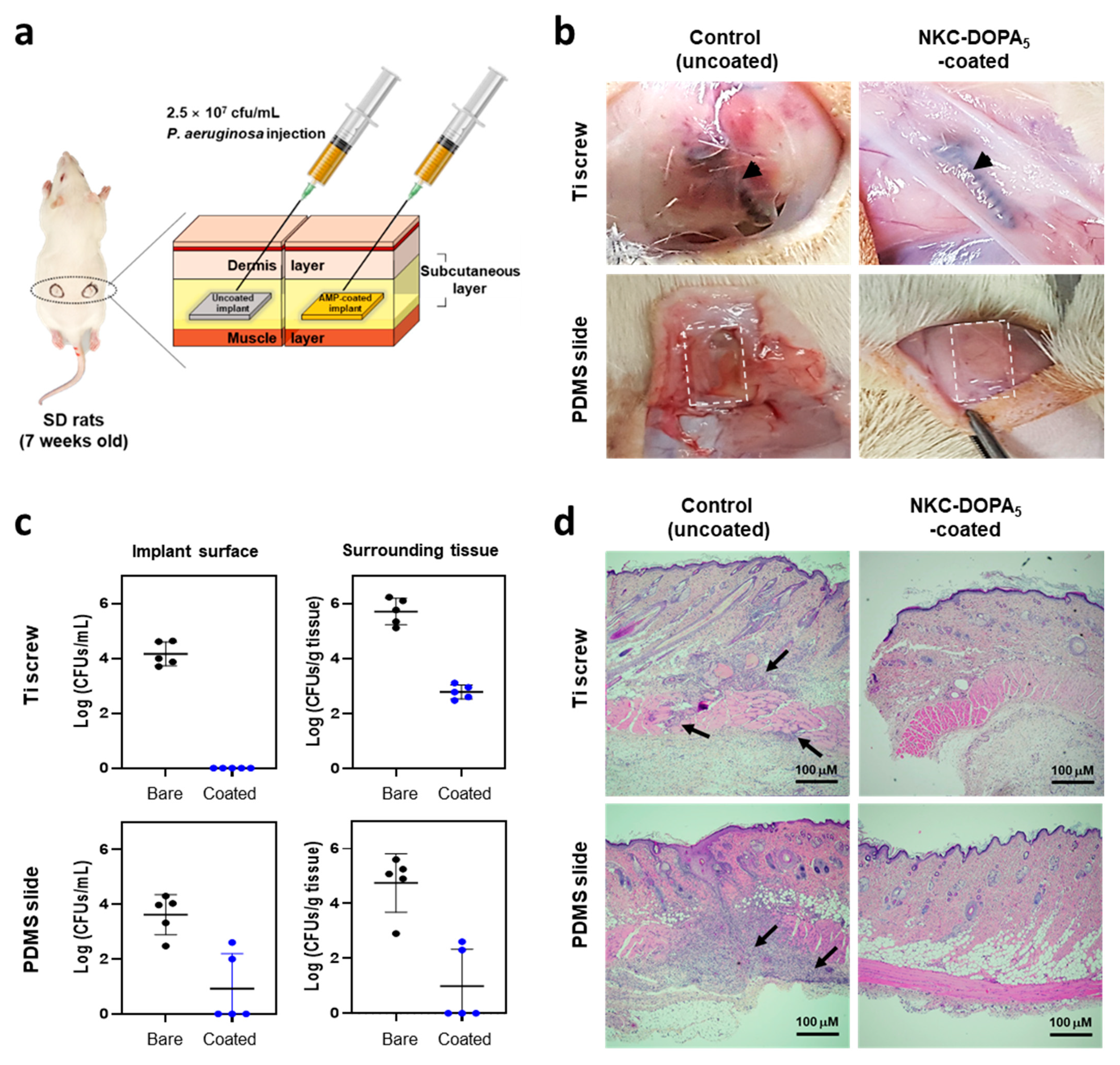
2.6. In Vivo Antimicrobial Activity of NKC-DOPA5
3. Materials and Methods
3.1. Peptides, Strains, Reagents, and Substrates
3.2. Minimum Inhibitory Concentration (MIC) Assay
3.3. AMP Coating
3.4. Quantification of Immobilized Peptides
3.5. Surface Characterization
3.6. Antibacterial Activity of Peptide-Coated Surfaces
3.7. Bacterial Adhesion Assay
3.8. Stability Assessment
3.9. Cytotoxicity Assay
3.10. In Vivo Antimicrobial Activity in a Rat Subcutaneous Infection Model
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and Application Perspectives on Designing an Antimicrobial Surface. ACS Appl. Mater. Interfaces 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent Progress in Polymer Research to Tackle Infections and Antimicrobial Resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Vreuls, C.; Zocchi, G.; Garitte, G.; Archambeau, C.; Martial, J.; Van de Weerdt, C. Biomolecules in multilayer film for antimicrobial and easy-cleaning stainless steel surface applications. Biofouling 2010, 26, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, Y.; Wang, Y.; Zhang, J.; Zhao, S.F.; Yang, G.L. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 2015, 5, 16336. [Google Scholar] [CrossRef] [Green Version]
- Saha, A.; Nir, S.; Reches, M. Amphiphilic Peptide with Dual Functionality Resists Biofouling. Langmuir 2020, 36, 4201–4206. [Google Scholar] [CrossRef]
- Parreira, P.; Monteiro, C.; Graca, V.; Gomes, J.; Maia, S.; Gomes, P.; Goncalves, I.C.; Martins, M.C.L. Surface Grafted MSI-78A Antimicrobial Peptide has High Potential for Gastric Infection Management. Sci. Rep. 2019, 9, 18212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.C.; Chen, J.J.; Hu, G.S.; Wang, L.; Zheng, J.; Zhan, J.Z.; Zhu, Y.C.; Zhong, C.T.; Shi, X.T.; Liu, S.; et al. Immobilization of an antimicrobial peptide on silicon surface with stable activity by click chemistry. J. Mater. Chem. B 2018, 6, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial Properties of hLf1–11 Peptide onto Titanium Surfaces: A Comparison Study Between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.Z.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.Q.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.Z.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhan, W.J.; Cao, L.M.; Hu, C.M.; Qu, Y.C.; Yu, Q.; Chen, H. Multifunctional and Regenerable Antibacterial Surfaces Fabricated by a Universal Strategy. ACS Appl. Mater. Interfaces 2016, 8, 30048–30057. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mitra, D.; Kang, E.T.; Lau, T.; Chiong, E.; Neoh, K.G. Thiol-ol Chemistry for Grafting of Natural Polymers to Form Highly Stable and Efficacious Antibacterial Coatings. ACS Appl. Mater. Int. 2017, 9, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.S.; Zhang, C.; Guo, H.S.; Zheng, W.W.; Yang, J.; Zhou, X.; Zhang, L. Bioinspired Multifunctional Protein Coating for Antifogging, Self-Cleaning, and Antimicrobial Properties. ACS Appl. Mater. Int. 2019, 11, 24504–24511. [Google Scholar] [CrossRef]
- Sundaram, H.S.; Han, X.; Nowinski, A.K.; Ella-Menye, J.R.; Wimbish, C.; Marek, P.; Senecal, K.; Jiang, S.Y. One-Step Dip Coating of Zwitterionic Sulfobetaine Polymers on Hydrophobic and Hydrophilic Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 6664–6671. [Google Scholar] [CrossRef]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.H.; Khalilpour, A.; Li, B.Y.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.R.; Qin, M.; Li, Y.; Cao, Y.; Wang, W. Single Molecule Evidence for the Adaptive Binding of DOPA to Different Wet Surfaces. Langmuir 2014, 30, 4358–4366. [Google Scholar] [CrossRef]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. The Chemistry of Bioinspired Catechol(amine)-Based Coatings. ACS Biomater. Sci. Eng. 2019, 5, 2708–2724. [Google Scholar] [CrossRef]
- Lim, K.Y.; Chua, R.R.Y.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, Y.W.; Park, M.K.; Shin, J.R.; Lim, K.J.; Cho, J.H.; Kim, S.C. Efficacy of the designer antimicrobial peptide SHAP1 in wound healing and wound infection (vol 46, pg 2333, 2014). Amino Acids 2014, 46, 2345. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.S.; Sung, B.H.; Park, M.K.; Lee, J.H.; Lim, K.J.; Park, S.C.; Kim, S.L.; Kim, H.K.; Sohn, J.H.; Kim, H.M.; et al. Recombinant lipase engineered with amphipathic and coiled-coil peptides. ACS Catal. 2015, 5, 5016–5025. [Google Scholar] [CrossRef]
- Park, I.Y.; Cho, J.H.; Kim, K.S.; Kim, Y.B.; Kim, M.S.; Kim, S.C. Helix stability confers salt resistance upon helical antimicrobial peptides. J. Biol. Chem. 2004, 279, 13896–13901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Gao, Y.; Wang, Y.; Pan, G. Mussel-inspired peptide mimicking: An emerging strategy for surface bioengineering of medical implants. Smart Mater. Med. 2021, 2, 299–304. [Google Scholar] [CrossRef]
- Zhi, Z.L.; Su, Y.J.; Xi, Y.W.; Tian, L.; Xu, M.; Wang, Q.Q.; Padidan, S.; Li, P.; Huang, W. Dual-Functional Polyethylene Glycol-b-polyhexanide Surface Coating with in Vitro and in Vivo Antimicrobial and Antifouling Activities. ACS Appl. Mater. Interfaces 2017, 9, 10383–10397. [Google Scholar] [CrossRef] [PubMed]
- Riordan, L.; Smith, E.F.; Mills, S.; Hudson, J.; Stapley, S.; Nikoi, N.D.; Edmondson, S.; Blair, J.; Peacock, A.F.A.; Scurr, D.; et al. Directly bonding antimicrobial peptide mimics to steel and the real world applications of these materials. Mat. Sci. Eng. C-Mater. 2019, 102, 299–304. [Google Scholar] [CrossRef]
- Valiei, A.; Okshevsky, M.; Lin, N.; Tufenkji, N. Anodized Aluminum with Nanoholes Impregnated with Quaternary Ammonium Compounds Can Kill Pathogenic Bacteria within Seconds of Contact. ACS Appl. Mater. Interfaces 2018, 10, 41207–41214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, D.; Li, M.; Kang, E.T.; Neoh, K.G. Transparent Copper-Based Antibacterial Coatings with Enhanced Efficacy against Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2019, 11, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Tang, L.; Wee, K.H.; Zhao, Y.H.; Bai, R.B. Immobilization of silver in polypropylene membrane for anti-biofouling performance. Biofouling 2011, 27, 773–786. [Google Scholar] [CrossRef]
- Chen, J.X.; Howell, C.; Haller, C.A.; Patel, M.S.; Ayala, P.; Moravec, K.A.; Dai, E.B.; Liu, L.Y.; Sotiri, I.; Aizenberg, M.; et al. An immobilized liquid interface prevents device associated bacterial infection in vivo. Biomaterials 2017, 113, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajoie, M.J.; Rovner, A.J.; Goodman, D.B.; Aerni, H.R.; Haimovich, A.D.; Kuznetsov, G.; Mercer, J.A.; Wang, H.H.; Carr, P.A.; Mosberg, J.A.; et al. Genomically recoded organisms expand biological functions. Science 2013, 342, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredens, J.; Wang, K.; de la Torre, D.; Funke, L.F.H.; Robertson, W.E.; Christova, Y.; Chia, T.; Schmied, W.H.; Dunkelmann, D.L.; Beranek, V.; et al. Total synthesis of Escherichia coli with a recoded genome. Nature 2019, 569, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Shin, Y.M.; Jun, I.; Lim, Y.M.; Rhim, T.; Shin, H. Bio-inspired Immobilization of Cell-Adhesive Ligands on Electrospun Nanofibrous Patches for Cell Delivery. Macromol. Mater. Eng. 2013, 298, 555–564. [Google Scholar] [CrossRef]
- Alves, D.; Pereira, M.O. Bio-Inspired Coating Strategies for the Immobilization of Polymyxins to Generate Contact-Killing Surfaces. Macromol. Biosci. 2016, 16, 1450–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Gao, C.Y. Influences of surface coating of PLGA nanoparticles on immune activation of macrophages. J. Mater. Chem. B 2018, 6, 2065–2077. [Google Scholar] [CrossRef]
- He, S.; Zhou, P.; Wang, L.X.; Xiong, X.L.; Zhang, Y.F.; Deng, Y.; Wei, S.C. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Gao, P.; Tan, J.Y.; Xiong, K.Q.; Maitz, M.F.; Pan, C.J.; Wu, H.K.; Chen, Y.; Yang, Z.L.; Huang, N. Assembly of metal phenolic/catecholamine networks for synergistically anti-inflammatory, antimicrobial, and anticoagulant coatings. ACS Appl. Mater. Interfaces 2018, 10, 40844–40853. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef] [PubMed]
| Name | Amino Acid Sequence * | MW (Da) | Reference |
|---|---|---|---|
| NKC | APKAMKLLKKLLKLQKKGI | 2148.8 | Yang et al. [23] |
| NKC-L | APKAMKLLKKLLKLQKKGIGGGGSGGGGS | 2781.2 | This study |
| NKC-DOPA1 | APKAMKLLKKLLKLQKKGIGGGGSGGGGSY | 2959.6 | This study |
| NKC-DOPA3 | APKAMKLLKKLLKLQKKGIGGGGSGGGGSYYY | 3318.0 | This study |
| NKC-DOPA5 | APKAMKLLKKLLKLQKKGIGGGGSGGGGSYYYYY | 3676.3 | This study |
| NKC-DOPA7 | APKAMKLLKKLLKLQKKGIGGGGSGGGGSYYYYYYY | 4034.7 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.E.; Im, S.; Kim, H.; Sohn, J.-H.; Cho, B.-K.; Cho, J.H.; Sung, B.H.; Kim, S.C. Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating. Int. J. Mol. Sci. 2021, 22, 11915. https://doi.org/10.3390/ijms222111915
Hwang YE, Im S, Kim H, Sohn J-H, Cho B-K, Cho JH, Sung BH, Kim SC. Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating. International Journal of Molecular Sciences. 2021; 22(21):11915. https://doi.org/10.3390/ijms222111915
Chicago/Turabian StyleHwang, Young Eun, Seonghun Im, Hyun Kim, Jung-Hoon Sohn, Byung-Kwan Cho, Ju Hyun Cho, Bong Hyun Sung, and Sun Chang Kim. 2021. "Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating" International Journal of Molecular Sciences 22, no. 21: 11915. https://doi.org/10.3390/ijms222111915
APA StyleHwang, Y. E., Im, S., Kim, H., Sohn, J.-H., Cho, B.-K., Cho, J. H., Sung, B. H., & Kim, S. C. (2021). Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating. International Journal of Molecular Sciences, 22(21), 11915. https://doi.org/10.3390/ijms222111915







