Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells
Abstract
1. Introduction
2. Results
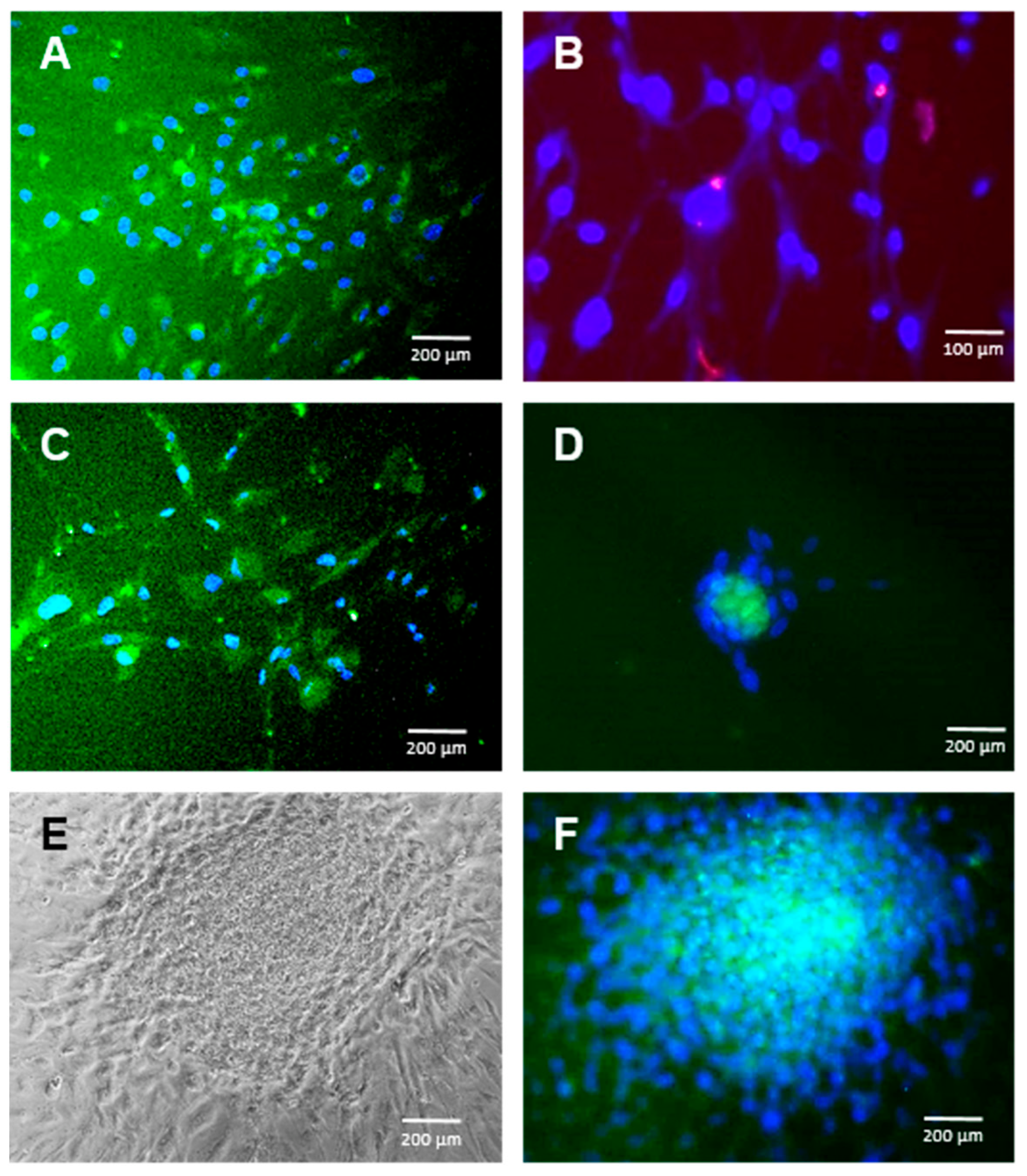
2.1. hADSC Differentiation into NCCs
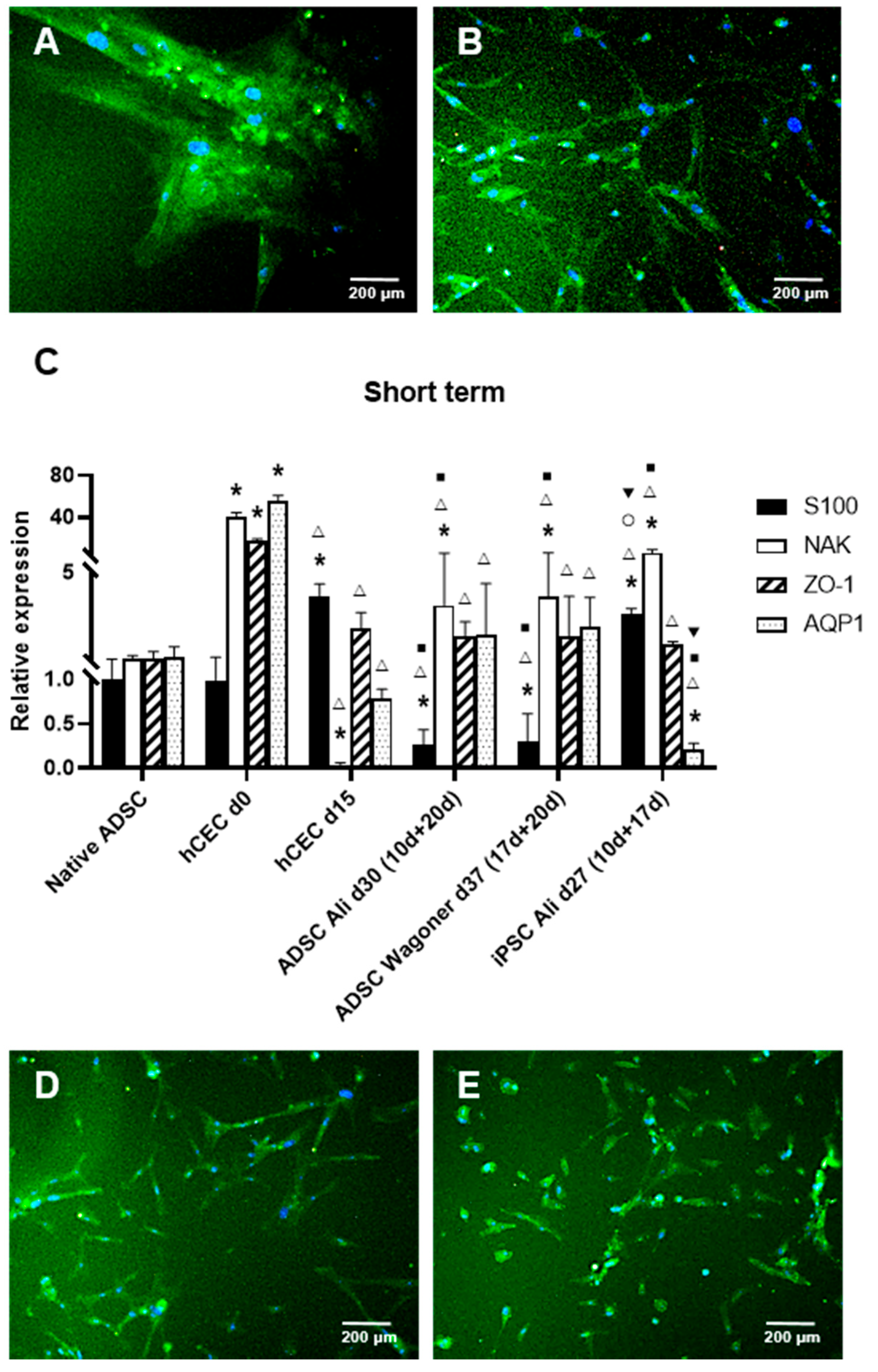
2.2. hADSC Differentiation into CEC Cells
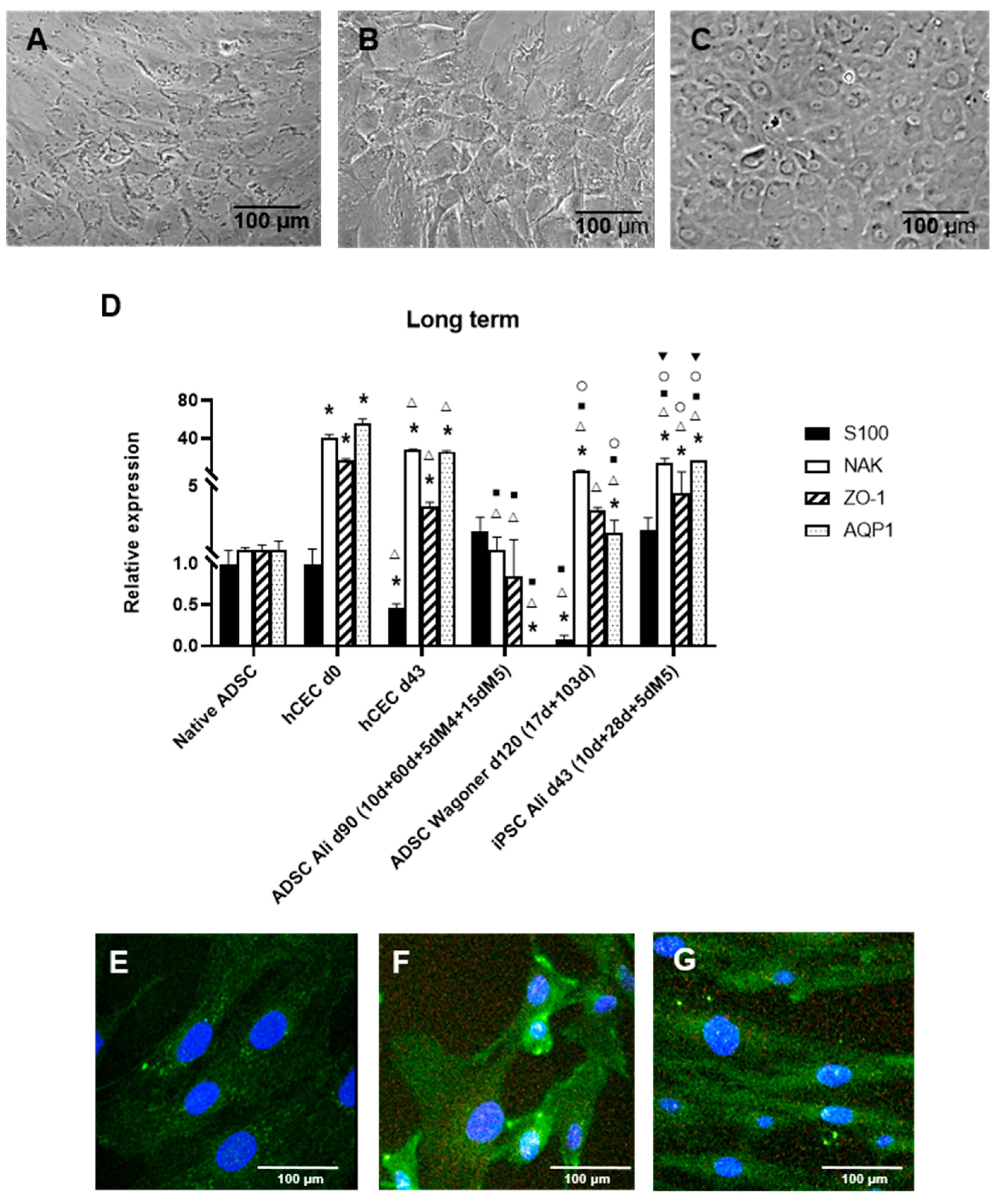
2.3. Long-Term Culture and CEC Characterization
3. Discussion
4. Materials and Methods
4.1. Isolation of hADSCs
4.2. Differentiation Protocols
4.3. Quantitative Real Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
4.4. Immunocytochemistry
4.5. Statistical Analysis
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Resnikoff, S. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull. World Health Organ. 2008, 86, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Dunn, S.P.; Gal, R.L.; Kollman, C.; Raghinaru, D.; Dontchev, M.; Blanton, C.L.; Holland, E.J.; Lass, J.H.; Kenyon, K.R.; Mannis, M.J.; et al. Corneal Graft Rejection 10 Years after Penetrating Keratoplasty in the Cornea Donor Study. Cornea 2014, 33, 1003–1009. [Google Scholar] [CrossRef]
- Coster, D.J.; Williams, K.A. The Australian Corneal Graft Registry (ACGR). Klin. Mon. für Augenheilkd. 1994, 205, 271–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mimura, T.; Yamagami, S.; Amano, S. Corneal endothelial regeneration and tissue engineering. Prog. Retin. Eye Res. 2013, 35, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Peh, G.; Mehta, J.S. Translational issues for human corneal endothelial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 11, 2425–2442. [Google Scholar] [CrossRef]
- Møller-Pedersen, T.; Hartmann, U.; Ehlers, N.; Engelmann, K. Evaluation of potential organ culture media for eye banking using a human corneal endothelial cell growth assay. Graefe Arch. Clin. Exp. Ophthalmol. 2001, 239, 778–782. [Google Scholar] [CrossRef]
- Bednarz, J.; Doubilei, V.; Wollnik, P.C.M.; Engelmann, K. Effect of three different media on serum free culture of donor corneas and isolated human corneal endothelial cells. Br. J. Ophthalmol. 2001, 85, 1416–1420. [Google Scholar] [CrossRef]
- Jäckel, T.; Knels, L.; Valtink, M.; Funk, R.H.W.; Engelmann, K. Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br. J. Ophthalmol. 2011, 95, 123–130. [Google Scholar] [CrossRef]
- Peh, G.; Toh, K.-P.; Wu, F.-Y.; Tan, D.T.; Mehta, J.S. Cultivation of Human Corneal Endothelial Cells Isolated from Paired Donor Corneas. PLoS ONE 2011, 6, e28310. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.; Chng, Z.; Ang, H.-P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.-Y.; George, B.L.; Toh, K.-P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of Human Corneal Endothelial Cells: A Novel Dual Media Approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Ang, H.-P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.-Y.; Lin, S.-J.; Bhogal, M.; Liu, Y.-C.; Tan, D.T.; et al. Regulatory Compliant Tissue-Engineered Human Corneal Endothelial Grafts Restore Corneal Function of Rabbits with Bullous Keratopathy. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.-I.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef]
- Wongvisavavit, R.; Parekh, M.; Ahmad, S.; Daniels, J.T. Challenges in corneal endothelial cell culture. Regen. Med. 2021, 16, 871–891. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Moratilla, A.; Fuentes-Julián, S.; Aparicio, V.; Martin, M.C.; Peh, G.; Mehta, J.S.; Adnan, K.; Porrua, L.; Pérez-Sarriegui, A.; et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS ONE 2019, 14, e0225480. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Forest, F.; Bernard, A.; Gauthier, A.-S.; Montard, R.; Peoc’h, M.; Jumelle, C.; Courrier, E.; Perrache, C.; Gain, P.; et al. Cutting and Decellularization of Multiple Corneal Stromal Lamellae for the Bioengineering of Endothelial Grafts. Investig. Opthalmology Vis. Sci. 2016, 57, 6639–6651. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.; Jaime, G.R.L.; Barrientos, C.A.R.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef]
- Zhang, K.; Pang, K.; Wu, X. Isolation and Transplantation of Corneal Endothelial Cell–Like Cells Derived from In-Vitro-Differentiated Human Embryonic Stem Cells. Stem Cells Dev. 2014, 23, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.L.; Kunzevitzky, N.; Chiswell, B.P.; Xia, X.; Goldberg, J.L.; Lanza, R. Efficient Generation of Human Embryonic Stem Cell-Derived Corneal Endothelial Cells by Directed Differentiation. PLoS ONE 2015, 10, e0145266. [Google Scholar] [CrossRef]
- Zhao, J.J.; Afshari, N.A. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Investig. Opthalmology Vis. Sci. 2016, 57, 6878–6884. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.D.; Bohrer, L.R.; Aldrich, B.T.; Greiner, M.; Mullins, R.; Worthington, K.; Tucker, B.; Wiley, L.A. Feeder-free differentiation of cells exhibiting characteristics of corneal endothelium from human induced pluripotent stem cells. Biol. Open 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, S.Y.; Vasanth, S.; Ahmed, M.R.; Chen, R.; Na, C.H.; Thomson, J.J.; Qiu, C.; Gottsch, J.D.; Riazuddin, S.A. Generation and Proteome Profiling of PBMC-Originated, iPSC-Derived Corneal Endothelial Cells. Investig. Opthalmology Vis. Sci. 2018, 59, 2437–2444. [Google Scholar] [CrossRef]
- Jang, S.; Cho, H.-H.; Cho, Y.-B.; Park, J.-S.; Jeong, H.-S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef]
- Zavan, B.; Michelotto, L.; Lancerotto, L.; Della Puppa, A.; D’Avella, D.; Abatangelo, G.; Vindigni, V.; Cortivo, R. Neural potential of a stem cell population in the adipose and cutaneous tissues. Neurol. Res. 2010, 32, 47–54. [Google Scholar] [CrossRef]
- Hatou, S.; Shimmura, S. Review: Corneal endothelial cell derivation methods from ES/iPS cells. Inflamm. Regen. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Lee, H.T.; Kay, E.P. FGF-2 induced reorganization and disruption of actin cytoskeleton through PI 3-kinase, Rho, and Cdc42 in corneal endothelial cells. Mol. Vis. 2003, 9, 624–634. [Google Scholar]
- Sabater, A.L.; Andreu, E.J.; Guzmán, M.G.; López, T.; Abizanda, G.; Perez, V.L.; Moreno-Montañés, J.; Prósper, F. Combined PI3K/Akt and Smad2 Activation Promotes Corneal Endothelial Cell Proliferation. Investig. Opthalmology Vis. Sci. 2017, 58, 745–754. [Google Scholar] [CrossRef]
- Chen, P.; Chen, J.-Z.; Shao, C.-Y.; Li, C.-Y.; Zhang, Y.-D.; Lu, W.-J.; Fu, Y.; Gu, P.; Fan, X. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp. Ther. Med. 2015, 9, 351–360. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2003, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-H.; Zhao, N.; Feng, X.; Jie, Y.; Jin, Z.-B. Conversion of mouse embryonic fibroblasts into neural crest cells and functional corneal endothelia by defined small molecules. Sci. Adv. 2021, 7, eabg5749. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Yue, B.Y.; Niedra, R.; Baum, J.L. Effects of ascorbic acid on cultured rabbit corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1471–1476. [Google Scholar]
- Penhallow, R.C.; Brown-Mason, A.; Woodworth, R.C. Comparative studies of the binding and growth-supportive ability of mammalian transferrins in human cells. J. Cell. Physiol. 1986, 128, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Arnalich-Montiel, F.; Pastor, S.; Blázquez-Martínez, A.; Fernandez-Delgado, J.; Nistal, M.; Alio, J.L.; De Miguel, M.P. Adipose-Derived Stem Cells Are a Source for Cell Therapy of the Corneal Stroma. Stem Cells 2008, 26, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Huerta, V.; Flores-Soto, M.E.; Sigala, M.E.M.; de León, J.C.B.; Lemus-Varela, M.D.L.; Torres-Mendoza, B.M.; Beas-Zárate, C. Proinflammatory Cytokines, Enolase and S-100 as Early Biochemical Indicators of Hypoxic-Ischemic Encephalopathy Following Perinatal Asphyxia in Newborns. Pediatr. Neonatol. 2017, 58, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.A.; Johar, K.; Gajjar, D.U.; Ganatra, A.D.; Kayastha, F.B.; Pal, A.K.; Patel, A.R.; Rajkumar, S.; Vasavada, A.R. Cx43, ZO-1, alpha-catenin and beta-catenin in cataractous lens epithelial cells. J. Biosci. 2012, 37, 979–987. [Google Scholar] [CrossRef]
- López-Iglesias, P.; Blázquez-Martínez, A.; Fernández-Delgado, J.; Regadera, J.; Nistal, M.; De Miguel, M.P. Fate of human AMSCs over the short and long term after subcutaneous injection in immunodeficient mice. World J. Stem Cells 2011, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marta, C.-M.; Adrian, M.; Jorge, F.-D.; Francisco, A.-M.; De Miguel, M.P. Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11982. https://doi.org/10.3390/ijms222111982
Marta C-M, Adrian M, Jorge F-D, Francisco A-M, De Miguel MP. Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(21):11982. https://doi.org/10.3390/ijms222111982
Chicago/Turabian StyleMarta, Cadenas-Martin, Moratilla Adrian, Fernández-Delgado Jorge, Arnalich-Montiel Francisco, and Maria P. De Miguel. 2021. "Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells" International Journal of Molecular Sciences 22, no. 21: 11982. https://doi.org/10.3390/ijms222111982
APA StyleMarta, C.-M., Adrian, M., Jorge, F.-D., Francisco, A.-M., & De Miguel, M. P. (2021). Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells. International Journal of Molecular Sciences, 22(21), 11982. https://doi.org/10.3390/ijms222111982




