High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
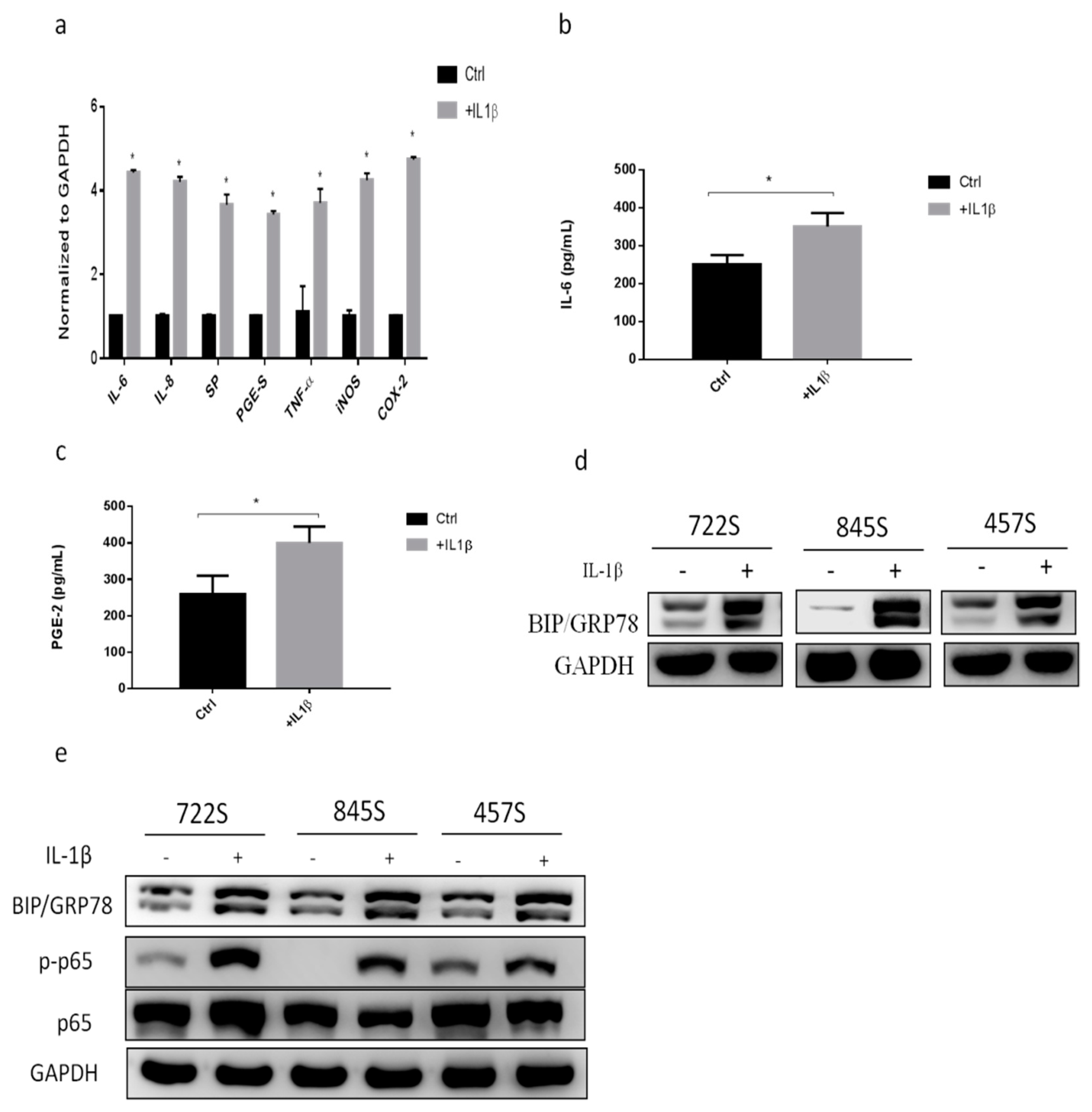
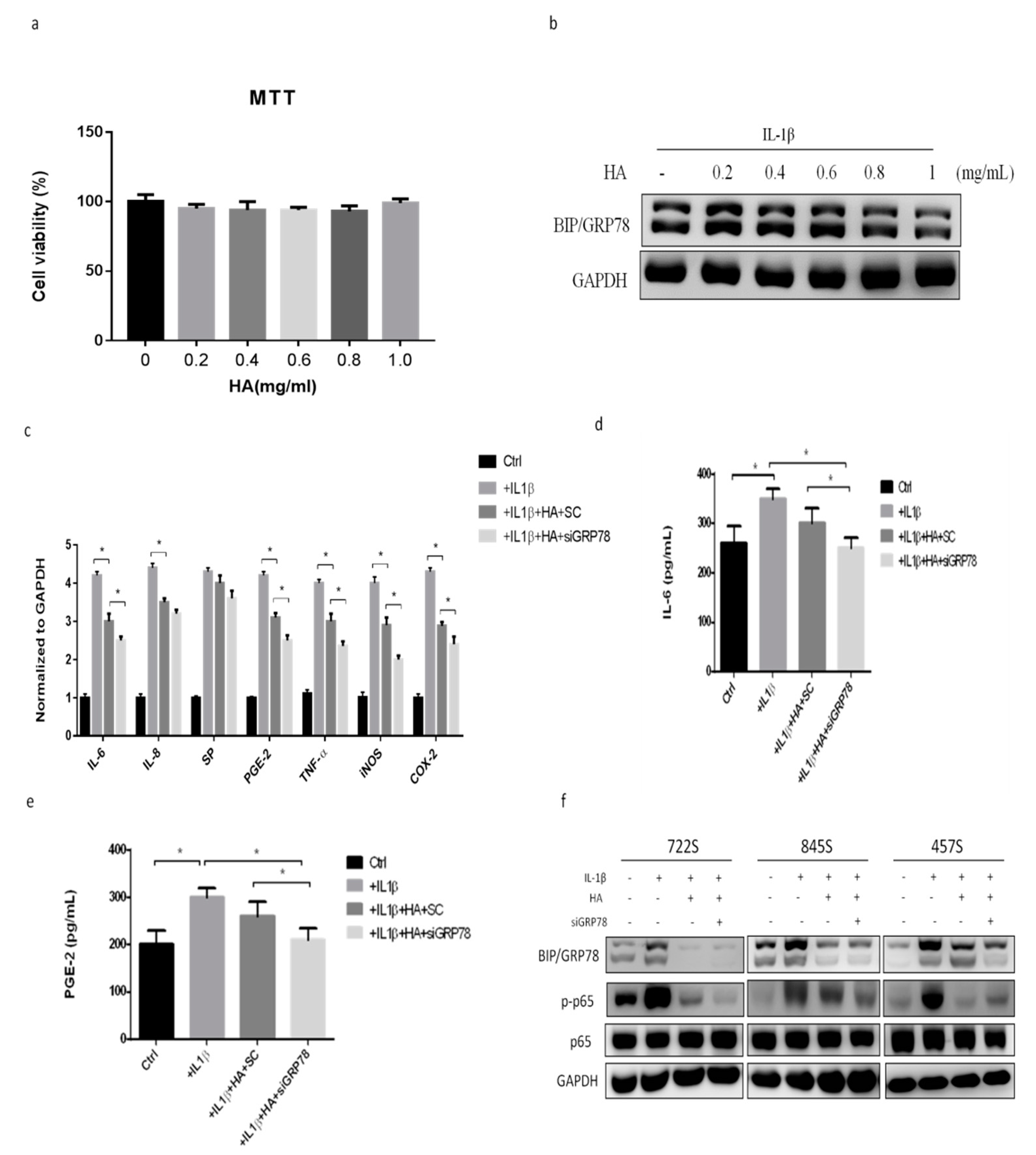
2.1. IL-1β Treatment Increased Inflammatory Responses and GRP78 Expression
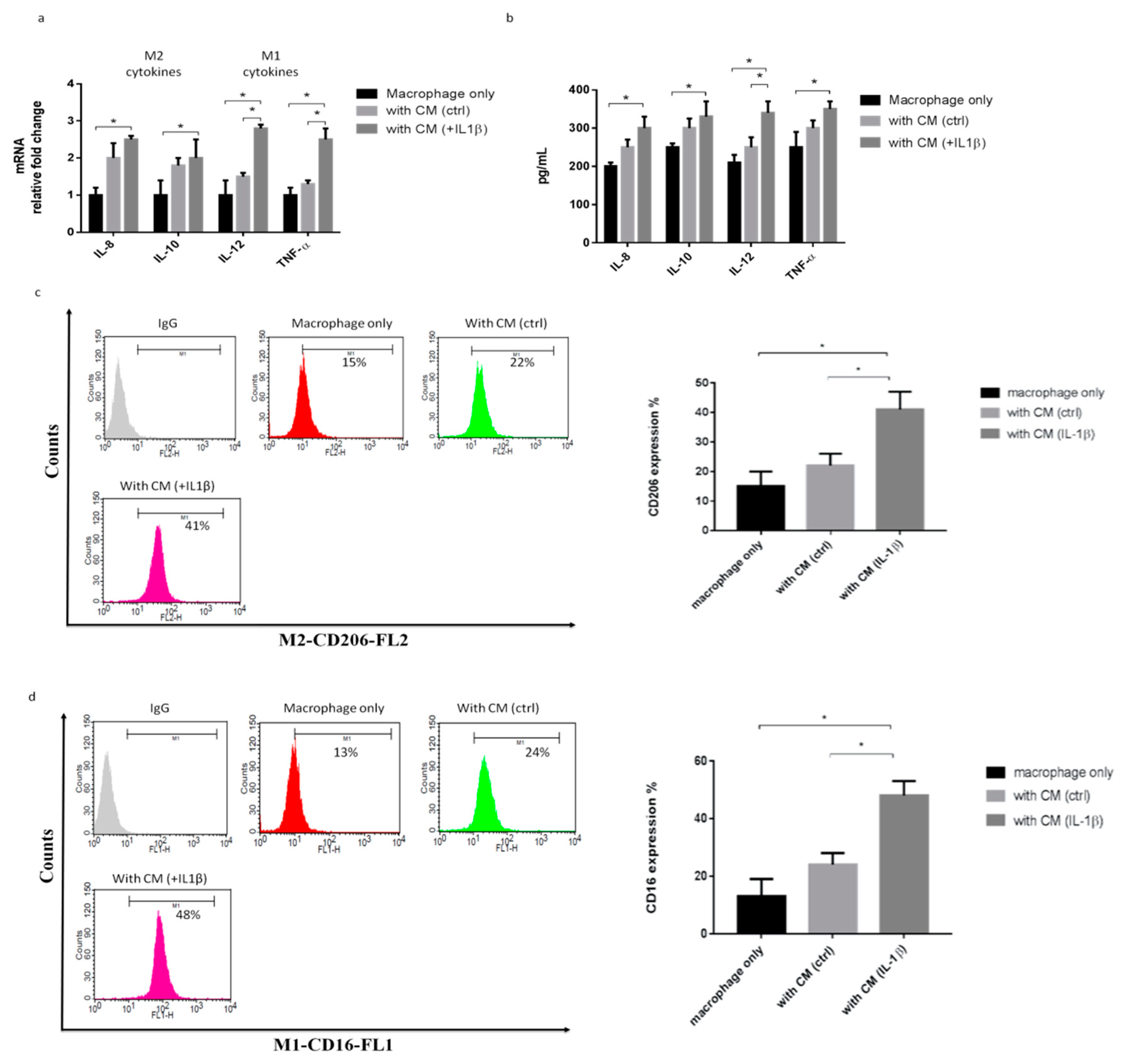
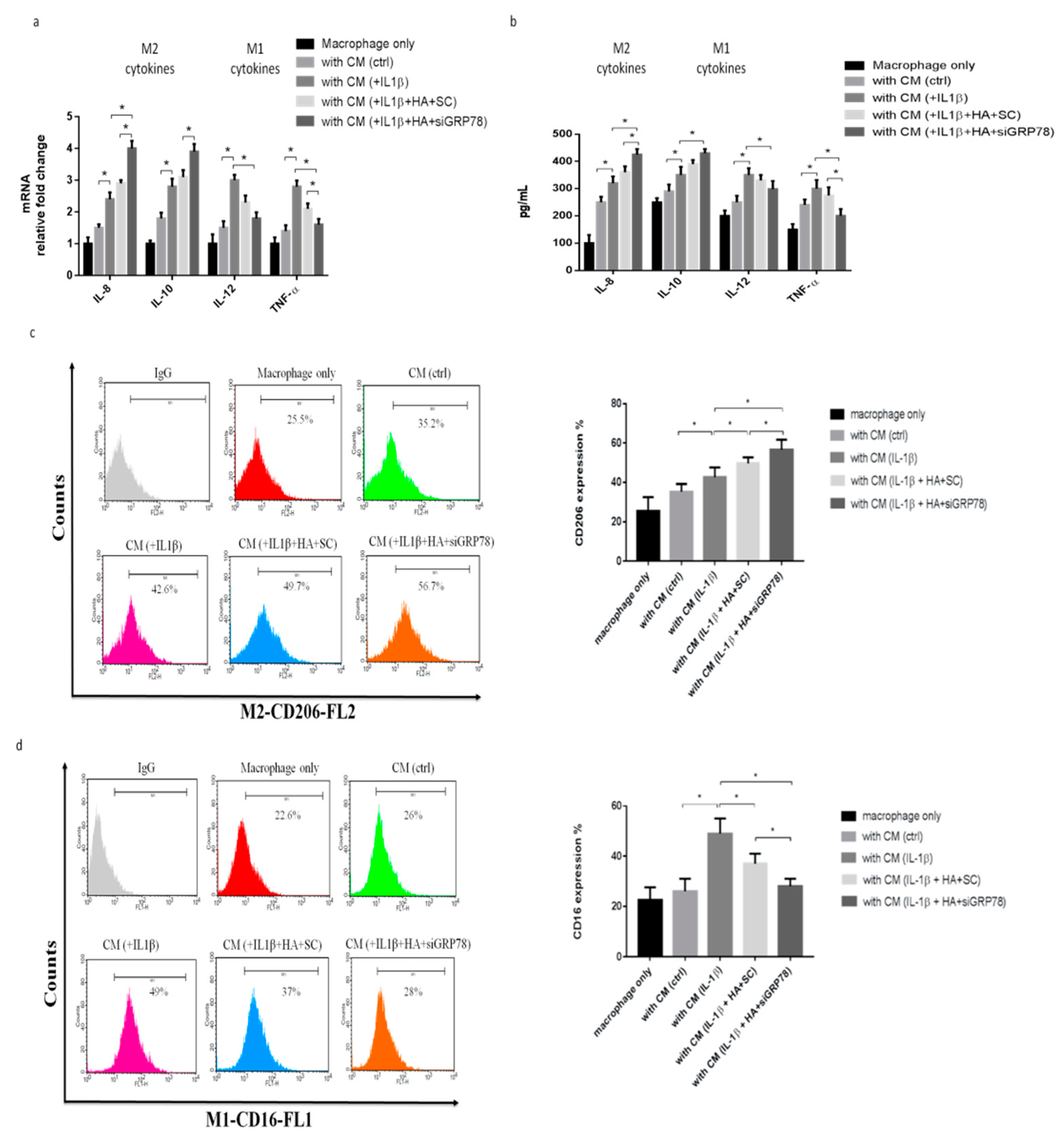
2.2. IL-1β-Treated Synoviocytes Affected the Polarization of Macrophages
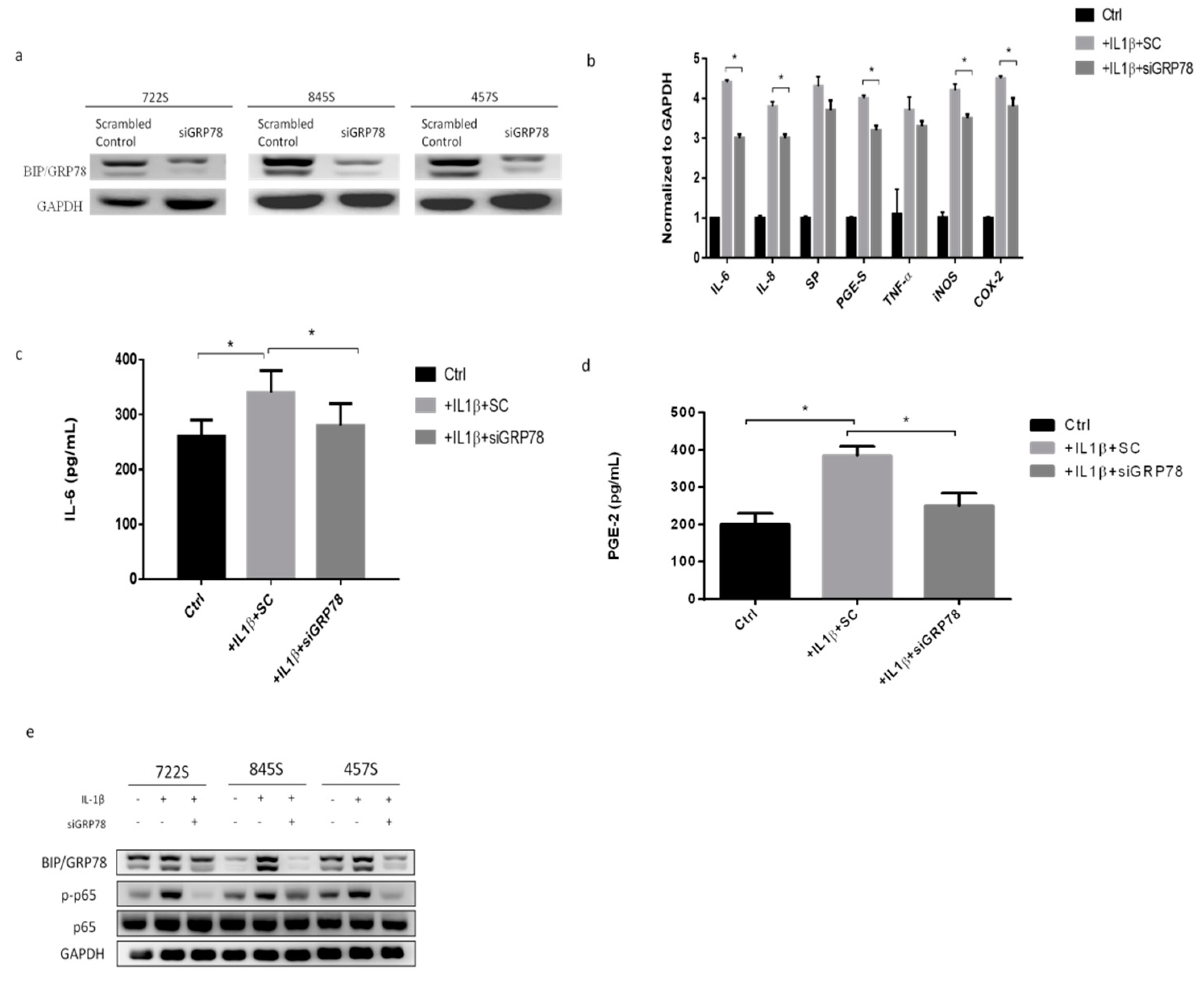
2.3. Knockdown GRP78 in Synoviocytes Decreased Inflammatory Responses and Promoted M2 Polarization
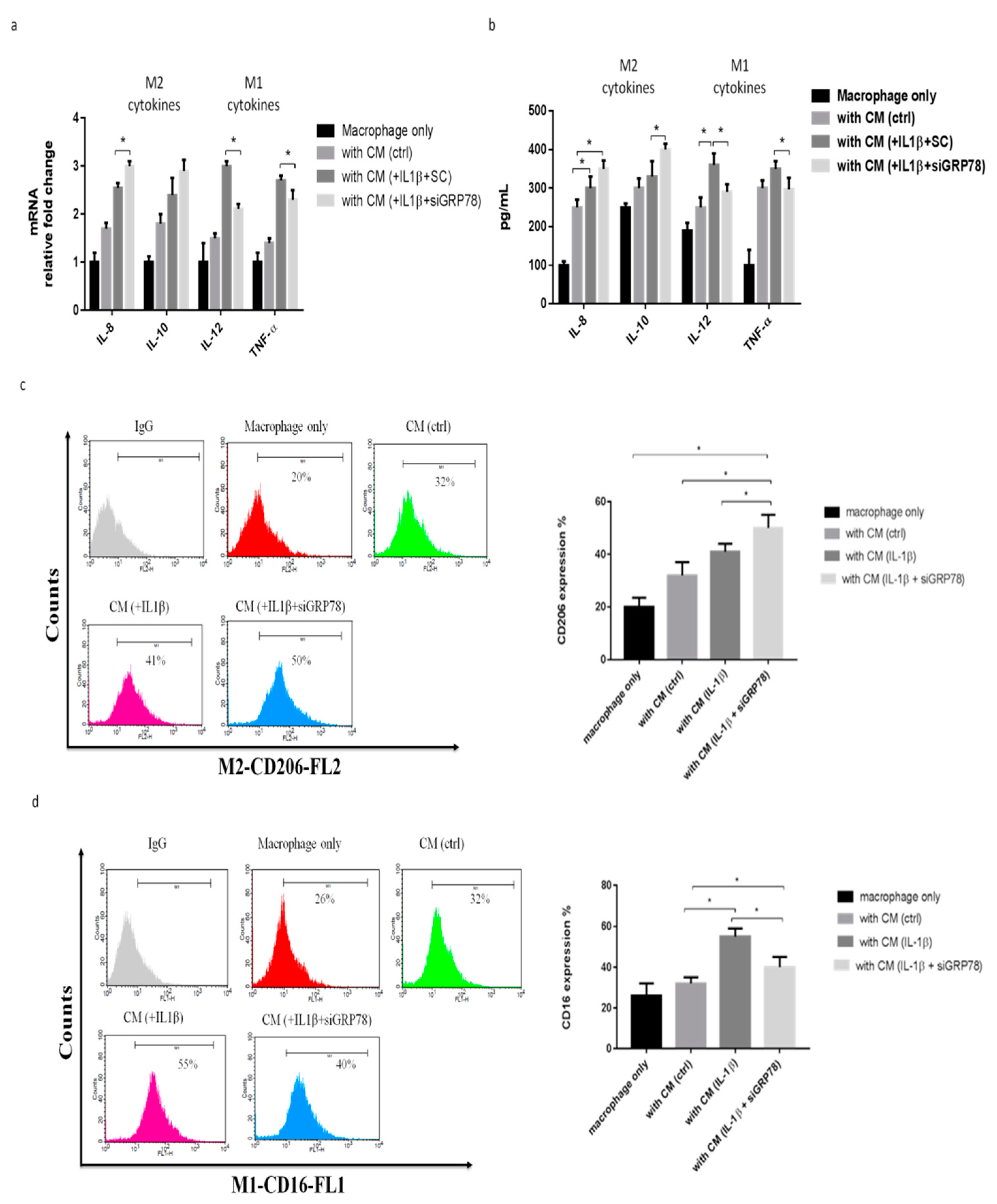
2.4. HMW-HA Treatment Improves Synovium Inflammation and Macrophage Polarization through GRP78 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Source and Culture of Human Primary Synoviocytes
4.3. Preparation of Conditioned Medium (CM) from Synoviocytes
4.4. Quantitative Polymerase Chain Reaction Analysis
4.5. Western Blotting
4.6. siRNA Transfection
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Fluorescence-Activated Cell Sorter Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenham, C.Y.; Conaghan, P.G. The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Guan, P.P.; Guo, C.; Zhu, F.; Konstantopoulos, K.; Wang, Z.Y. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 2013, 27, 4664–4677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, A.R.; Friis, T.; Sekar, S.; Crawford, R.; Xiao, Y.; Prasadam, I. Is Synovial Macrophage Activation the Inflammatory Link Between Obesity and Osteoarthritis? Curr. Rheumatol. Rep. 2016, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Spath, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddell, D.D. Viscosupplementation with hyaluronans for osteoarthritis of the knee: Clinical efficacy and economic implications. Drugs Aging 2007, 24, 629–642. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P. The role of hyaluronic acid (hyaluronan) in health and disease: Interactions with cells, cartilage and components of synovial fluid. Clin. Exp. Rheumatol. 1994, 12, 75–82. [Google Scholar]
- Chang, C.C.; Hsieh, M.S.; Liao, S.T.; Chen, Y.H.; Cheng, C.W.; Huang, P.T.; Lin, Y.F.; Chen, C.H. Hyaluronan regulates PPARgamma and inflammatory responses in IL-1beta-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr. Polym. 2012, 90, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Prestipino, V.; Scuruchi, M.; Nastasi, G.; Calatroni, A.; Campo, S. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. Biofactors 2012, 38, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Hsu, H.C.; Yang, K.C.; Yao, C.H.; Lin, F.H. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J. Trauma 2010, 68, 146–152. [Google Scholar] [CrossRef]
- Chabane, N.; Zayed, N.; Afif, H.; Mfuna-Endam, L.; Benderdour, M.; Boileau, C.; Martel-Pelletier, J.; Pelletier, J.P.; Duval, N.; Fahmi, H. Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthr. Cartil. 2008, 16, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husa, M.; Petursson, F.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. C/EBP homologous protein drives pro-catabolic responses in chondrocytes. Arthritis Res. Ther. 2013, 15, R218. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein-Wieringa, I.R.; de Lange-Brokaar, B.J.; Yusuf, E.; Andersen, S.N.; Kwekkeboom, J.C.; Kroon, H.M.; Van Osch, G.J.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Nelissen, R.G.; et al. Inflammatory Cells in Patients with Endstage Knee Osteoarthritis: A Comparison between the Synovium and the Infrapatellar Fat Pad. J. Rheumatol. 2016, 43, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Zhao, L.; Xu, C.; Zhang, L.; Zhao, H. High Molecular Weight Hyaluronan Suppresses Macrophage M1 Polarization and Enhances IL-10 Production in PM2.5-Induced Lung Inflammation. Molecules 2019, 24, 1766. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-P.; Zhang, X.; Tan, Q.-L.; Xu, W.-X.; Zhou, C.-Y.; Luo, M.; Li, X.; Huang, R.-Y.; Zeng, X. NF-kappaB pathways are involved in M1 polarization of RAW 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLoS ONE 2017, 12, e0188317. [Google Scholar]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Aoki, T.; Frȍsen, J.; Fukuda, M.; Bando, K.; Shioi, G.; Tsuji, K.; Ollikainen, E.; Nozaki, K.; Laakkonen, J.; Narumiya, S. Prostaglandin E2-EP2-NF-kappaB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal. 2017, 10, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, Z.; Haqqi, T.M. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2alpha, p38-MAPK and NF-kappaB in advanced glycation end products stimulated human chondrocytes. Biochim. Biophys. Acta 2012, 1823, 2179–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, K.; Normington, K.; Sambrook, J.; Gething, M.J.; Mori, K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol. Cell. Biol. 1993, 13, 877–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Carlson, S.G.; McBurney, D.; Horton, W.E., Jr. Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J. Biol. Chem. 2005, 280, 31156–31165. [Google Scholar] [CrossRef] [Green Version]
- Nugent, A.E.; Speicher, D.M.; Gradisar, I.; McBurney, D.L.; Baraga, A.; Doane, K.J.; Horton, W.E. Advanced osteoarthritis in humans is associated with altered collagen VI expression and upregulation of ER-stress markers Grp78 and bag-1. J. Histochem. Cytochem. 2009, 57, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Oliver, B.L.; Cronin, C.G.; Zhang-Benoit, Y.; Goldring, M.B.; Tanzer, M.L. Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J. Cell Physiol. 2005, 204, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.J.; Xiong, Z.; Lu, X.; Ye, M.; Han, X.; Jiang, R. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cell Signal 2014, 26, 332–342. [Google Scholar] [CrossRef]
- Uehara, Y.; Hirose, J.; Yamabe, S.; Okamoto, N.; Okada, T.; Oyadomari, S.; Mizuta, H. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthr. Cartil. 2014, 22, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Olivotto, E.; Otero, M.; Marcu, K.B.; Goldring, M.B. Pathophysiology of osteoarthritis: Canonical NF-kappaB/IKKbeta-dependent and kinase-independent effects of IKKalpha in cartilage degradation and chondrocyte differentiation. RMD Open. 2015, 1, e000061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Nordskog, A.W. Significance of body weight as a performance parameter. Poult. Sci. 1982, 61, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambarus, C.A.; Noordenbos, T.; de Hair, M.J.; Tak, P.P.; Baeten, D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [Green Version]
- Berenbaum, F. Signaling transduction: Target in osteoarthritis. Curr. Opin. Rheumatol. 2004, 16, 616–622. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef]
- Barboza, E.; Hudson, J.; Chang, W.; Kovats, S.; Towner, R.A.; Silasi, R.; Lupu, F.; Kent, C.; Griffin, T.M. Profibrotic Infrapatellar Fat Pad Remodeling Without M1 Macrophage Polarization Precedes Knee Osteoarthritis in Mice with Diet-Induced Obesity. Arthritis Rheumatol. 2017, 69, 1221–1232. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Ren, H.; Zhou, G.; Yuan, X.; Shi, X. ER-stress regulates macrophage polarization through pancreatic EIF-2alpha kinase. Cell Immunol. 2019, 336, 40–47. [Google Scholar] [CrossRef]
- Shan, B.; Wang, X.; Wu, Y.; Xu, C.; Xia, Z.; Dai, J.; Shao, M.; Zhao, F.; He, S.; Yang, L.; et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017, 18, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Gao, J.; Ishigaki, Y.; Kondo, K.; Sawada, S.; Izumi, T.; Uno, K.; Kaneko, K.; Tsukita, S.; Takahashi, K.; et al. ER Stress Protein CHOP Mediates Insulin Resistance by Modulating Adipose Tissue Macrophage Polarity. Cell Rep. 2017, 18, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-T.; Sheu, M.-T.; Lin, Y.-F.; Lan, J.; Chin, Y.-P.; Hsieh, M.-S.; Cheng, C.-W.; Chen, C.-H. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. BMC Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Sasaki, K.; Konttinen, Y.T.; Santavirta, S.; Takahara, M.; Takei, H.; Ogino, T.; Takagi, M. Hyaluronate inhibits the interleukin-1beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J. Exp. Med. 2004, 204, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Majors, A.K.; Austin, R.C.; de la Motte, C.A.; Pyeritz, R.E.; Hascall, V.C.; Kessler, S.P.; Sen, G.; Strong, S.A. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J. Biol. Chem. 2003, 278, 47223–47231. [Google Scholar] [CrossRef] [Green Version]
- Schinzel, R.T.; Higuchi-Sanabria, R.; Shalem, O.; Moehle, E.A.; Webster, B.M.; Joe, L.; Bar-Ziv, R.; Frankino, P.A.; Durieux, J.; Pender, C.; et al. The Hyaluronidase, TMEM2, Promotes ER Homeostasis and Longevity Independent of the UPRER. Cell 2019, 179, 1306–1318.e1318. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Sedighi, M.; Bauleth-Ramos, T.; Kant, K.; Correia, A.; Poursina, N.; Sarmento, B.; Hirvonen, J.; Santos, H.A. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega 2018, 3, 18444–18455. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Samà, D.; Calatroni, A. The antioxidant effect exerted by TGF-1beta-stimulated hyaluronan production reduced NF-kB activation and apoptosis in human fibroblasts exposed to FeSo4 plus ascorbate. Mol. Cell. Biochem. 2008, 31, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Deedwania, R.; Pizzo, S.V. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem. 2006, 281, 13694–13707. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Hiramatsu, N.; Hayakawa, K.; Saito, Y.; Kato, H.; Huang, T.; Yao, J.; Paton, A.W.; Paton, J.C.; Kitamura, M. Selective abrogation of BiP/GRP78 blunts activation of NF-kappaB through the ATF6 branch of the UPR: Involvement of C/EBPbeta and mTOR-dependent dephosphorylation of Akt. Mol. Cell Biol. 2011, 31, 1710–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.F.; Chao, T.T.; Su, Y.F.; Hsu, C.C.; Chien, C.Y.; Chiu, K.C.; Shiah, S.G.; Lee, C.H.; Liu, S.Y.; Shieh, Y.S. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-kappaB signaling. Oncotarget 2017, 8, 20706–20718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moe, S.M.; Singh, G.K.; Bailey, A.M. beta2-microglobulin induces MMP-1 but not TIMP-1 expression in human synovial fibroblasts. Kidney Int. 2000, 57, 2023–2034. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-W.; Kim, K.-E.; Choi, J.-W.; Lee, C.Y.; Lee, J.; Seo, H.-H.; Lim, K.H.; Lim, S.; Lee, S.; Kim, S.W.; et al. Proteomic Analysis and Identification of Paracrine Factors in Mesenchymal Stem Cell-Conditioned Media under Hypoxia. Cell. Physiol. Biochem. 2016, 40, 400–410. [Google Scholar] [CrossRef] [Green Version]
- Wright Muelas, M.; Ortega, F.; Breitling, R.; Bendtsen, C.; Westerhoff, H.V. Rational cell culture optimization enhances experimental reproducibility in cancer cells. Sci. Rep. 2018, 8, 3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciria, M.; Garcia, N.A.; Ontoria-Oviedo, I.; Gonzalez-King, H.; Carrero, R.; De La Pompa, J.L.; Montero, J.A.; Sepulveda, P. Mesenchymal Stem Cell Migration and Proliferation Are Mediated by Hypoxia-Inducible Factor-1α Upstream of Notch and SUMO Pathways. Stem Cells Dev. 2017, 26, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Alzoubi, K.H.; Khabour, O.F.; Banihani, M.N.; Al-Balas, Q.A.; Swaidan, S. GRP78 regulates sensitivity of human colorectal cancer cells to DNA targeting agents. Cytotechnology 2016, 68, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Chiang, C.-F.; Kuo, F.-C.; Su, S.-C.; Huang, C.-L.; Liu, J.-S.; Lu, C.-H.; Hsieh, C.-H.; Wang, C.-C.; Lee, C.-H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. https://doi.org/10.3390/ijms222111917
Lee C-H, Chiang C-F, Kuo F-C, Su S-C, Huang C-L, Liu J-S, Lu C-H, Hsieh C-H, Wang C-C, Lee C-H, et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(21):11917. https://doi.org/10.3390/ijms222111917
Chicago/Turabian StyleLee, Chien-Hsing, Chi-Fu Chiang, Feng-Chih Kuo, Sheng-Chiang Su, Chia-Luen Huang, Jhih-Syuan Liu, Chieh-Hua Lu, Chang-Hsun Hsieh, Chih-Chien Wang, Chian-Her Lee, and et al. 2021. "High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway" International Journal of Molecular Sciences 22, no. 21: 11917. https://doi.org/10.3390/ijms222111917
APA StyleLee, C.-H., Chiang, C.-F., Kuo, F.-C., Su, S.-C., Huang, C.-L., Liu, J.-S., Lu, C.-H., Hsieh, C.-H., Wang, C.-C., Lee, C.-H., & Shen, P.-H. (2021). High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. International Journal of Molecular Sciences, 22(21), 11917. https://doi.org/10.3390/ijms222111917





