Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis
Abstract
:1. Introduction
2. Mechanism of Signaling Pathways in Skeletal Fluorosis
2.1. Effect of Wnt/β-Catenin Signaling Pathway on Skeletal Fluorosis
2.2. Effect of Notch Signaling Pathway on Skeletal Fluorosis
2.3. Effect of PI3K/Akt/mTOR Signaling Pathway on Skeletal Fluorosis
2.4. Effect of Hedgehog Signaling Pathway on Skeletal Fluorosis
2.5. Effect of Hormones and Their Receptor Signaling Pathways on Skeletal Fluorosis
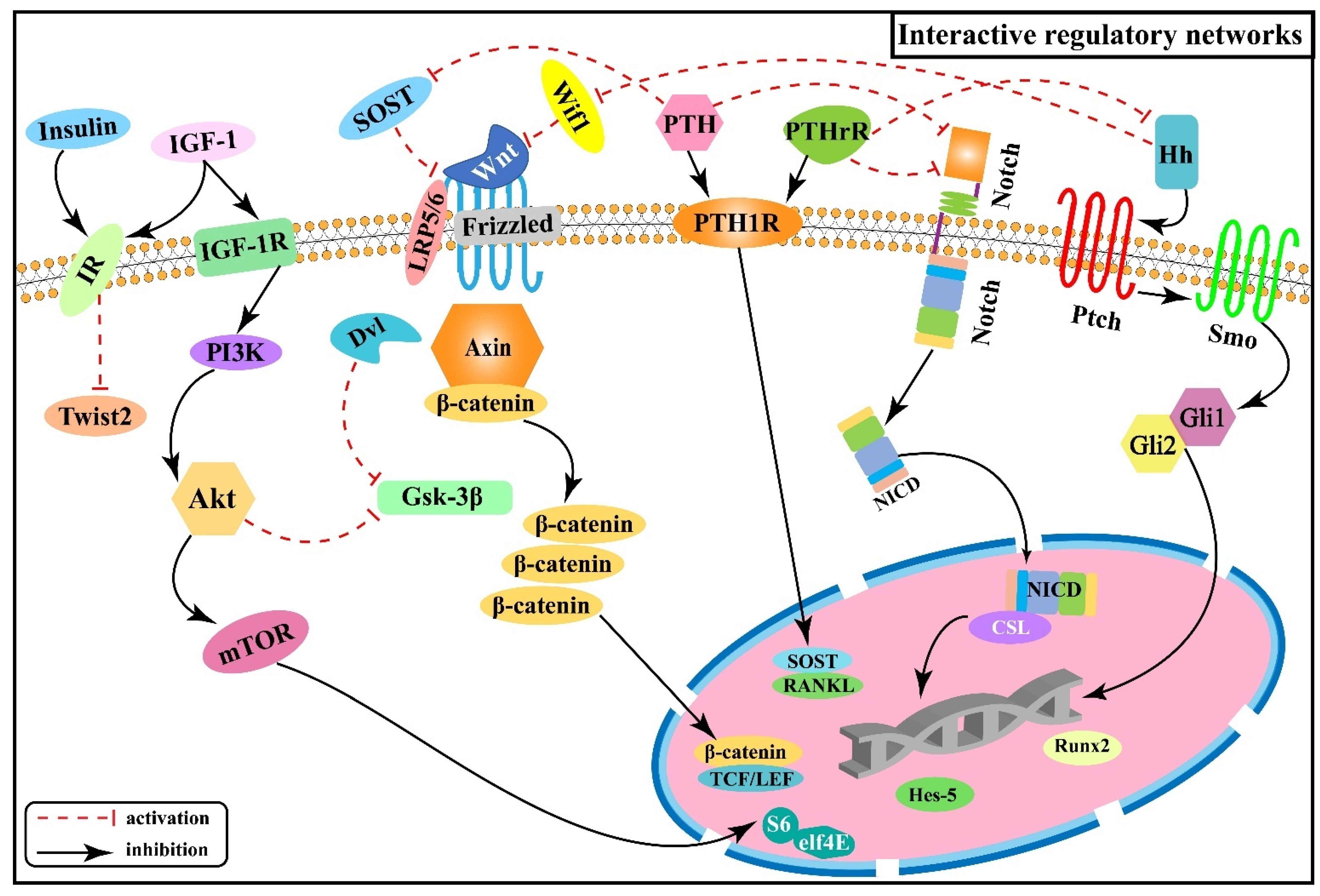
2.6. Interactive Regulatory Networks among Signaling Pathways Involved in Skeletal Fluorosis
3. Mechanism of Stress Pathways in Skeletal Fluorosis
3.1. Effect of Endoplasmic Reticulum Stress on Skeletal Fluorosis
3.2. Effect of Oxidative Stress on Skeletal Fluorosis
4. Mechanism of Epigenetics in Skeletal Fluorosis
4.1. Effect of DNA Methylation on Skeletal Fluorosis
4.2. Effect of Non-Coding RNAs on Skeletal Fluorosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.Y. The Progress about the influence of Fluorine on Bone. Med. Recapitul. 2006, 12, 1092–1094. [Google Scholar]
- Srivastava, S.; Flora, S.J.S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.P.; Gao, Y.H.; Wang, W.; Gong, H.Q.; Guo, M.; Zhao, S.C.; Liu, X.H.; Yu, B.; Sun, D.J. Prevalence of Brick Tea-Type Fluorosis in the Tibet Autonomous Region. J. Epidemiol. 2016, 26, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Izuora, K.; Twombly, J.G.; Whitford, G.M.; Demertzis, J.; Pacifici, R.; Whyte, M.P. Skeletal fluorosis from brewed tea. J. Clin. Endocrinol. Metab. 2011, 96, 2318–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, M.; Tadano, M.; Asanuma, S.; Tamura, K.; Matsushima, S.; Watanabe, T.; Kondo, T.; Sakurai, S.; Ji, R.D.; Liang, C.K.; et al. Health effects of indoor fluoride pollution from coal burning in China. Environ. Health Perspect. 1998, 106, 239–244. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Huang, H.; Zeng, Q.B.; Yu, C.; Yao, M.L.; Hong, F.; Luo, P.; Pan, X.L.; Zhang, A.H. The effect of elemental content on the risk of dental fluorosis and the exposure of the environment and population to fluoride produced by coal-burning. Environ. Toxicol. Pharmacol. 2017, 56, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.P.; Yan, H.; Zhang, L.H. Progress in molecular mechanism of skeletal fluorosis. Chin. Foreign Med. Res. 2011, 9, 158–160. [Google Scholar]
- Noël, C.; Gosselin, B.; Dracon, M.; Pagniez, D.; Lemaguer, D.; Lemaître, L.; Dhondt, J.L.; Lelièvre, G.; Tacquet, A. Risk of bone disease as a result of fluoride intake in chronic renal insufficiency. Nephrologie 1985, 6, 181–185. [Google Scholar]
- Wei, W.; Pang, S.J.; Sun, D.J. The pathogenesis of endemic fluorosis: Research progress in the last 5 years. J. Cell Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef] [Green Version]
- Boivin, G.; Chavassieux, P.; Chapuy, M.C.; Baud, C.A.; Meunier, P.J. Skeletal fluorosis: Histomorphometric findings. J. Bone Miner. Res. 1990, 5, S185–S189. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef] [Green Version]
- Day, T.F.; Guo, X.Z.; Garrett-Beal, L.; Yang, Y.Z. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.S.; Karsenty, G. Regulation of bone formation and vision by LRP5. N. Engl. J. Med. 2002, 346, 1572–1574. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.R.; Gao, Y.H.; Yang, Y.M.; Liu, Y.; Guo, N.; Wang, L.M.; Huang, W.; Wu, L.W.; Sun, D.J.; Gu, W.K. β-catenin mediates fluoride-induced aberrant osteoblasts activity and osteogenesis. Environ. Pollut. 2020, 265, 114734. [Google Scholar] [CrossRef]
- Karner, C.M.; Long, F.X. Wnt signaling and cellular metabolism in osteoblasts. Cell. Mol. Life Sci. 2017, 74, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Rawadi, G.; Roman-Roman, S. Wnt signaling pathway: A new target for the treatment of osteoporosis. Expert Opin. Ther. Targets 2005, 9, 1063–1077. [Google Scholar] [CrossRef]
- Sun, D.J.; Gao, Y.H. Molecular mechanism of pathogenesis of osteofluorosis: A discussion in the view of bony turnover. Chin. J. Endemiol. 2008, 27, 239–241. [Google Scholar]
- Chen, X.S.; Yu, Y.N.; Yi, W.; Wan, L.B.; Xie, Y. Effect of fluoride on expression of mRNA and protein of Wnt3a and β-catenin in osteoblast of rats. Chin. J. Endemiol. 2013, 32, 140–145. [Google Scholar]
- Wang, W.P.; Xu, J.; Liu, K.J.; Liu, X.L.; Li, C.C.; Cui, C.Y.; Zhang, Y.Z.; Li, H.B. Suppression of Sclerostin and Dickkopf-1 levels in patients with fluorine bone injury. Environ. Toxicol. Pharmacol. 2013, 35, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, C.C.; Liu, K.J.; Cui, C.Y.; Zhang, Y.Z.; Liu, Y. The influence of fluoride on the expression of inhibitors of Wnt/β-catenin signaling pathway in rat skin fibroblast Cells. Biol. Trace Elem. Res. 2012, 148, 117–121. [Google Scholar] [CrossRef]
- Zeng, Q.B.; Xu, Y.Y.; Yu, X.; Yang, J.; Hong, F.; Zhang, A.H. Silencing GSK3β instead of DKK1 can inhibit osteogenic differentiation caused by co-exposure to fluoride and arsenic. Bone 2019, 123, 196–203. [Google Scholar] [CrossRef]
- Yu, J.; Canalis, E. Notch and the regulation of osteoclast differentiation and function. Bone 2020, 138, 115474. [Google Scholar] [CrossRef]
- Schroeter, E.H.; Kisslinger, J.A.; Kopan, R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998, 393, 382–386. [Google Scholar] [CrossRef]
- Canalis, E. Notch in skeletal physiology and disease. Osteoporos. Int. 2018, 29, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int. 2012, 90, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilton, M.J.; Tu, X.; Wu, X.; Bai, S.; Zhao, H.; Kobayashi, T.; Kronenberg, H.M.; Teitelbaum, S.L.; Ross, F.P.; Kopan, R.; et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med. 2008, 14, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, P.; Osipo, C.; Foreman, K.; Golde, T.; Osborne, B.; Miele, L. Rational targeting of Notch signaling in cancer. Oncogene 2008, 27, 5124–5131. [Google Scholar] [CrossRef] [Green Version]
- Zanotti, S.; Canalis, E. Notch Signaling and the Skeleton. Endocr. Rev. 2016, 37, 223–253. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Kawashima, N.; Sakamoto, K.; Katsube, K.I.; Shindo, K.; Suda, H.; Shi, J.N. Osteogenic differentiation of murine mesenchymal progenitor cells Kusa-A1 is promoted by CBF1. Basic Clin. Med. 2006, 26, 409–414. [Google Scholar]
- Wang, S.C.; Kawashima, N.; Sakamoto, K.; Suda, H.; Shi, J.N. Expression of Notch-related Genes in the Differentiation of Mesenchymal Progenitor Cell, Kusa-A1. J. Oral Sci. Res. 2005, 21, 389–392. [Google Scholar]
- Chen, X.W.; Wan, C.W.; Xie, C.; Wei, Y.; Wu, Y.; Wan, W. Fluoride Inhibits Expressions of Notch3 and Jag1 Proteins in Rat Bone Tissues. J. Environ. Occup. Med. 2016, 33, 494–498. [Google Scholar]
- Chen, X.W.; Wan, C.W.; Xie, C.; Yang, X.X.; Wu, Y.; Wan, W. Influence of fluoride on RBPJ and related genes in bone tissue of rats. Chin. J. Public Health 2016, 32, 195–198. [Google Scholar]
- Zhang, Z.D.; Zhang, X.Z.; Zhao, D.W.; Liu, B.Y.; Wang, B.J.; Yu, W.T.; Li, J.L.; Yu, X.B.; Cao, F.; Zheng, G.S.; et al. TGF-β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol. Med. Rep. 2019, 19, 3505–3518. [Google Scholar] [CrossRef]
- Ma, J.; Du, D.; Liu, J.; Guo, L.; Li, Y.C.; Chen, A.; Ye, T.W. Hydrogen sulphide promotes osteoclastogenesis by inhibiting autophagy through the PI3K/AKT/mTOR pathway. J. Drug Target. 2020, 28, 176–185. [Google Scholar] [CrossRef]
- Guan, Y.J.; Yang, X.; Yang, W.T.; Charbonneau, C.; Chen, Q. Mechanical activation of mammalian target of rapamycin pathway is required for cartilage development. FASEB J. 2014, 28, 4470–4481. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.N.; Yang, D.; Zhu, H.Z.; Deng, C.N.; Guan, Z.Z. Expression of mRNA and protein of p38, Osx, PI3K and Akt1 in rat bone with chronic fluorosis. Chin. J. Pathol. 2012, 41, 622–626. [Google Scholar]
- Chen, R.; Yu, Y.N.; Xu, L.; Deng, C.N. Role of mTOR autophagy signaling in rats cartilages with fluorosis-caused damage. Chin. J. Control Endem. Dis. 2017, 32, 18–19. [Google Scholar]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.X. Fluoride Inhibits the Proliferation and Differentiation of ATDC5 Cells via the PI3K/AKT/mTOR Signaling Pathway. Master’s Thesis, China Medical University, Shenyang, China, 2020. [Google Scholar]
- Wang, Y.; Han, C.; Lu, L.; Magliato, S.; Wu, T. Hedgehog Signaling Pathway Regulates Autophagy in Human Hepatocellular Carcinoma Cells. Hepatology 2013, 58, 995–1010. [Google Scholar] [CrossRef] [Green Version]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [Green Version]
- Long, F.X.; Zhang, X.M.; Karp, S.; Yang, Y.Z.; McMahon, A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001, 128, 5099–5108. [Google Scholar] [CrossRef]
- Deng, C.N. The Mechanism and Relationship of Hedgehog Signaling in the Bone Injury and Bone Microenvironment of Chronic Fluorosis Rats. Ph.D. Thesis, Guiyang Medical College, Guiyang, China, 2014. [Google Scholar]
- Gui, C.Z.; Wang, C.S.; Yu, Y.N.; Tang, J.J.; Liu, J.J. Influence of fluoride on the growth and apoptosis of cultured cartilage and antagonizing effect of superoxide dismutase (SOD). Guizhou Med. J. 2004, 28, 291–293. [Google Scholar]
- Zhang, J.Y.; Xu, S.J.; Wang, K. The Role of Free Radicals in the Pathological Process of Kaschin-Beck Disease Ⅵ. Damage of Type Ⅰ Collagen Induced by Active Oxygen Free Radicals and the Mineralization of Hydroxyapatite in the Damaced Collagen. J. Beijing Med. Univ. 1991, 23, 231–234. [Google Scholar]
- Fitch, P.M.; Howie, S.E.M.; Wallace, W.A.H. Oxidative damage and TGF-b differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: Implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int. J. Exp. Pathol. 2011, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.J.; Yu, Y.N.; Chen, R.; Huang, X.L. Role of hedgehog signaling pathway on cartilage tissue damage in chronic fluorosis rats. Chin. J. Public Health 2018, 34, 241–245. [Google Scholar]
- Datta, N.S.; Abou-Samra, A.B. PTH and PTHrP signaling in osteoblasts. Cell. Signal. 2009, 21, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fermor, B.; Skerry, T.M. PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J. Bone Miner. Res. 1995, 10, 1935–1943. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Q.Y.; Zhang, J.M.; Zhang, H.; Li, G.S. Elevation of PTH and PTHrp induced by excessive fluoride in rats on a calcium-deficient diet. Biol. Trace Elem. Res. 2010, 137, 79–87. [Google Scholar] [CrossRef]
- Yu, X.H.; Yu, H.L.; Jiang, N.N.; Zhang, X.Y.; Zhang, M.M.; Xu, H. PTH (1-34) affects bone turnover governed by osteocytes exposed to fluoride. Toxicol. Lett. 2018, 288, 25–34. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kuroshima, S.; Uto, Y.; Kanai, R.; Inoue, M.; Suzue, M.; Sawase, T. Intermittent administration of parathyroid hormone improves bone quality and quantity around implants in rat tibiae. J. Oral. Biosci. 2020, 62, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.M.; Wan, C.; Chen, D.Q.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010, 142, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.Y.; Ren, L.Q.; Li, X.N.; Wu, N.; Li, G.S.; Liu, Q.Y.; Xu, H. Effect of fluoride on insulin level of rats and insulin receptor expression in the MC3T3-E1 cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.M.; Li, Y.G.; Wang, Y.; Mao, W.X.; Gao, Y.; Xu, H. Streptozotocin Aggravated Osteopathology and Insulin Induced Osteogenesis Through Co-treatment with Fluoride. Biol. Trace Elem. Res. 2015, 168, 453–461. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Liu, H.; Yu, X.H.; Wang, Y.; Yang, C.; Xu, H. Analysis of the Role of Insulin Signaling in Bone Turnover Induced by Fluoride. Biol. Trace Elem. Res. 2016, 171, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, X.Y.; Yang, S.R.; Li, Z.W.; Jia, Y. Mechanism and cross-talk of signaling pathways associated with bone damage in fluorosis. J. Environ. Occup. Med. 2021, 38, 794–800. [Google Scholar]
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.G.; Bilezikian, J.P. Sclerostin: Therapeutic horizons based upon its actions. Curr. Osteoporos. Rep. 2012, 10, 64–72. [Google Scholar] [CrossRef]
- Kramer, I.; Keller, H.; Leupin, O.; Kneissel, M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol. Metab. 2010, 21, 237–244. [Google Scholar] [CrossRef]
- Gui, F.Z. Effects of Fluoride on the Expression of GAG Components and Related Signaling Pathways FGFR3 and Ihh/PTHrP in Rat Growth Plate Cartilage. Master’s Thesis, China Medical College, Shenyang, China, 2019. [Google Scholar]
- Wang, W.D. Effects of PTH and Notch Signaling Pathway on the Differentiation of Bone Mesenchymal Stem Cells into Osteoblasts. Master’s Thesis, Nanjing Medical College, Nanjing, China, 2015. [Google Scholar]
- Lin, F.T.; Xia, C.; Zhang, B.; Huang, J.G.; Zheng, X.P.; Yi, T.T.; Zhao, H.H.; Zhang, Y.B. Effects of PTHrP and Notch signaling on the proliferation of epiphysis stem cells. Natl. Med. J. China 2011, 91, 2073–2076. [Google Scholar]
- Day, T.F.; Yang, Y. Wnt and hedgehog signaling pathways in bone development. J. Bone Joint Surg. Am. 2008, 90, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hilton, M.J.; Tu, X.; Yu, K.; Ornitz, D.M.; Long, F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005, 132, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, R.C.; Matsumaru, D.; Ho, A.S.; Garcia-Barceló, M.M.; Yuan, Z.W.; Smith, D.; Kodjabachian, L.; Tam, P.K.; Yamada, G.; Lui, V.C. Dysregulation of Wnt inhibitory factor 1 (Wif1) expression resulted in aberrant Wnt-β-catenin signaling and cell death of the cloaca endoderm, and anorectal malformations. Cell Death Differ. 2014, 21, 978–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [Green Version]
- Surmann-Schmitt, C.; Widmann, N.; Dietz, U.; Saeger, B.; Eitzinger, N.; Nakamura, Y.; Rattel, M.; Latham, R.; Hartmann, C.; von der Mark, H.; et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J. Cell Sci. 2009, 122, 3627–3637. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Saidak, Z.; Le Henaff, C.; Azzi, S.; Marty, C.; Da Nascimento, S.; Sonnet, P.; Marie, P.J. Wnt/β-catenin signaling mediates osteoblast differentiation triggered by peptide-induced α5β1 integrin priming in mesenchymal skeletal cells. J. Biol. Chem. 2015, 290, 6903–6912. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Bikle, D.D. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J. Cell Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Rivas, A.; Vidal, R.L.; Hetz, C. Targeting the unfolded protein response for disease intervention. Expert Opin. Ther. Targets 2015, 19, 1203–1218. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Invest. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.A.; Hollien, J. The unfolded protein response in secretory cell function. Annu. Rev. Genet. 2012, 46, 165–183. [Google Scholar] [CrossRef]
- Wu, J.; Kaufman, R.J. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006, 13, 374–384. [Google Scholar] [CrossRef]
- Shen, X.H.; Zhang, K.Z.; Kaufman, R.J. The unfolded protein response-a stress signaling pathway of the endoplasmic reticulum. J. Chem. Neuroanat. 2004, 28, 79–92. [Google Scholar] [CrossRef]
- Xu, H.; Jing, L.; Zhang, C.W.; Qi, L.; Li, G.S. Analysis of proteins in osteoblast exposed to fluoride by two-dimensional electrophoresis and mass spectrometry. Chin. J. Endemiol. 2006, 25, 35–38. [Google Scholar]
- Maurel, M.; Chevet, E. Endoplasmic reticulum stress signaling: The microRNA connection. Am. J. Physiol. Cell Physiol. 2013, 304, C1117–C1126. [Google Scholar] [CrossRef] [Green Version]
- Mujcic, H.; Nagelkerke, A.; Rouschop, K.M.; Chung, S.; Chaudary, N.; Span, P.N.; Clarke, B.; Milosevic, M.; Sykes, J.; Hill, R.P.; et al. Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin. Cancer Res. 2013, 19, 6126–6137. [Google Scholar] [CrossRef] [Green Version]
- Salaroglio, I.C.; Panada, E.; Moiso, E.; Buondonno, I.; Provero, P.; Rubinstein, M.; Kopecka, J.; Riganti, C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol. Cancer 2017, 16, 91. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Sun, Q.; Zhao, C.Y.; Ling, S.K.; Li, Q.; Chang, Y.Z.; Li, Y.X. HDAC4 protects cells from ER stress induced apoptosis through interaction with ATF4. Cell. Signal. 2014, 26, 556–563. [Google Scholar] [CrossRef]
- Harding, H.P.; Novoa, I.; Zhang, Y.H.; Zeng, H.Q.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhou, Y.L.; Zhang, X.Y.; Lu, P.; Li, G.S. Activation of PERK signaling through fluoride-mediated endoplasmic reticulum stress in OS732 cells. Toxicology 2010, 277, 1–5. [Google Scholar] [CrossRef]
- Sun, F.; Li, X.N.; Yang, C.; Lv, P.; Li, G.S.; Xu, H. A role for PERK in the mechanism underlying fluoride-induced bone turnover. Toxicology 2014, 325, 52–66. [Google Scholar] [CrossRef]
- He, P.; Zhang, M.; He, W.H.; Xia, T.; Yang, K.D.; Wang, A.G. Effects of fluoride on oxidative stress and apoptosis in primary rat hippocampal neurons. Chin. J. Endemiol. 2006, 25, 264–267. [Google Scholar]
- Shanthakumari, D.; Srinivasalu, S.; Subramanian, S. Effect of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. Toxicology 2004, 204, 219–228. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.H.; Zhao, Z.T.; Zhang, W.B.; Li, G.S. Role of oxidative stress in osteoblasts exposed to sodium fluoride. Biol. Trace Elem. Res. 2008, 123, 109–115. [Google Scholar] [CrossRef]
- Shi, C.L. Protective Effect and Mechanism of Gastrodin on Rats with Chronic Fluorosis and Bone Damage. Master’s Thesis, China Medical University, Shenyang, China, 2019. [Google Scholar]
- Zhong, Y.F. The Effects of Fluoride on Nrf2-ARE Signal Pathway in Rat Osteoblasts. Master’s Thesis, Guangdong School of Pharmacy, Guangzhou, China, 2011. [Google Scholar]
- Wang, Z.; Yang, X.; Yang, S.; Ren, G.; Ferreri, M.; Su, Y.; Chen, L.; Han, B. Sodium fluoride suppress proliferation and induce apoptosis through decreased insulin-like growth factor-I expression and oxidative stress in primary cultured mouse osteoblasts. Arch. Toxicol. 2011, 85, 1407–1417. [Google Scholar] [CrossRef]
- Park, K.H.; Park, B.; Yoon, D.S.; Kwon, S.H.; Shin, D.M.; Lee, J.W.; Lee, H.G.; Shim, J.H.; Park, J.H.; Lee, J.M. Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun. Signal. 2013, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yu, Y.N.; Wan, L.B.; Chen, X.S. Effect of fluoride on expression of CaN mRNA and protein in bone tissue of rats. Chin. J. Pathol. 2012, 41, 761–764. [Google Scholar]
- Pei, J.R.; Li, B.Y.; Li, Z.W.; Wei, W.; Yao, Y.J.; Xu, J.X.; Gao, Y.H. The effect of fluoride on osteoclast in bone tissue of rats and its mechanism. Chin. J. Endemiol. 2017, 36, 714–718. [Google Scholar]
- He, H.; Liu, X.; Lv, L.; Liang, H.; Leng, B.; Zhao, D.; Zhang, Y.; Du, Z.; Chen, X.; Li, S.; et al. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death Dis. 2014, 5, e997. [Google Scholar] [CrossRef] [PubMed]
- Neganova, I.; Lako, M. G1 to S phase cell cycle transition in somatic and embryonic stem cells. J. Anat. 2008, 213, 30–44. [Google Scholar] [CrossRef]
- Zang, J.J.; Xie, F.; Xu, J.F.; Qin, Y.Y.; Shen, R.X.; Yang, J.M.; He, J. P16 gene hypermethylation and hepatocellular carcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2011, 17, 3043–3048. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, A.H.; Pan, X.L. The effects of fluoride on hypermethylation, transcription and expression of p16 gene in osteoblasts of rats. Chin. J. Endem. 2016, 35, 89–93. [Google Scholar]
- Daiwile, A.P.; Tarale, P.; Sivanesan, S.; Naoghare, P.K.; Bafana, A.; Parmar, D.; Kannan, K. Role of fluoride induced epigenetic alterations in the development of skeletal fluorosis. Ecotoxicol. Environ. Saf. 2019, 169, 410–417. [Google Scholar] [CrossRef]
- Lv, H.H.; Tang, X.L.; Fu, S.B. Puerarin reduces methylation of estrogen receptorαpromoter in osteoblasts and regulates its proliferation and osteoblastic differentiation. J. Hebei Med. Univ. 2015, 36, 385–390. [Google Scholar]
- Zhang, Y.L.; Huang, H.; Gong, B.; Duan, L.Z.; Sun, L.; He, T.K.; Cheng, X.M.; Li, Z.Y.; Cui, L.X.; Ba, Y. Do Environmental Fluoride Exposure and ESRα Genetic Variation Modulate Methylation Modification on Bone Changes in Chinese Farmers? Chem. Res. Toxicol. 2017, 30, 1302–1308. [Google Scholar] [CrossRef]
- Chen, T.; Liu, J. Advances in epigenetic pathogenesis of fluorosis. Chin. J. Endemiol. 2020, 39, 698–702. [Google Scholar]
- Jiang, Y.T.; Yang, Y.M.; Wang, H.G.; Darko, G.M.; Sun, D.J.; Gao, Y.H. Identification of miR-200c-3p as a major regulator of SaoS2 cells activation induced by fluoride. Chemosphere 2018, 199, 694–701. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; Chen, M.; Lin, X.L.; Lv, Z.Q.; Liang, R.Y.; Huang, L.J. Triptolide inhibits PDGF-induced proliferation of ASMCs through G0/G1 cell cycle arrest and suppression of the AKT/NF-κB/cyclinD1 signaling pathway. Eur. J. Pharmacol. 2020, 867, 172811. [Google Scholar] [CrossRef]
- Ouyang, T.; Qin, Y.; Luo, K.K.; Han, X.; Yu, C.; Zhang, A.H.; Pan, X.L. miR-486-3p regulates CyclinD1 and promotes fluoride-induced osteoblast proliferation and activation. Environ. Toxicol. 2021, 36, 1817–1828. [Google Scholar] [CrossRef]
- Gao, J.Y.; Qin, Y.; Luo, K.K.; Wang, X.L.; Yu, C.; Zhang, A.H.; Pan, X.L. Downregulation of miR-4755-5p promotes fluoride-induced osteoblast activation via tageting Cyclin D1. J. Trace. Elem. Med. Biol. 2020, 62, 126626. [Google Scholar] [CrossRef]
- Luo, K.K.; Qin, Y.; Ouyang, T.; Wang, X.L.; Zhang, A.H.; Luo, P.; Pan, X.L. Let-7c-5p Regulates CyclinD1 in Fluoride-Mediated Osteoblast Proliferation and Activation. Toxicol. Sci. 2021, 182, 275–287. [Google Scholar]
- Kapinas, K.; Kessler, C.; Ricks, T.; Gronowicz, G.; Delany, A.M. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010, 285, 25221–25231. [Google Scholar] [CrossRef] [Green Version]
- Kapinas, K.; Kessler, C.B.; Delany, A.M. miR-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical Wnt signaling. J. Cell Biochem. 2009, 108, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Xu, Z. miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem. Biophys. Res. Commun. 2010, 402, 186–189. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Xu, H. Treatment and Prevention of Skeletal Fluorosis. Biomed. Environ. Sci. 2017, 30, 147–149. [Google Scholar] [PubMed]
- Wang, W.Y.; Gui, C.Z.; Guan, Z.Z. Research progress on antagonists of fluorosis. Occup. Health 2021, 37, 2433–2438. [Google Scholar]
- Gupta, S.K.; Gupta, R.C.; Seth, A.K.; Gupta, A. Reversal of fluorosis in children. Acta Paediatr. Jpn. 1996, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Q.; Yu, M.J.; Shen, H.P.; Wang, D.; Yuan, Z.H.; Cheng, J.F. Histomorphometry Effect of Compound Traditional Chinese Medicine on Rats Skeletal Fluorosis. Mod. Prev. Med. 2016, 43, 1471–1475. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.; Liu, X.; He, Y.; Zhang, J.; Huang, H.; Bian, W.; Chilufya, M.M.; Zhao, Y.; Han, J. Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis. Int. J. Mol. Sci. 2021, 22, 11932. https://doi.org/10.3390/ijms222111932
Qiao L, Liu X, He Y, Zhang J, Huang H, Bian W, Chilufya MM, Zhao Y, Han J. Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis. International Journal of Molecular Sciences. 2021; 22(21):11932. https://doi.org/10.3390/ijms222111932
Chicago/Turabian StyleQiao, Lichun, Xuan Liu, Yujie He, Jiaheng Zhang, Hao Huang, Wenming Bian, Mumba Mulutula Chilufya, Yan Zhao, and Jing Han. 2021. "Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis" International Journal of Molecular Sciences 22, no. 21: 11932. https://doi.org/10.3390/ijms222111932







