The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia–Reperfusion Injury
Abstract
:1. Introduction
2. Nrf2 Signaling and Cellular Redox Homeostasis
3. Regulation of Nrf2 Transcriptional Activity
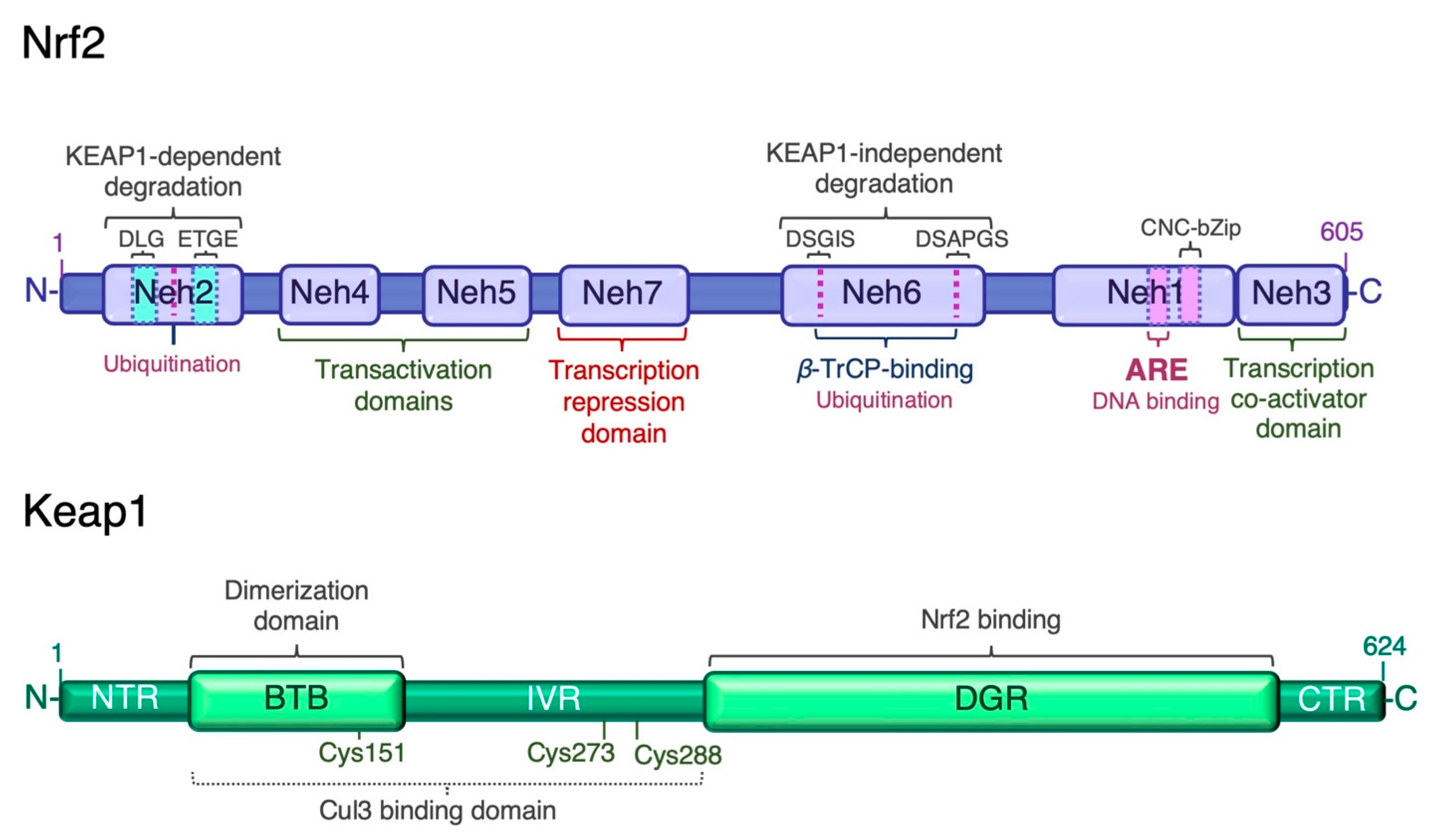
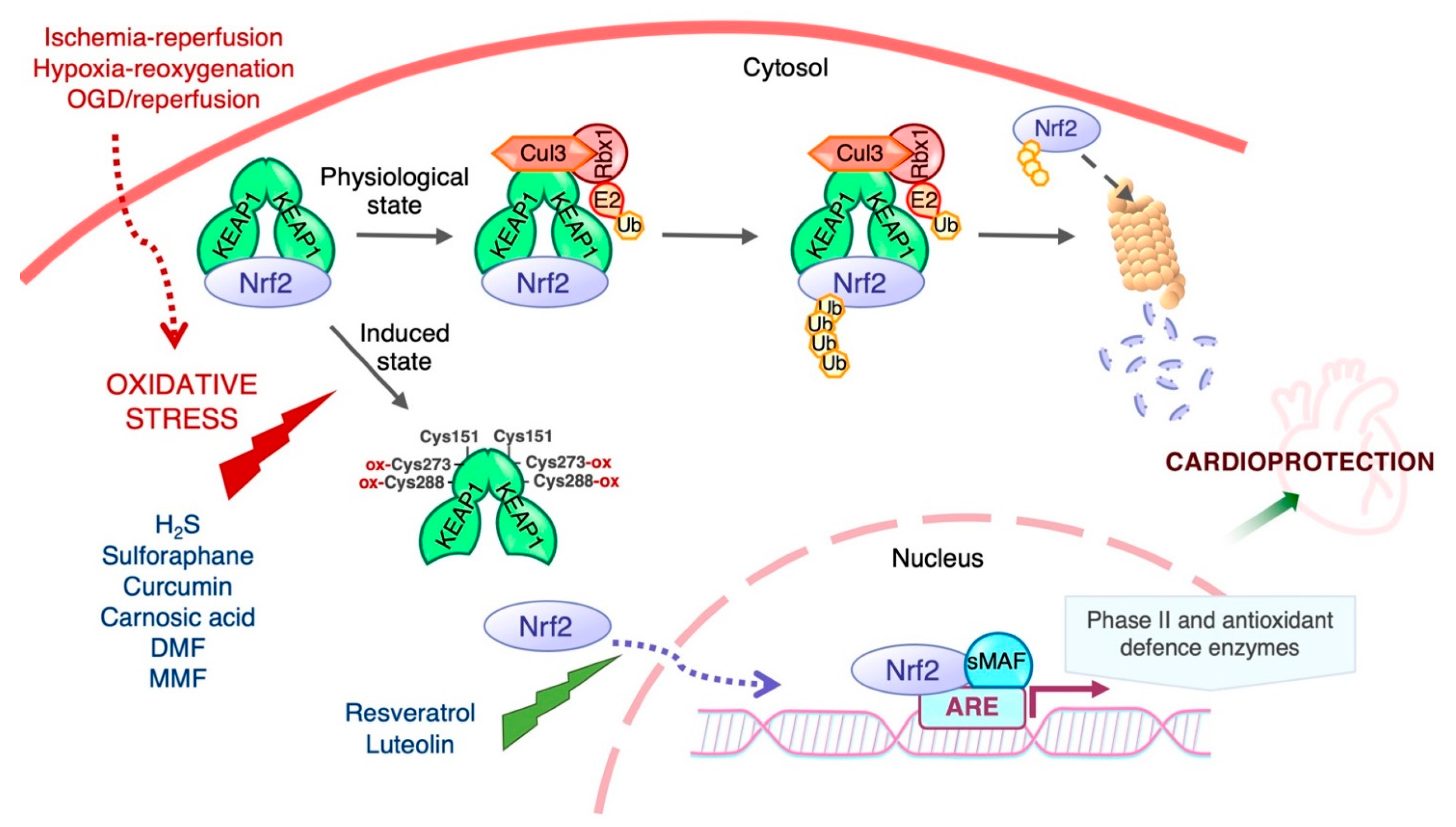
3.1. Keap1-Dependent Proteasomal Degradation of Nrf2
3.2. Keap1-Independent Regulation of Nrf2
3.3. Post-Transcriptional Regulation of Nrf2
4. Cardiac Ischemia–Reperfusion Injury
5. Role of Nrf2 in Ischemia–Reperfusion Injury
6. Involvement of Nrf2 in Protective Ischemic Conditioning
7. Activators of Nrf2 and Their Protective Role against Ischemia–Reperfusion Injury
8. Concluding Remarks and Future Perspectives
Funding
Conflicts of Interest
References
- Murphy, M.P. Understanding and Preventing Mitochondrial Oxidative Damage. Biochem. Soc. Trans. 2016, 44, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and Characterization of a Novel Erythroid Cell-Derived CNC Family Transcription Factor Heterodimerizing with the Small Maf Family Proteins. Mol. Cell Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-Related Factor 2 (Nrf2), a NF-E2-like Basic Leucine Zipper Transcriptional Activator That Binds to the Tandem NF-E2/AP1 Repeat of the Beta-Globin Locus Control Region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Han, X.L.; Kan, Y.W. Cloning of Nrf1, an NF-E2-Related Transcription Factor, by Genetic Selection in Yeast. Proc. Natl. Acad. Sci. USA 1993, 90, 11371–11375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, A.; Ito, E.; Toki, T.; Kogame, K.; Takahashi, S.; Igarashi, K.; Hayashi, N.; Yamamoto, M. Molecular Cloning and Functional Characterization of a New Cap’n’ Collar Family Transcription Factor Nrf3. J. Biol. Chem. 1999, 274, 6443–6452. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C.; Erdjument-Bromage, H.; Davidson, M.B.; Tempst, P.; Orkin, S.H. Erythroid Transcription Factor NF-E2 Is a Haematopoietic-Specific Basic-Leucine Zipper Protein. Nature 1993, 362, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Oyake, T.; Itoh, K.; Motohashi, H.; Hayashi, N.; Hoshino, H.; Nishizawa, M.; Yamamoto, M.; Igarashi, K. Bach Proteins Belong to a Novel Family of BTB-Basic Leucine Zipper Transcription Factors That Interact with MafK and Regulate Transcription through the NF-E2 Site. Mol. Cell Biol. 1996, 16, 6083–6095. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The Cap’n’Collar Basic Leucine Zipper Transcription Factor Nrf2 (NF-E2 P45-Related Factor 2) Controls Both Constitutive and Inducible Expression of Intestinal Detoxification and Glutathione Biosynthetic Enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The Antioxidant Responsive Element. Activation by Oxidative Stress and Identification of the DNA Consensus Sequence Required for Functional Activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a Novel Nrf2-Regulated Antioxidant Response Element (ARE) in the Mouse NAD(P)H:Quinone Oxidoreductase 1 Gene: Reassessment of the ARE Consensus Sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Cui, T.; Lai, Y.; Janicki, J.S.; Wang, X. Nuclear Factor Erythroid-2 Related Factor 2 (Nrf2)-Mediated Protein Quality Control in Cardiomyocytes. Front. Biosci. 2016, 21, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond Repression of Nrf2: An Update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the Heart of Oxidative Stress and Cardiac Protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Erkens, R.; Suvorava, T.; Sutton, T.R.; Fernandez, B.O.; Mikus-Lelinska, M.; Barbarino, F.; Flögel, U.; Kelm, M.; Feelisch, M.; Cortese-Krott, M.M. Nrf2 Deficiency Unmasks the Significance of Nitric Oxide Synthase Activity for Cardioprotection. Oxid. Med. Cell. Longev. 2018, 2018, 8309698. [Google Scholar] [CrossRef] [Green Version]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Wang, X.; Castellani, L.W.; Reue, K.; Lusis, A.J.; et al. NF-E2-Related Factor 2 Promotes Atherosclerosis by Effects on Plasma Lipoproteins and Cholesterol Transport That Overshadow Antioxidant Protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, H.; Mathew, R.O.; Cui, T. The Dark Side of Nrf2 in the Heart. Front. Physiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-Regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar] [PubMed]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Agbaga, M.-P.; Anderson, R.E. Upregulation of Thioredoxin System via Nrf2-Antioxidant Responsive Element Pathway in Adaptive-Retinal Neuroprotection in Vivo and in Vitro. Free Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, A.K.; McMahon, M.; Plummer, S.M.; Higgins, L.G.; Penning, T.M.; Igarashi, K.; Hayes, J.D. Characterization of the Cancer Chemopreventive NRF2-Dependent Gene Battery in Human Keratinocytes: Demonstration That the KEAP1-NRF2 Pathway, and Not the BACH1-NRF2 Pathway, Controls Cytoprotection against Electrophiles as Well as Redox-Cycling Compounds. Carcinogenesis 2009, 30, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agyeman, A.S.; Chaerkady, R.; Shaw, P.G.; Davidson, N.E.; Visvanathan, K.; Pandey, A.; Kensler, T.W. Transcriptomic and Proteomic Profiling of KEAP1 Disrupted and Sulforaphane-Treated Human Breast Epithelial Cells Reveals Common Expression Profiles. Breast Cancer Res. Treat. 2012, 132, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and Glutathione Have Complementary Antioxidant and Cytoprotective Roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, J.M.; Dieter, M.Z.; Aleksunes, L.M.; Slitt, A.L.; Guo, G.; Tanaka, Y.; Scheffer, G.L.; Chan, J.Y.; Manautou, J.E.; Chen, Y.; et al. Oxidative and Electrophilic Stress Induces Multidrug Resistance-Associated Protein Transporters via the Nuclear Factor-E2-Related Factor-2 Transcriptional Pathway. Hepatology 2007, 46, 1597–1610. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The Mitochondrially Targeted Antioxidant MitoQ Protects the Intestinal Barrier by Ameliorating Mitochondrial DNA Damage via the Nrf2/ARE Signaling Pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced Expression of the Transcription Factor Nrf2 by Cancer Chemopreventive Agents: Role of Antioxidant Response Element-like Sequences in the Nrf2 Promoter. Mol. Cell Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 Is Controlled by Two Distinct β-TrCP Recognition Motifs in Its Neh6 Domain, One of Which Can Be Modulated by GSK-3 Activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/{beta}-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobón-Velasco, J.C.; Devijver, H.; García-Mayoral, M.F.; Van Leuven, F.; et al. Structural and Functional Characterization of Nrf2 Degradation by the Glycogen Synthase Kinase 3/β-TrCP Axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Chiba, T.; Suzuki, T.; Fujita, T.; Ikenoue, T.; Omata, M.; Furuichi, K.; Shikama, H.; Tanaka, K. Homodimer of Two F-Box Proteins BetaTrCP1 or BetaTrCP2 Binds to IkappaBalpha for Signal-Dependent Ubiquitination. J. Biol. Chem. 2000, 275, 2877–2884. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Xu, G.; Schulman, B.A.; Jeffrey, P.D.; Harper, J.W.; Pavletich, N.P. Structure of a Beta-TrCP1-Skp1-Beta-Catenin Complex: Destruction Motif Binding and Lysine Specificity of the SCF(Beta-TrCP1) Ubiquitin Ligase. Mol. Cell 2003, 11, 1445–1456. [Google Scholar] [CrossRef]
- Motohashi, H.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Small Maf Proteins Serve as Transcriptional Cofactors for Keratinocyte Differentiation in the Keap1-Nrf2 Regulatory Pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 6379–6384. [Google Scholar] [CrossRef] [Green Version]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two Domains of Nrf2 Cooperatively Bind CBP, a CREB Binding Protein, and Synergistically Activate Transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα Inhibits the NRF2-ARE Signaling Pathway through a Direct Interaction with the Neh7 Domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Chowdhry, S.; Dinkova-Kostova, A.T.; Sutherland, C. Dual Regulation of Transcription Factor Nrf2 by Keap1 and by the Combined Actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015, 43, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional Regulation of NF-E2 P45-Related Factor (NRF2) Expression by the Aryl Hydrocarbon Receptor-Xenobiotic Response Element Signaling Pathway: Direct Cross-Talk between Phase I and II Drug-Metabolizing Enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; McMahon, M. Cross-Talk between Transcription Factors AhR and Nrf2: Lessons for Cancer Chemoprevention from Dioxin. Toxicol. Sci. 2009, 111, 199–201. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sugawara, A.; Taniyama, Y.; Uruno, A.; Igarashi, K.; Arima, S.; Ito, S.; Takeuchi, K. Suppression of Rat Thromboxane Synthase Gene Transcription by Peroxisome Proliferator-Activated Receptor Gamma in Macrophages via an Interaction with NRF2. J. Biol. Chem. 2000, 275, 33142–33150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.Y.; Cho, I.J.; Kim, S.G. Transactivation of the PPAR-Responsive Enhancer Module in Chemopreventive Glutathione S-Transferase Gene by the Peroxisome Proliferator-Activated Receptor-Gamma and Retinoid X Receptor Heterodimer. Cancer Res. 2004, 64, 3701–3713. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, S.A.; Zaitseva, L.; Murray, M.Y.; Shah, N.M.; Bowles, K.M.; MacEwan, D.J. The High Nrf2 Expression in Human Acute Myeloid Leukemia Is Driven by NF-ΚB and Underlies Its Chemo-Resistance. Blood 2012, 120, 5188–5198. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription Factors NRF2 and NF-ΚB Are Coordinated Effectors of the Rho Family, GTP-Binding Protein RAC1 during Inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [Green Version]
- Siswanto, F.M.; Oguro, A.; Imaoka, S. Sp1 Is a Substrate of Keap1 and Regulates the Activity of CRL4AWDR23 Ubiquitin Ligase toward Nrf2. J. Biol. Chem. 2021, 296, 100704. [Google Scholar] [CrossRef]
- Tung, M.-C.; Lin, P.-L.; Wang, Y.-C.; He, T.-Y.; Lee, M.-C.; Yeh, S.D.; Chen, C.-Y.; Lee, H. Mutant P53 Confers Chemoresistance in Non-Small Cell Lung Cancer by Upregulating Nrf2. Oncotarget 2015, 6, 41692–41705. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-Induced Nrf2 Transcription Promotes ROS Detoxification and Tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Levy, S.; Forman, H.J. C-Myc Is a Nrf2-Interacting Protein That Negatively Regulates Phase II Genes through Their Electrophile Responsive Elements. IUBMB Life 2010, 62, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Baniasadi, P.S.; Harris, I.S.; Silvester, J.; Inoue, S.; Snow, B.; Joshi, P.A.; Wakeham, A.; Molyneux, S.D.; Martin, B.; et al. BRCA1 Interacts with Nrf2 to Regulate Antioxidant Signaling and Cell Survival. J. Exp. Med. 2013, 210, 1529–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihvola, V.; Levonen, A.-L. Keap1 as the Redox Sensor of the Antioxidant Response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the Cysteine-Based Mammalian Intracellular Sensor for Electrophiles and Oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- Katoh, Y.; Iida, K.; Kang, M.-I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary Conserved N-Terminal Domain of Nrf2 Is Essential for the Keap1-Mediated Degradation of the Protein by Proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, D.; Killeen, E.; Naquin, R.; Alam, S.; Alam, J. Degradation of Transcription Factor Nrf2 via the Ubiquitin-Proteasome Pathway and Stabilization by Cadmium. J. Biol. Chem. 2003, 278, 2396–2402. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different Electrostatic Potentials Define ETGE and DLG Motifs as Hinge and Latch in Oxidative Stress Response. Mol. Cell Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-Mediated Transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [Green Version]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhao, F.; Gao, B.; Tan, C.; Yagishita, N.; Nakajima, T.; Wong, P.K.; Chapman, E.; Fang, D.; Zhang, D.D. Hrd1 Suppresses Nrf2-Mediated Cellular Protection during Liver Cirrhosis. Genes Dev. 2014, 28, 708–722. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Hong, Y.B.; Kim, H.J.; Bae, I. CR6-Interacting Factor 1 (CRIF1) Regulates NF-E2-Related Factor 2 (NRF2) Protein Stability by Proteasome-Mediated Degradation. J. Biol. Chem. 2010, 285, 21258–21268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by P300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef] [Green Version]
- Malloy, M.T.; McIntosh, D.J.; Walters, T.S.; Flores, A.; Goodwin, J.S.; Arinze, I.J. Trafficking of the Transcription Factor Nrf2 to Promyelocytic Leukemia-Nuclear Bodies: Implications for Degradation of NRF2 in the Nucleus. J. Biol. Chem. 2013, 288, 14569–14583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurinna, S.; Werner, S. NRF2 and MicroRNAs: New but Awaited Relations. Biochem. Soc. Trans. 2015, 43, 595–601. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Ivanova-Radkevich, V.I.; Chernov, N.N. Role of MicroRNAs in the Regulation of Redox-Dependent Processes. Biochemistry 2019, 84, 1233–1246. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of MicroRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Papp, D.; Lenti, K.; Módos, D.; Fazekas, D.; Dúl, Z.; Türei, D.; Földvári-Nagy, L.; Nussinov, R.; Csermely, P.; Korcsmáros, T. The NRF2-Related Interactome and Regulome Contain Multifunctional Proteins and Fine-Tuned Autoregulatory Loops. FEBS Lett. 2012, 586, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. MiR-28 Regulates Nrf2 Expression through a Keap1-Independent Mechanism. Breast Cancer Res. Treat. 2011, 129, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen Peroxide Responsive MiR153 Targets Nrf2/ARE Cytoprotection in Paraquat Induced Dopaminergic Neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Inoue, J.; Kawano, T.; Kozaki, K.; Omura, K.; Inazawa, J. The Impact of MiRNA-Based Molecular Diagnostics and Treatment of NRF2-Stabilized Tumors. Mol. Cancer Res. 2014, 12, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Ronghe, A.M.; Chatterjee, A.; Bhat, N.K.; Bhat, H.K. MicroRNA-93 Regulates NRF2 Expression and Is Associated with Breast Carcinogenesis. Carcinogenesis 2013, 34, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Sangokoya, C.; Telen, M.J.; Chi, J.-T. MicroRNA MiR-144 Modulates Oxidative Stress Tolerance and Associates with Anemia Severity in Sickle Cell Disease. Blood 2010, 116, 4338–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of Novel MicroRNAs in Post-Transcriptional Control of Nrf2 Expression and Redox Homeostasis in Neuronal, SH-SY5Y Cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef] [Green Version]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric Restriction Confers Persistent Anti-Oxidative, pro-Angiogenic, and Anti-Inflammatory Effects and Promotes Anti-Aging MiRNA Expression Profile in Cerebromicrovascular Endothelial Cells of Aged Rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H292–H306. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Jones, M.A.; Abdelrahman, A.A.; Powell, F.L.; Thounaojam, M.C.; Gutsaeva, D.; Bartoli, M.; Martin, P.M. Inhibiting MicroRNA-144 Potentiates Nrf2-Dependent Antioxidant Signaling in RPE and Protects against Oxidative Stress-Induced Outer Retinal Degeneration. Redox Biol. 2020, 28, 101336. [Google Scholar] [CrossRef]
- Luo, R.; Jin, H.; Li, L.; Hu, Y.-X.; Xiao, F. Long Noncoding RNA MEG3 Inhibits Apoptosis of Retinal Pigment Epithelium Cells Induced by High Glucose via the MiR-93/Nrf2 Axis. Am. J. Pathol. 2020, 190, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of Novel NRF2-Regulated Genes by ChIP-Seq: Influence on Retinoid X Receptor Alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [Green Version]
- Kurinna, S.; Schäfer, M.; Ostano, P.; Karouzakis, E.; Chiorino, G.; Bloch, W.; Bachmann, A.; Gay, S.; Garrod, D.; Lefort, K.; et al. A Novel Nrf2-MiR-29-Desmocollin-2 Axis Regulates Desmosome Function in Keratinocytes. Nat. Commun. 2014, 5, 5099. [Google Scholar] [CrossRef] [Green Version]
- Joo, M.S.; Lee, C.G.; Koo, J.H.; Kim, S.G. MiR-125b Transcriptionally Increased by Nrf2 Inhibits AhR Repressor, Which Protects Kidney from Cisplatin-Induced Injury. Cell Death Dis. 2013, 4, e899. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.M.; Zaitseva, L.; Bowles, K.M.; MacEwan, D.J.; Rushworth, S.A. NRF2-Driven MiR-125B1 and MiR-29B1 Transcriptional Regulation Controls a Novel Anti-Apoptotic MiRNA Regulatory Network for AML Survival. Cell Death Differ. 2015, 22, 654–664. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Kevin, L.G.; Camara, A.K.S.; Riess, M.L.; Novalija, E.; Stowe, D.F. Ischemic Preconditioning Alters Real-Time Measure of O2 Radicals in Intact Hearts with Ischemia and Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H566–H574. [Google Scholar] [CrossRef] [Green Version]
- Braunersreuther, V.; Jaquet, V. Reactive Oxygen Species in Myocardial Reperfusion Injury: From Physiopathology to Therapeutic Approaches. Curr. Pharm. Biotechnol. 2012, 13, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Kuppusamy, P.; Williams, R.; Rayburn, B.K.; Smith, D.; Weisfeldt, M.L.; Flaherty, J.T. Measurement and Characterization of Postischemic Free Radical Generation in the Isolated Perfused Heart. J. Biol. Chem. 1989, 264, 18890–18895. [Google Scholar] [CrossRef]
- Henry, T.D.; Archer, S.L.; Nelson, D.; Weir, E.K.; From, A.H. Enhanced Chemiluminescence as a Measure of Oxygen-Derived Free Radical Generation during Ischemia and Reperfusion. Circ. Res. 1990, 67, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Zweier, J.L.; Talukder, M.A.H. The Role of Oxidants and Free Radicals in Reperfusion Injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Di Lisa, F.; Bernardi, P. Modulation of Mitochondrial Permeability Transition in Ischemia-Reperfusion Injury of the Heart. Advantages and Limitations. Curr. Med. Chem. 2015, 22, 2480–2487. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Rodriguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J.; Agulló, L.; Cabestrero, A. The End-Effectors of Preconditioning Protection against Myocardial Cell Death Secondary to Ischemia-Reperfusion. Cardiovasc. Res. 2006, 70, 274–285. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Clarke, S.J.; Javadov, S.A. Mitochondrial Permeability Transition Pore Opening during Myocardial Reperfusion—A Target for Cardioprotection. Cardiovasc. Res. 2004, 61, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Meana, M.; Abellán, A.; Miró-Casas, E.; Agulló, E.; Garcia-Dorado, D. Role of Sarcoplasmic Reticulum in Mitochondrial Permeability Transition and Cardiomyocyte Death during Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1281–H1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solaini, G.; Harris, D.A. Biochemical Dysfunction in Heart Mitochondria Exposed to Ischaemia and Reperfusion. Biochem. J. 2005, 390, 377–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisarenko, O.; Studneva, I.; Khlopkov, V.; Solomatina, E.; Ruuge, E. An Assessment of Anaerobic Metabolism during Ischemia and Reperfusion in Isolated Guinea Pig Heart. Biochim. Biophys. Acta 1988, 934, 55–63. [Google Scholar] [CrossRef]
- Peuhkurinen, K.J.; Takala, T.E.; Nuutinen, E.M.; Hassinen, I.E. Tricarboxylic Acid Cycle Metabolites during Ischemia in Isolated Perfused Rat Heart. Am. J. Physiol. 1983, 244, H281–H288. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pell, V.R.; Chouchani, E.T.; Frezza, C.; Murphy, M.P.; Krieg, T. Succinate Metabolism: A New Therapeutic Target for Myocardial Reperfusion Injury. Cardiovasc. Res. 2016, 111, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.J.; Murphy, M.P. Animal and Human Studies with the Mitochondria-Targeted Antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.J.; Murphy, M.P.; Sammut, I.A. Targeting an Antioxidant to Mitochondria Decreases Cardiac Ischemia-Reperfusion Injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef]
- Andrienko, T.; Pasdois, P.; Rossbach, A.; Halestrap, A.P. Real-Time Fluorescence Measurements of ROS and [Ca2+] in Ischemic/Reperfused Rat Hearts: Detectable Increases Occur Only after Mitochondrial Pore Opening and Are Attenuated by Ischemic Preconditioning. PLoS ONE 2016, 11, e0167300. [Google Scholar] [CrossRef] [Green Version]
- Andrienko, T.N.; Pasdois, P.; Pereira, G.C.; Ovens, M.J.; Halestrap, A.P. The Role of Succinate and ROS in Reperfusion Injury—A Critical Appraisal. J. Mol. Cell Cardiol. 2017, 110, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, M.J.; Llwyd, O.; Morin, D.; de Paulis, D.; Arnoux, T.; Gouarné, C.; Koul, S.; Engblom, H.; Bordet, T.; Tissier, R.; et al. Differences in the Profile of Protection Afforded by TRO40303 and Mild Hypothermia in Models of Cardiac Ischemia/Reperfusion Injury. Eur. J. Pharmacol. 2015, 760, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Valls-Lacalle, L.; Barba, I.; Miró-Casas, E.; Alburquerque-Béjar, J.J.; Ruiz-Meana, M.; Fuertes-Agudo, M.; Rodríguez-Sinovas, A.; García-Dorado, D. Succinate Dehydrogenase Inhibition with Malonate during Reperfusion Reduces Infarct Size by Preventing Mitochondrial Permeability Transition. Cardiovasc. Res. 2016, 109, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhang, J.; Strom, J.; Lee, S.; Chen, Q.M. Myocardial Ischemic Reperfusion Induces de Novo Nrf2 Protein Translation. Biochim. Biophys. Acta 2014, 1842, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Leonard, M.O.; Kieran, N.E.; Howell, K.; Burne, M.J.; Varadarajan, R.; Dhakshinamoorthy, S.; Porter, A.G.; O’Farrelly, C.; Rabb, H.; Taylor, C.T. Reoxygenation-Specific Activation of the Antioxidant Transcription Factor Nrf2 Mediates Cytoprotective Gene Expression in Ischemia-Reperfusion Injury. FASEB J. 2006, 20, 2624–2626. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, Z.; Lin, V.; May, A.; Shaw, D.H.; Wang, Z.; Che, B.; Tran, K.; Du, H.; Shaw, P.X. MicroRNA MiR-24-3p Reduces Apoptosis and Regulates Keap1-Nrf2 Pathway in Mouse Cardiomyocytes Responding to Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2018, 2018, 7042105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Hou, W.; Guo, L. MiR-153 Regulates Cardiomyocyte Apoptosis by Targeting Nrf2/HO-1 Signaling. Chromosome Res. 2019, 27, 167–178. [Google Scholar] [CrossRef]
- Hou, W.; Zhu, X.; Liu, J.; Ma, J. Inhibition of MiR-153 Ameliorates Ischemia/Reperfusion-Induced Cardiomyocytes Apoptosis by Regulating Nrf2/HO-1 Signaling in Rats. Biomed. Eng. Online 2020, 19, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anedda, A.; López-Bernardo, E.; Acosta-Iborra, B.; Saadeh Suleiman, M.; Landázuri, M.O.; Cadenas, S. The Transcription Factor Nrf2 Promotes Survival by Enhancing the Expression of Uncoupling Protein 3 under Conditions of Oxidative Stress. Free Radic. Biol. Med. 2013, 61, 395–407. [Google Scholar] [CrossRef]
- Zhang, Y.; Sano, M.; Shinmura, K.; Tamaki, K.; Katsumata, Y.; Matsuhashi, T.; Morizane, S.; Ito, H.; Hishiki, T.; Endo, J.; et al. 4-Hydroxy-2-Nonenal Protects against Cardiac Ischemia-Reperfusion Injury via the Nrf2-Dependent Pathway. J. Mol. Cell Cardiol. 2010, 49, 576–586. [Google Scholar] [CrossRef]
- López-Bernardo, E.; Anedda, A.; Sánchez-Pérez, P.; Acosta-Iborra, B.; Cadenas, S. 4-Hydroxynonenal Induces Nrf2-Mediated UCP3 Upregulation in Mouse Cardiomyocytes. Free Radic. Biol. Med. 2015, 88, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 Protects against Maladaptive Cardiac Responses to Hemodynamic Stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef]
- Fukunaga, N.; Kawajiri, H.; Badiwala, M.V.; Butany, J.; Li, R.-K.; Billia, F.; Rao, V. Protective Role of Nrf2 against Ischemia Reperfusion Injury and Cardiac Allograft Vasculopathy. Am. J. Transpl. 2020, 20, 1262–1271. [Google Scholar] [CrossRef]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef] [Green Version]
- Makkos, A.; Ágg, B.; Petrovich, B.; Varga, Z.V.; Görbe, A.; Ferdinandy, P. Systematic Review and Network Analysis of MicroRNAs Involved in Cardioprotection against Myocardial Ischemia/Reperfusion Injury and Infarction: Involvement of Redox Signalling. Free Radic. Biol. Med. 2021, 172, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the Regulation of Cellular Redox Status and Its Implications in Myocardial Ischemia-Reperfusion Injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Mutharasan, R.K.; Nagpal, V.; Ichikawa, Y.; Ardehali, H. MicroRNA-210 Is Upregulated in Hypoxic Cardiomyocytes through Akt- and P53-Dependent Pathways and Exerts Cytoprotective Effects. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1519–H1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tingle, S.J.; Sewpaul, A.; Bates, L.; Thompson, E.R.; Shuttleworth, V.; Figueiredo, R.; Ibrahim, I.K.; Ali, S.; Wilson, C.; Sheerin, N.S. Dual MicroRNA Blockade Increases Expression of Antioxidant Protective Proteins: Implications for Ischemia-Reperfusion Injury. Transplantation 2020, 104, 1853–1861. [Google Scholar] [CrossRef]
- Sun, X.; Zuo, H.; Liu, C.; Yang, Y. Overexpression of MiR-200a Protects Cardiomyocytes against Hypoxia-Induced Apoptosis by Modulating the Kelch-like ECH-Associated Protein 1-Nuclear Factor Erythroid 2-Related Factor 2 Signaling Axis. Int. J. Mol. Med. 2016, 38, 1303–1311. [Google Scholar] [CrossRef]
- Shah, Z.A.; Li, R.-C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of Reactive Oxygen Species in Modulation of Nrf2 Following Ischemic Reperfusion Injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, A.Y.; Li, P.; Murphy, T.H. A Small-Molecule-Inducible Nrf2-Mediated Antioxidant Response Provides Effective Prophylaxis against Cerebral Ischemia in Vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Luo, D.; Xia, W.; Gu, C.; Lahm, T.; Xu, X.; Qiu, Q.; Zhang, Z. Nuclear Factor (Erythroid-Derived 2)-Like 2 (Nrf2) Contributes to the Neuroprotective Effects of Histone Deacetylase Inhibitors In Retinal Ischemia-Reperfusion Injury. Neuroscience 2019, 418, 25–36. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Fragoulis, A.; Rosen, C.; Kan, Y.W.; Sönmez, T.T.; Pufe, T.; Wruck, C.J. Nrf2 Protects against TWEAK-Mediated Skeletal Muscle Wasting. Sci. Rep. 2014, 4, 3625. [Google Scholar] [CrossRef] [Green Version]
- Kudoh, K.; Uchinami, H.; Yoshioka, M.; Seki, E.; Yamamoto, Y. Nrf2 Activation Protects the Liver from Ischemia/Reperfusion Injury in Mice. Ann. Surg. 2014, 260, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.-Y.; Harberg, C.; Matkowskyj, K.A.; Cook, S.; Roenneburg, D.; Werner, S.; Johnson, J.; Foley, D.P. Overactivation of the Nuclear Factor (Erythroid-Derived 2)-like 2-Antioxidant Response Element Pathway in Hepatocytes Decreases Hepatic Ischemia/Reperfusion Injury in Mice. Liver Transpl. 2016, 22, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yue, S.; Ke, B.; Zhu, J.; Shen, X.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Nuclear Factor Erythroid 2-Related Factor 2 Regulates Toll-like Receptor 4 Innate Responses in Mouse Liver Ischemia-Reperfusion Injury through Akt-Forkhead Box Protein O1 Signaling Network. Transplantation 2014, 98, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, B.; Shen, X.-D.; Zhang, Y.; Ji, H.; Gao, F.; Yue, S.; Kamo, N.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; et al. KEAP1-NRF2 Complex in Ischemia-Induced Hepatocellular Damage of Mouse Liver Transplants. J. Hepatol. 2013, 59, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription Factor Nrf2 Is Protective during Ischemic and Nephrotoxic Acute Kidney Injury in Mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nezu, M.; Souma, T.; Yu, L.; Suzuki, T.; Saigusa, D.; Ito, S.; Suzuki, N.; Yamamoto, M. Transcription Factor Nrf2 Hyperactivation in Early-Phase Renal Ischemia-Reperfusion Injury Prevents Tubular Damage Progression. Kidney Int. 2017, 91, 387–401. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic Conditioning and Reperfusion Injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-S.; Chen, H.-P.; Yu, H.-H.; Yan, Y.-F.; Liao, Z.-P.; Huang, Q.-R. Nrf2-Dependent Upregulation of Antioxidative Enzymes: A Novel Pathway for Hypoxic Preconditioning-Mediated Delayed Cardioprotection. Mol. Cell Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Z.; Yao, J.; Zhao, G.; Fa, X.; Niu, J. Participation of Protein Kinase C in the Activation of Nrf2 Signaling by Ischemic Preconditioning in the Isolated Rabbit Heart. Mol. Cell Biochem. 2013, 372, 169–179. [Google Scholar] [CrossRef]
- Buelna-Chontal, M.; Guevara-Chávez, J.-G.; Silva-Palacios, A.; Medina-Campos, O.-N.; Pedraza-Chaverri, J.; Zazueta, C. Nrf2-Regulated Antioxidant Response Is Activated by Protein Kinase C in Postconditioned Rat Hearts. Free Radic. Biol. Med. 2014, 74, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Motori, E.; Fabbri, D.; Malaguti, M.; Leoncini, E.; Lorenzini, A.; Hrelia, S. H2O2 Preconditioning Modulates Phase II Enzymes through P38 MAPK and PI3K/Akt Activation. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2196–H2205. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.B.; Bolli, R.; Dawn, B.; Sanganalmath, S.K.; Zhu, Y.; Wang, O.-L.; Guo, Y.; Motterlini, R.; Xuan, Y.-T. Carbon Monoxide Induces a Late Preconditioning-Mimetic Cardioprotective and Antiapoptotic Milieu in the Myocardium. J. Mol. Cell Cardiol. 2012, 52, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.H.; Caldarone, C.A.; Guan, R.; Wen, X.-Y.; Ailenberg, M.; Kapus, A.; Szaszi, K.; Rotstein, O.D. Nuclear Factor (Erythroid-Derived 2)-Like 2 Regulates the Hepatoprotective Effects of Remote Ischemic Conditioning in Hemorrhagic Shock. Antioxid. Redox Signal. 2019, 30, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Shokeir, A.A.; Barakat, N.; Hussein, A.M.; Awadalla, A.; Harraz, A.M.; Khater, S.; Hemmaid, K.; Kamal, A.I. Activation of Nrf2 by Ischemic Preconditioning and Sulforaphane in Renal Ischemia/Reperfusion Injury: A Comparative Experimental Study. Physiol. Res. 2015, 64, 313–323. [Google Scholar] [CrossRef]
- Tanaka, Y.; Maher, J.M.; Chen, C.; Klaassen, C.D. Hepatic Ischemia-Reperfusion Induces Renal Heme Oxygenase-1 via NF-E2-Related Factor 2 in Rats and Mice. Mol. Pharmacol. 2007, 71, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, A.; Srivastava, S.; Siow, R.C.M.; Cash, D.; Modo, M.; Duchen, M.R.; Fraser, P.A.; Williams, S.C.R.; Mann, G.E. Sulforaphane Preconditioning of the Nrf2/HO-1 Defense Pathway Protects the Cerebral Vasculature against Blood-Brain Barrier Disruption and Neurological Deficits in Stroke. Free Radic. Biol. Med. 2013, 65, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Perez-Pinzon, M.A. Ischemic Preconditioning Protects Astrocytes against Oxygen Glucose Deprivation Via the Nuclear Erythroid 2-Related Factor 2 Pathway. Transl. Stroke Res. 2018, 9, 99–109. [Google Scholar] [CrossRef]
- Bell, K.F.; Al-Mubarak, B.; Fowler, J.H.; Baxter, P.S.; Gupta, K.; Tsujita, T.; Chowdhry, S.; Patani, R.; Chandran, S.; Horsburgh, K.; et al. Mild Oxidative Stress Activates Nrf2 in Astrocytes, Which Contributes to Neuroprotective Ischemic Preconditioning. Proc. Natl. Acad. Sci. USA 2011, 108, E1–E2. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sun, Y.; Mao, L.; Zhang, M.; Li, Q.; Zhang, L.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Brain Ischemic Preconditioning Protects against Ischemic Injury and Preserves the Blood-Brain Barrier via Oxidative Signaling and Nrf2 Activation. Redox Biol. 2018, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Li, Q.; Li, S.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Ischemic Preconditioning Provides Long-Lasting Neuroprotection against Ischemic Stroke: The Role of Nrf2. Exp. Neurol. 2020, 325, 113142. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, K.; Xue, L.; Cheng, J.; Bai, Y. Preconditioning of H2S Inhalation Protects against Cerebral Ischemia/Reperfusion Injury by Induction of HSP70 through PI3K/Akt/Nrf2 Pathway. Brain Res. Bull. 2016, 121, 68–74. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Liu, M.; Chen, H. Hydrogen Sulfide Attenuates Renal I/R-induced Activation of the Inflammatory Response and Apoptosis via Regulating Nrf2-mediated NLRP3 Signaling Pathway Inhibition. Mol. Med. Rep. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane Protects from Myocardial Ischemia-Reperfusion Damage through the Balanced Activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Ping, Z.; Liu, W.; Kang, Z.; Cai, J.; Wang, Q.; Cheng, N.; Wang, S.; Wang, S.; Zhang, J.H.; Sun, X. Sulforaphane Protects Brains against Hypoxic–Ischemic Injury through Induction of Nrf2-Dependent Phase 2 Enzyme. Brain Res. 2010, 1343, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane Reduces Infarct Volume Following Focal Cerebral Ischemia in Rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef]
- Srivastava, S.; Alfieri, A.; Siow, R.C.M.; Mann, G.E.; Fraser, P.A. Temporal and Spatial Distribution of Nrf2 in Rat Brain Following Stroke: Quantification of Nuclear to Cytoplasmic Nrf2 Content Using a Novel Immunohistochemical Technique. J. Physiol. 2013, 591, 3525–3538. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.M.; Fraser, P.A.; Mann, G.E. Nrf2-Regulated Redox Signaling in Brain Endothelial Cells Adapted to Physiological Oxygen Levels: Consequences for Sulforaphane Mediated Protection against Hypoxia-Reoxygenation. Redox Biol. 2020, 37, 101708. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-Y.; Kang, N.-I.; Lee, H.-K.; Jang, K.Y.; Park, J.-W.; Park, B.-H. Sulforaphane Protects Kidneys against Ischemia-Reperfusion Injury through Induction of the Nrf2-Dependent Phase 2 Enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef]
- Kocak, C.; Kocak, F.E.; Akcilar, R.; Isiklar, O.O.; Kocak, H.; Bayat, Z.; Simsek, H.; Taser, F.; Altuntas, I. Molecular and Biochemical Evidence on the Protective Effects of Embelin and Carnosic Acid in Isoproterenol-Induced Acute Myocardial Injury in Rats. Life Sci. 2016, 147, 15–23. [Google Scholar] [CrossRef]
- Sahu, B.D.; Putcha, U.K.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Carnosic Acid Promotes Myocardial Antioxidant Response and Prevents Isoproterenol-Induced Myocardial Oxidative Stress and Apoptosis in Mice. Mol. Cell Biochem. 2014, 394, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Kosaka, K.; Itoh, K.; Kobayashi, A.; Yamamoto, M.; Shimojo, Y.; Kitajima, C.; Cui, J.; Kamins, J.; Okamoto, S.; et al. Carnosic Acid, a Catechol-Type Electrophilic Compound, Protects Neurons Both in Vitro and in Vivo through Activation of the Keap1/Nrf2 Pathway via S-Alkylation of Targeted Cysteines on Keap1. J. Neurochem. 2008, 104, 1116–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-X.; Zhang, L.-Y.; Chen, Y.-L.; Yu, S.-S.; Zhao, Y.; Zhao, J. Curcumin Pretreatment and Post-Treatment Both Improve the Antioxidative Ability of Neurons with Oxygen-Glucose Deprivation. Neural Regen. Res. 2015, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by Down-Regulating NF-KB and Elevating Nrf2, Reduces Brain Edema and Neurological Dysfunction after Cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin Upregulates Transcription Factor Nrf2, HO-1 Expression and Protects Rat Brains against Focal Ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.G.; El-Emam, S.Z.; Mohamed, E.A.; Abd Ellah, M.F. Dimethyl Fumarate and Curcumin Attenuate Hepatic Ischemia/Reperfusion Injury via Nrf2/HO-1 Activation and Anti-Inflammatory Properties. Int. Immunopharmacol. 2020, 80, 106131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Xia, M.-L.; Wang, J.; Zhou, X.-R.; Lou, Y.-Y.; Tang, L.-H.; Zhang, F.-J.; Yang, J.-T.; Qian, L.-B. Luteolin Attenuates Cardiac Ischemia/Reperfusion Injury in Diabetic Rats by Modulating Nrf2 Antioxidative Function. Oxidative Med. Cell. Longev. 2019, 2019, e2719252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-R.; Ru, X.-C.; Xiao, C.; Pan, J.; Lou, Y.-Y.; Tang, L.-H.; Yang, J.-T.; Qian, L.-B. Sestrin2 Is Involved in the Nrf2-Regulated Antioxidative Signaling Pathway in Luteolin-Induced Prevention of the Diabetic Rat Heart from Ischemia/Reperfusion Injury. Food Funct. 2021, 12, 3562–3571. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol Attenuates Inflammation and Oxidative Stress Induced by Myocardial Ischemia-Reperfusion Injury: Role of Nrf2/ARE Pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar]
- Li, J.; Li, L.; Wang, S.; Zhang, C.; Zheng, L.; Jia, Y.; Xu, M.; Zhu, T.; Zhang, Y.; Rong, R. Resveratrol Alleviates Inflammatory Responses and Oxidative Stress in Rat Kidney Ischemia-Reperfusion Injury and H2O2-Induced NRK-52E Cells via the Nrf2/TLR4/NF-ΚB Pathway. CPB 2018, 45, 1677–1689. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Tang, L.; Pan, Y.; Liu, Z.; Zeng, C.; Wang, J.; Wei, T.; Liang, G. Novel Curcumin Analogue 14p Protects against Myocardial Ischemia Reperfusion Injury through Nrf2-Activating Anti-Oxidative Activity. Toxicol. Appl. Pharmacol. 2015, 282, 175–183. [Google Scholar] [CrossRef]
- Ashrafian, H.; Czibik, G.; Bellahcene, M.; Aksentijević, D.; Smith, A.C.; Mitchell, S.J.; Dodd, M.S.; Kirwan, J.; Byrne, J.J.; Ludwig, C.; et al. Fumarate Is Cardioprotective via Activation of the Nrf2 Antioxidant Pathway. Cell Metab. 2012, 15, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective Effect of Dimethyl Fumarate on Oxidative Damage and Signaling in Cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, W.; Liu, Z.; Han, W.; Shi, K.; Shen, Y.; Li, H.; Liu, Q.; Fu, Y.; Huang, D.; et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016, 7, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Vollmer, M.K.; Kelly, M.G.; Fernandez, V.M.; Fernandez, T.G.; Kim, H.; Doré, S. Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury. Mol. Neurobiol. 2020, 57, 105–117. [Google Scholar] [CrossRef]
- Gendy, A.; Soubh, A.; Al-Mokaddem, A.; Kotb El-Sayed, M. Dimethyl Fumarate Protects against Intestinal Ischemia/Reperfusion Lesion: Participation of Nrf2/HO-1, GSK-3β and Wnt/β-Catenin Pathway. Biomed. Pharmacother. 2021, 134, 111130. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Hartsock, M.J.; Xu, Z.; He, M.; Duh, E.J. Monomethyl Fumarate Promotes Nrf2-Dependent Neuroprotection in Retinal Ischemia-Reperfusion. J. Neuroinflammation 2015, 12, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, B.; Cao, Q.; Wu, L.; Yang, G. Hydrogen Sulfide Mediates the Anti-Survival Effect of Sulforaphane on Human Prostate Cancer Cells. Toxicol. Appl. Pharmacol. 2011, 257, 420–428. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Sellke, F.W. The Effects of Therapeutic Sulfide on Myocardial Apoptosis in Response to Ischemia—Reperfusion Injury. Eur. J. Cardiothorac. Surg. 2008, 33, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide and Ischemia-Reperfusion Injury. Pharmacol. Res. 2010, 62, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic Activities of Sulforaphane and Structurally Related Synthetic Norbornyl Isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective Effect of Sulforaphane against Oxidative Stress: Recent Advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane—Role in Aging and Neurodegeneration. GeroScience 2019, 41, 655–670. [Google Scholar] [CrossRef]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Gao, S.; Lee, G.-H.; Kim, D.S.; Park, B.-H.; Chae, S.W.; Chae, H.-J.; Kim, S.H. Sulforaphane Protects Ischemic Injury of Hearts through Antioxidant Pathway and Mitochondrial KATP Channels. Pharmacol. Res. 2010, 61, 342–348. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Rada, P.; Cuadrado, A.; López, M.G.; García, A.G.; León, R. Melatonin-Sulforaphane Hybrid ITH12674 Induces Neuroprotection in Oxidative Stress Conditions by a “drug-Prodrug” Mechanism of Action. Br. J. Pharmacol. 2015, 172, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Cekauskas, A.; Bruns, H.; Manikas, M.; Herr, I.; Gross, M.-L.; Zorn, M.; Jankevicius, F.; Strupas, K.; Schemmer, P. Sulforaphane Decreases Kidney Injury after Transplantation in Rats: Role of Mitochondrial Damage. Ann. Transplant. 2013, 18, 488–496. [Google Scholar] [CrossRef]
- Li, Z.; Galli, U.; Becker, L.E.; Bruns, H.; Nickkolgh, A.; Hoffmann, K.; Karck, M.; Schemmer, P. Sulforaphane Protects Hearts from Early Injury after Experimental Transplantation. Ann. Transplant. 2013, 18, 558–566. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The Emerging Role of Redox-Sensitive Nrf2–Keap1 Pathway in Diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoncini, E.; Malaguti, M.; Angeloni, C.; Motori, E.; Fabbri, D.; Hrelia, S. Cruciferous Vegetable Phytochemical Sulforaphane Affects Phase II Enzyme Expression and Activity in Rat Cardiomyocytes through Modulation of Akt Signaling Pathway. J. Food Sci. 2011, 76, H175–H181. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane Reduces Hepatic Glucose Production and Improves Glucose Control in Patients with Type 2 Diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli Sprouts Powder Could Improve Serum Triglyceride and Oxidized LDL/LDL-Cholesterol Ratio in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef]
- Liu, P.; Dong, J. Protective Effects of Carnosic Acid against Mitochondria-mediated Injury in H9c2 Cardiomyocytes Induced by Hypoxia/Reoxygenation. Exp. Ther. Med. 2017, 14, 5629–5634. [Google Scholar] [CrossRef]
- Yoshida, H.; Mimura, J.; Imaizumi, T.; Matsumiya, T.; Ishikawa, A.; Metoki, N.; Tanji, K.; Ota, K.; Hayakari, R.; Kosaka, K.; et al. Edaravone and Carnosic Acid Synergistically Enhance the Expression of Nerve Growth Factor in Human Astrocytes under Hypoxia/Reoxygenation. Neurosci. Res. 2011, 69, 291–298. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective Effect of the Antioxidant Curcumin: Recent Findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Ghoneim, A.I.; Abdel-naim, A.B.; Khalifa, A.E.; El-denshary, E.S. Protective Effects of Curcumin against Ischaemia/Reperfusion Insult in Rat Forebrain. Pharmacol. Res. 2002, 46, 273–279. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Sharma, S.S. Neuroprotective Effect of Curcumin in Middle Cerebral Artery Occlusion Induced Focal Cerebral Ischemia in Rats. Life Sci. 2004, 74, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, Y.; Zheng, W.; Lu, Y.; Feng, G.; Yu, S. Neuroprotective Effect of Curcumin on Transient Focal Cerebral Ischemia in Rats. Brain Res. 2008, 1229, 224–232. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective Mechanisms of Curcumin against Cerebral Ischemia-Induced Neuronal Apoptosis and Behavioral Deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qiao, Z.; Xu, Y. Protective Effect of Curcumin against Myocardium Injury in Ischemia Reperfusion Rats. Pharm. Biol. 2017, 55, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Na, L.-X.; Li, Y.; Pan, H.-Z.; Zhou, X.-L.; Sun, D.-J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids Modify Lipid Profile in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of Atherogenic Risk in Patients with Type 2 Diabetes by Curcuminoid Extract: A Randomized Controlled Trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano Curcumin Supplementation Reduced the Severity of Diabetic Sensorimotor Polyneuropathy in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo- Controlled Clinical Trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, H.; Ding, K.; Zhang, L.; Wang, C.; Li, T.; Wei, W.; Lu, X. Luteolin Provides Neuroprotection in Models of Traumatic Brain Injury via the Nrf2-ARE Pathway. Free Radic. Biol. Med. 2014, 71, 186–195. [Google Scholar] [CrossRef]
- Yang, D.; Tan, X.; Lv, Z.; Liu, B.; Baiyun, R.; Lu, J.; Zhang, Z. Regulation of Sirt1/Nrf2/TNF-α Signaling Pathway by Luteolin Is Critical to Attenuate Acute Mercuric Chloride Exposure Induced Hepatotoxicity. Sci. Rep. 2016, 6, 37157. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wu, M.-J.; Liu, I.Y.-C.; Su, J.-D.; Yen, J.-H. Neurotrophic and Cytoprotective Action of Luteolin in PC12 Cells through ERK-Dependent Induction of Nrf2-Driven HO-1 Expression. J. Agric. Food Chem. 2010, 58, 4477–4486. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Samukawa, Y.; Jiang, S.; Yamashita, Y.; Ashida, H. A Physiological Concentration of Luteolin Induces Phase II Drug-Metabolizing Enzymes through the ERK1/2 Signaling Pathway in HepG2 Cells. Arch. Biochem. Biophys. 2019, 663, 151–159. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, e803971. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhu, M.; Zhang, Q.; Wang, X.; Wang, Y.; Zhang, J.; Li, J.; Yang, L.; Liu, J.; et al. Resveratrol Attenuates Myocardial Ischemia/Reperfusion Injury through up-Regulation of Vascular Endothelial Growth Factor B. Free Radic. Biol. Med. 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Cheng, A.-S.; Cheng, Y.-H.; Chiou, C.-H.; Chang, T.-L. Resveratrol Upregulates Nrf2 Expression to Attenuate Methylglyoxal-Induced Insulin Resistance in Hep G2 Cells. J. Agric. Food Chem. 2012, 60, 9180–9187. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Jang, J.-H.; Li, M.-H.; Surh, Y.-J. Resveratrol Upregulates Heme Oxygenase-1 Expression via Activation of NF-E2-Related Factor 2 in PC12 Cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Song, W.; Wang, Z.; Wang, Z.; Jin, X.; Xu, J.; Bai, L.; Li, Y.; Cui, J.; Cai, L. Resveratrol Attenuates Testicular Apoptosis in Type 1 Diabetic Mice: Role of Akt-Mediated Nrf2 Activation and P62-Dependent Keap1 Degradation. Redox. Biol. 2018, 14, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-Β1-42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-ΚB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a Grape Extract Supplement Containing Resveratrol Decreases Oxidized LDL and ApoB in Patients Undergoing Primary Prevention of Cardiovascular Disease: A Triple-Blind, 6-Month Follow-up, Placebo-Controlled, Randomized Trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Cheng, Z.; Quan, X.; Xie, Z.; Zhang, L.; Ding, Z. Dimethyl Fumarate Protects Cardiomyocytes against Oxygen-Glucose Deprivation/Reperfusion (OGD/R)-Induced Inflammatory Response and Damages via Inhibition of Egr-1. Int. Immunopharmacol. 2020, 86, 106733. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [Green Version]
| Compound | Administration and Dose | Model | Outcome | Reference |
|---|---|---|---|---|
| Hydrogen sulfide | - NaHS intracardiac injection (100 μg/kg) at reperfusion and i.v. for 7 days | - Cardiac-specific transgenic mice overexpressing CGL with LCA occlusion | - Decreased oxidative stress - Attenuation of mitochondrial dysfunction and cardiac injury | [156] |
| Hydrogen sulfide | - NaHS i.v. injection (100 μg/kg) 24 h before IR | - Cardiac-specific transgenic mice overexpressing CGL and Nrf2-KO mice with LCA occlusion | - Antioxidant, antiapoptotic signaling - Nrf2 nuclear accumulation - Strong cardioprotection | [157] |
| Hydrogen sulfide | - Inhalation (air mixed with H2S at 40 ppm for 8 h) for 7 days before IR | - MCAO in Nrf2-KO mice | - Prevention of abnormal neurological function, inflammation and oxidative injury | [158] |
| Hydrogen sulfide | - NaHS i.p. injection (50 μmol/kg) before IR | - Renal IR model in Nrf2-KO mice | - Alleviation of inflammatory stress, cell apoptosis and renal injury | [159] |
| Sulforaphane | - Single injection in the left ventricle cavity (500 μg/kg) before IR | - Proximal LCA occlusion in rats | - Decreased oxidative stress and inflammation - Protective response in hearts | [160] |
| Sulforaphane | - Single i.p. injection (5 mg/kg) 30 min before hypoxia-ischemia | - Neonatal rat brain hypoxia-ischemia model | - Decreased abundance of apoptotic cells and cytotoxic oxygen radicals - Reduction of infarct volume | [161] |
| Sulforaphane | - Single i.p. injection (5 mg/kg) 1 h before IR | - CCA and MCAO in rats | - Improves redox-sensitive defenses in the brain - Limits the infarct volume and neurological deficits | [148,162] |
| Sulforaphane | - Single i.p. injection (5 mg/kg) 1 h before IR - In culture medium (2.5 μM) for 1–4 h | - MCAO in rats - bEnd.3 murine cells | - Increase Nrf2 nuclear accumulation before the infarct - Protects the brain against oxidative damage | [163] |
| Sulforaphane | - In culture medium (2.5 μM) for 24 h before H/R | - Hyperoxic and normoxic preconditioning and then H/R in bEnd.3 murine cells with Nrf2 silenced by siRNA | - Protection against the generation of ROS | [164] |
| Sulforaphane | - In culture medium (1-20 μM) for 12 h before H/R - Single i.v. injection (500 μg/kg) 24 h before IR | - H/R in HK2 human kidney cells- Renal IR rat model | - Cytoprotection against H/R toxicity - Reduction of renal dysfunction and injury | [165] |
| Carnosic acid | - Oral (50 mg/kg) for 5 days | - ISO-induced myocardial injury in rats | - Anti-inflammatory, antioxidant and antiapoptotic effect - Reduction in myocardial injury | [166] |
| Carnosic acid | - Oral (50 mg/kg) for 12 days | - ISO-induced myocardial stress in mice | - Reduction in oxidative stress, apoptotic status - Abolition of ISO-induced myocardial stress | [167] |
| Carnosic acid | - Single i.p. injection (1 mg/kg) before IR - In culture medium (10 μM) for 20 h | - MCAO/reperfusion in mice - PC12h cells with Nrf2 dominant-negative constructs | - Induction of phase II enzymes - Neuroprotection | [168] |
| Curcumin | - In culture medium (10 μM) for 24 h before OGD | - OGD model in rat cortical neurons | - Protection of neurons against cell damage - Activation of Nrf2/ARE | [169] |
| Curcumin | - Single i.p. injection (300 mg/kg) 30 min after ischemia | - MCAO/reperfusion in rats | - Antioxidation, anti-inflammatory and antiapoptotic - Reduction in brain edema and neurological dysfunction | [170] |
| Curcumin | - Single i.p. injection (50, 100 mg/kg) 15 min after ischemia | - MCAO in rats (permanent focal ischemia) | - Decreased infarct volume and improved brain edema | [171] |
| Curcumin | - Single i.p. injection (300 mg/kg) 1 h after IR - In culture medium (2.5–25 μM) for 24 h after OGD | - MCAO/reperfusion in rats - OGD/reoxygenation model in rat cortical neurons | - Reduction in infarct size and oxidative stress levels - Improved cell survival | [172] |
| Curcumin | - Oral (400 mg/kg) for 14 days before IR | - Hepatic IR in a rat model with vascular clamping | - Attenuation of inflammatory response - Improvement in hepatocyte proliferation and liver protection | [173] |
| Luteolin | - Intragastrical (100 mg/kg/day) for 2 consecutive weeks | - Isolated perfused rat heart (Langendorff) in a diabetic rat model | - Attenuation of cardiac injury, improved cardiac function and myocardial viability - Activation of Nrf2 antioxidative functions | [174,175] |
| Resveratrol | - Single i.v. injection (100 μmol/L) 5 min before reperfusion | - LAD coronary artery occlusion in rats | - Antioxidant and anti-inflammatory effects - Reduction in infarct area and improvement in cardiac function | [176] |
| Resveratrol | - Single intragastrical administration (0.23 μg/kg) 30 min before IR | - Renal IR in a rat model with vascular clamping | - Inhibition of inflammatory response - Reduction in oxidative stress and apoptosis - Renal protection | [177] |
| Curcumin analogue 14p | - Oral (10, 100 mg/kg) for 7 days before IR | - LAD coronary artery occlusion in mice model | - Reduction in oxidative stress and myocardial apoptosis- Decreased infarct size | [178] |
| Dimethyl fumarate | - Oral (15 mg/kg) twice daily for 5 days | - IR model in perfused hearts from Fh1 KO mice - Coronary artery ligation model in Nrf2-KO mice | - Increase of ARE gene expression - Reduction in myocardial infarct size | [179] |
| Dimethyl fumarate | - In culture medium (5–40 μM) for 24 h before OGD | - H9c2 rat cells cultured in an anaerobic chamber with glucose-free DMEM then in control conditions | - Reduction in ROS production, improvement in cellular viability and antiapoptotic effect | [180] |
| Dimethyl fumarate | -Oral (30, 45 mg/kg) twice daily for 7 days | - MCAO/reperfusion model in Nrf2-KO mice | - Reduction in neurological deficits - Decreased infarct volume, brain edema and cell death | [181] |
| Dimethyl fumarate | - Oral (100 mg/kg) for 7 days before hypoxia-ischemia | - Cerebral hypoxia-ischemia mouse model in Nrf2-KO mice | - Reduction in infarct size, brain edema and hippocampal neuronal degeneration - Activation of Nrf2 pathway | [182] |
| Dimethyl fumarate | - Oral (25 mg/kg) for 14 days before IR | - Hepatic IR in a rat model with vascular clamping | - Induction of antioxidant enzyme expression via Nrf2 - Liver protection | [173] |
| Dimethyl fumarate | - Oral (15, 25 mg/kg) for 14 days before IR | - Superior mesenteric artery occlusion/reperfusion rat model | - Reduction in oxidative stress and inflammatory response - Intact mucosa | [183] |
| Monomethyl fumarate | - Single i.p. injection (50 mg/kg) 2 days before IR and daily after IR | - Retinal IR with intraocular pressure increases and restoration model in Nrf2-KO mice | - Increase of Nrf2-regulated antioxidative gene expression - Inhibition of inflammatory gene expression - Decreased neuronal cell loss and improved retinal function | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, A.; Cadenas, S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11939. https://doi.org/10.3390/ijms222111939
Mata A, Cadenas S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia–Reperfusion Injury. International Journal of Molecular Sciences. 2021; 22(21):11939. https://doi.org/10.3390/ijms222111939
Chicago/Turabian StyleMata, Ana, and Susana Cadenas. 2021. "The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia–Reperfusion Injury" International Journal of Molecular Sciences 22, no. 21: 11939. https://doi.org/10.3390/ijms222111939
APA StyleMata, A., & Cadenas, S. (2021). The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia–Reperfusion Injury. International Journal of Molecular Sciences, 22(21), 11939. https://doi.org/10.3390/ijms222111939





