MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds
Abstract
:1. Introduction
2. Results
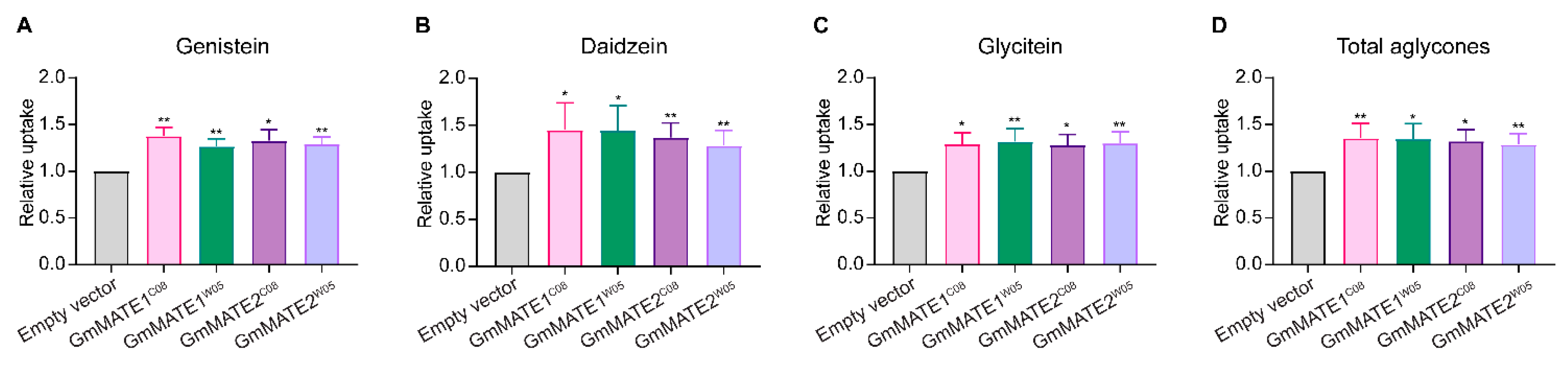
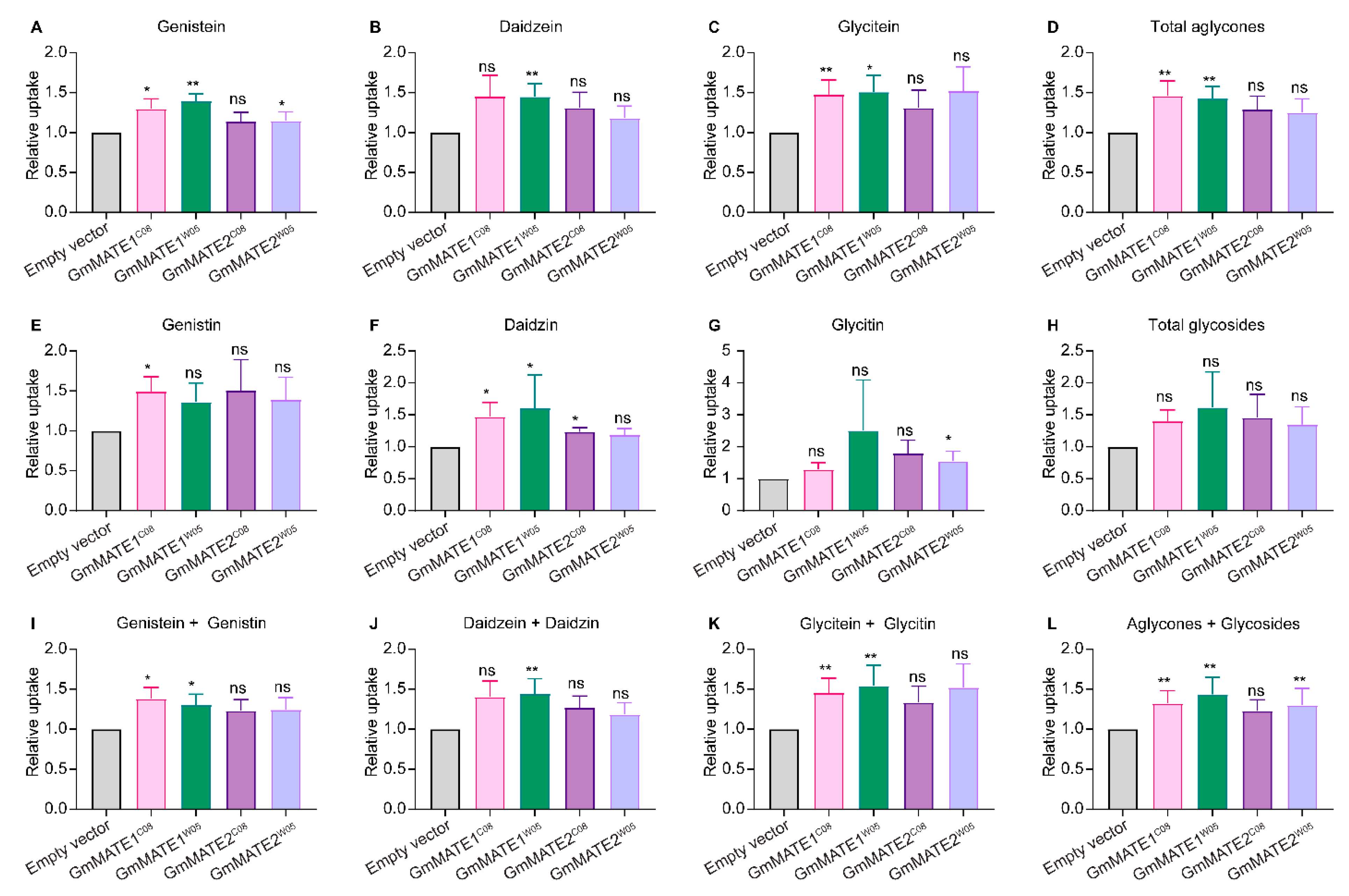
2.1. GmMATE1 and GmMATE2 Are Isoflavone Transporters in Soybeans
2.2. Both GmMATE1 and GmMATE2 Were Localized in the Vacuolar Membrane
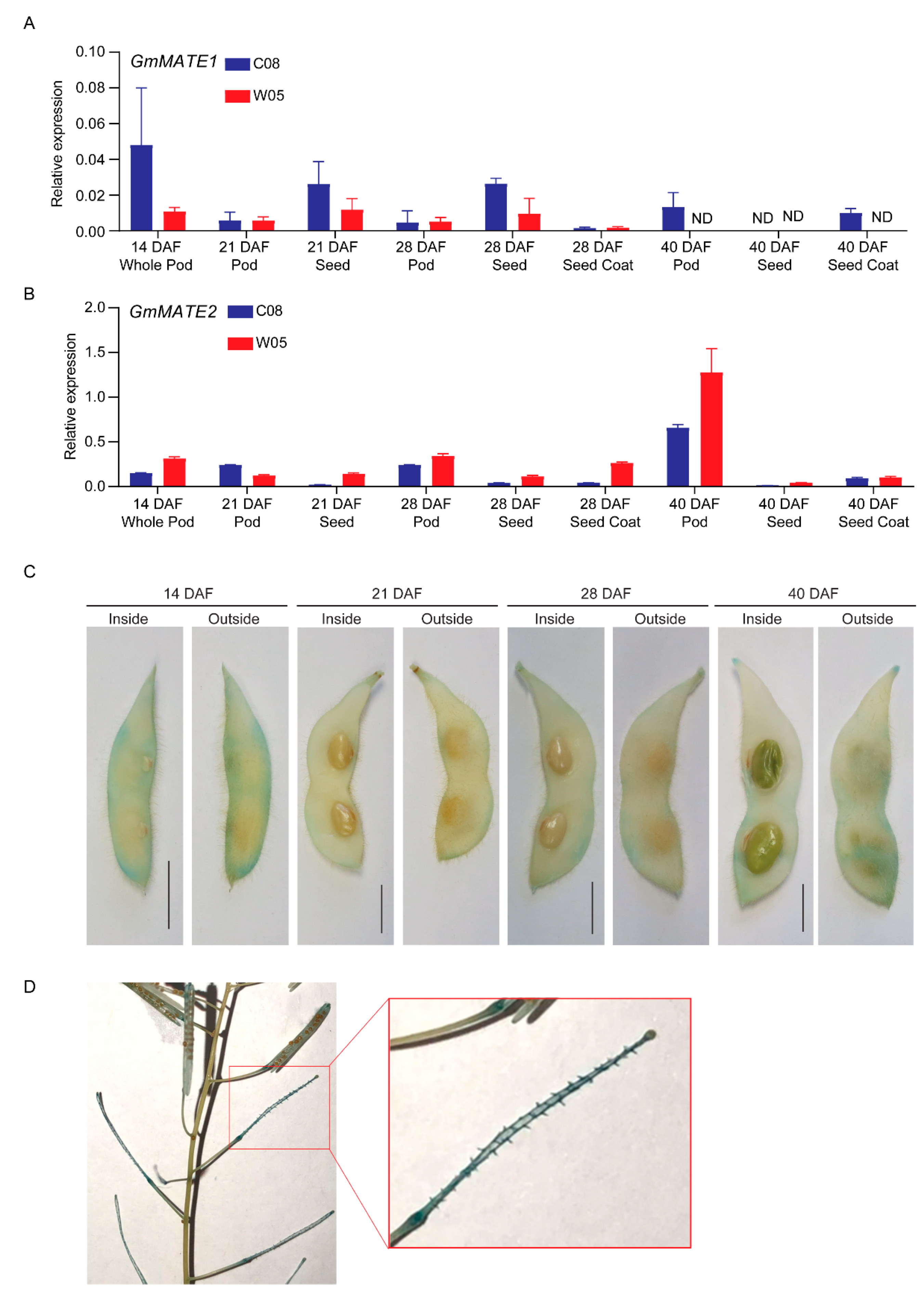
2.3. GmMATE1 and GmMATE2 Were Expressed in Developing Pods, Seeds, and the Seed Coat
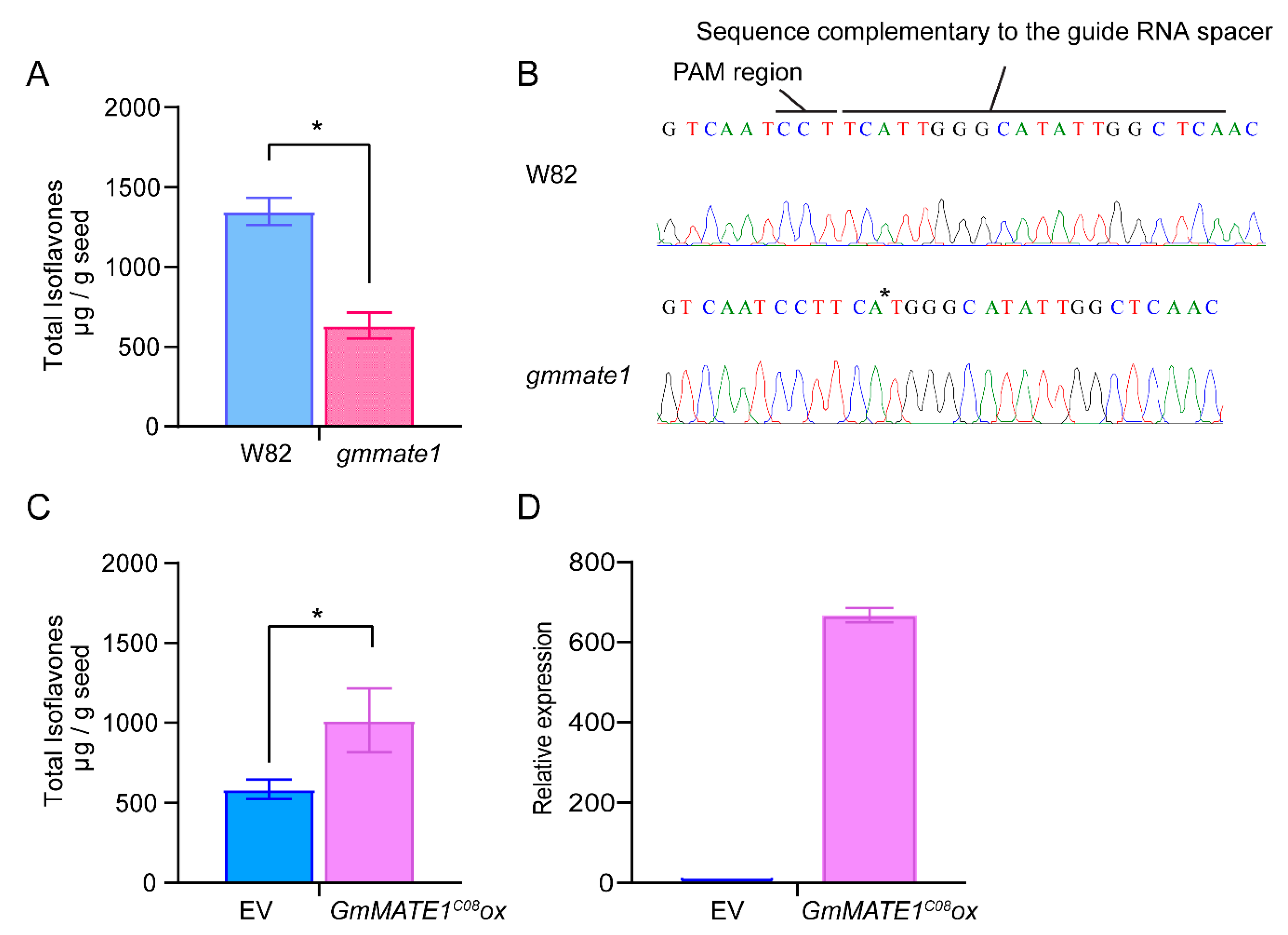
2.4. Manipulation of GmMATE1 in Soybean Significantly Altered the Isoflavone Contents in Seeds
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Coning, Vector Construction and Transformation
4.3. Yeast Uptake Assay
4.4. Extraction of Seed Isoflavones
4.5. Subcellular Localization of GmMATE1 and GmMATE2 in Tobacco BrightYellow-2 (BY-2) Cells
4.6. Expression of GmMATE1 and GmMATE2 in Pod, Seed, and Seed Coat
4.7. β-Glucuronidase (GUS) Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.H.; Ziegler, R.G.; Nomura, A.M.Y.; West, D.W.; Kolonel, L.N.; Horn-Ross, P.L.; Hoover, R.N.; Pike, M.C. Soy intake and risk of breast cancer in Asians and Asian Americans. Am. J. Clin. Nutr. 1998, 68, 1437S–1443S. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.; Thorpe, J.; Ambrosio, L.; Santin, M. The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. J. Nutr. 2006, 136, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadaishi, M.; Nishide, Y.; Tousen, Y.; Kruger, M.C.; Ishimi, Y. Cooperative effects of soy isoflavones and carotenoids on osteoclast formation. J. Clin. Biochem. Nutr. 2014, 54, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, N.S.; Foss, T.R.; Kelly, J.W. Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis. Proc. Natl. Acad. Sci. USA 2005, 102, 14545–14550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lira, M.A., Jr.; Nascimento, L.R.S.; Fracetto, G.G.M. Legume-rhizobia signal exchange: Promiscuity and environmental effects. Front. Microbiol. 2015, 6, 945. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Harrison, M.J.; Paiva, N.L. The isoflavonoid phytoalexin pathway: From enzymes to genes to transcription factors. Physiol. Plant. 1995, 93, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.Y.; Xiao, Z.X.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Wang, H.; Murphy, P.A. Isoflavone composition of American and Japanese soybeans in Iowa: Effects of variety, crop year, and location. J. Agric. Food Chem. 1994, 42, 1674–1677. [Google Scholar] [CrossRef]
- Jung, W.; Yu, O.; Lau, S.M.C.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, O.; Yu, D.Y. Polymorphisms of IFS1 and IFS2 gene are associated with isoflavone concentrations in soybean seeds. Plant Sci. 2008, 175, 505–512. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 2017, 58, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Wang, W.; Gai, J.; Li, Y. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genom. 2016, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Shen, J.; Gao, C.; Zhuang, X.; Wang, J.; Jiang, L. Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 2016, 9, 774–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uno, S.N.; Kamiya, M.; Morozumi, A.; Urano, Y. A green-light-emitting, spontaneously blinking fluorophore based on intramolecular spirocyclization for dual-colour super-resolution imaging. Chem. Comm. 2018, 54, 102–105. [Google Scholar] [CrossRef]
- Baião, D.D.S.; de Freitas, C.S.; Gomes, L.P.; da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from root, tubercles and grains cropped in Brazil: Chemical and nutritional characterization and their effects on human health and diseases. Nutrients 2017, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci. Rep. 2014, 4, 3964. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Fournier-Level, A.; Verriès, C.; Souquet, J.M.; Mazauric, J.P.; Klein, M.; Cheynier, V.; et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, Y.S.; Liu, H.D.; Kang, L.M.; Geng, J.M.; Gai, Y.Z.; Ding, Y.L.; Sun, H.Y.; Li, Y.D. Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS ONE 2015, 10, e0118578. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Hirata, S.; Vacha, M. Single-particle electroluminescence of CsPbBr3 perovskite nanocrystals reveals particle-selective recombination and blinking as key efficiency factors. Nat. Commun. 2019, 10, 4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Dixon, R.A. MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-Glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takanashi, K.; Shitan, N.; Yazaki, K. The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xu, S.; Li, J.; Hu, Y.; Lin, Z. Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep. 2006, 25, 705–710. [Google Scholar]
- Seo, P.J.; Park, J.; Park, M.J.; Kim, Y.S.; Kim, S.G.; Jung, J.H.; Park, C.M. A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem. J. 2012, 442, 551–561. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.S.; Rider, D.S.; Walsh, T.K.; De Vos, M.; Gordon, K.H.; Ponnala, L.; Macmil, S.L.; Roe, B.A.; Jander, G. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol. Biol. 2010, 19, 155–164. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Q.; Li, P.; Yang, L.; Sun, X.; Benedito, V.A.; Wen, J.; Chen, B.; Mysore, K.S.; Zhao, J. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J. 2017, 90, 79–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [Green Version]
- Li, M.W.; Munoz, N.B.; Wong, C.F.; Wong, F.L.; Wong, K.S.; Wong, J.W.H.; Qi, X.P.; Li, K.P.; Ng, M.S.; Lam, H.M. QTLs regulating the contents of antioxidants, phenolics, and flavonoids in soybean seeds share a common genomic region. Front. Plant Sci. 2016, 7, 854. [Google Scholar] [CrossRef]
- Ruohonen, L.; Aalto, M.K.; Keränen, S. Modifications to the ADH1 promoter of Saccharomyces cerevisiae for efficient production of heterologous proteins. J. Biotechnol. 1995, 39, 193–203. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Bolte, S.; Talbot, C.; Boutte, Y.; Catrice, O.; Read, N.D.; Satiat-Jeunemaitre, B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 2004, 214, 159–173. [Google Scholar] [CrossRef]
- Christiansen, L.C.; Dal Degan, F.; Ulvskov, P.; Borkhardt, B. Examination of the dehiscence zone in soybean pods and isolation of a dehiscence-related endopolygalacturonase gene. Plant Cell Environ. 2002, 25, 479–490. [Google Scholar] [CrossRef]
- Xie, H.; Wang, D.; Qin, Y.; Ma, A.; Fu, J.; Qin, Y.; Hu, G.; Zhao, J. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development. BMC Plant Biol. 2019, 19, 499. [Google Scholar] [CrossRef]
- Nakai, S.; Fujita, M.; Kamei, Y. Health promotion effects of soy isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Hsieh, O.A.L.; Chang, S.S. Characterization of the nonvolatile minor constituents responsible for the objectionable taste of defatted soybean flour. J. Food Sci. 1982, 47, 19–23. [Google Scholar] [CrossRef]
- Barz, W.; Welle, R. Biosynthesis and metabolism of isoflavones and pterocarpan phytoalexins in chickpea, soybean and phytopathogenic fungi. In Phenolic Metabolism in Plants; Stafford, H.A., Ibrahim, R.K., Eds.; Springer US: Boston, MA, USA, 1992; pp. 139–164. [Google Scholar]
- Bechtold, N.; Pelletier, G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. In Arabidopsis Protocols; Martinez-Zapater, J.M., Salinas, J., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 259–266. [Google Scholar]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Nemoto, Y.; Hasezawa, S. Tobacco BY-2 cell-line as the “Hela”-cell in the cell biology of higher-plants. Int. Rev. Cytol. 1992, 132, 1–30. [Google Scholar]
- Gietz, R.D.; Triggs-Raine, B.; Robbins, A.; Graham, K.C.; Woods, R.A. Identification of proteins that interact with a protein of interest: Applications of the yeast two-hybrid system. Mol. Cell Biochem. 1997, 172, 67–79. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.W.; Wong, F.L.; Luk, C.Y.; Chung, C.Y.L.; Yung, W.S.; Wang, Z.L.; Xie, M.; Song, S.K.; Chung, G.; et al. Increased copy number of gibberellin 2-oxidase 8 genes reduced trailing growth and shoot length during soybean domestication. Plant J. 2021, 107, 1739–1755. [Google Scholar] [CrossRef]
- Michno, J.M.; Wang, X.; Liu, J.; Curtin, S.J.; Kono, T.J.; Stupar, R.M. CRISPR/Cas mutagenesis of soybean and Medicago truncatula using a new web-tool and a modified Cas9 enzyme. GM Crops Food 2015, 6, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Hou, W.; Godo, I.; Wu, C.; Yu, Y.; Matityahu, I.; Hacham, Y.; Sun, S.; Han, T.; Amir, R. Soybean seeds expressing feedback-insensitive cystathionine γ-synthase exhibit a higher content of methionine. J. Exp. Bot. 2013, 64, 1917–1926. [Google Scholar] [CrossRef]
- Slatnar, A.; Jakopic, J.; Stampar, F.; Veberic, R.; Jamnik, P. The effect of bioactive compounds on in vitro and in vivo antioxidant activity of different berry juices. PLoS ONE 2012, 7, e47880. [Google Scholar] [CrossRef] [Green Version]
- Canelas, A.B.; ten Pierick, A.; Ras, C.; Seifar, R.M.; van Dam, J.C.; van Gulik, W.M.; Heijnen, J.J. Quantitative evaluation of intracellular metabolite extraction techniques for yeast metabolomics. Anal. Chem. 2009, 81, 7379–7389. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Odriozola-Serrano, I.; Bosch-Fusté, J.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Fast simultaneous determination of free and conjugated isoflavones in soy milk by UHPLC–UV. Food Chem. 2012, 135, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, C.; Nawaz, M.A.; Kurosaka, A.; Le, B.; Lee, J.D.; Son, E.; Yang, S.H.; Kurt, C.; Baloch, F.S.; Chung, G. Isoflavone profile diversity in Korean wild soybeans (Glycine soja Sieb. & Zucc.). Turk. J. Agric. For. 2018, 42, 248–261. [Google Scholar]
- Moscatelli, A.; Ciampolini, F.; Rodighiero, S.; Onelli, E.; Cresti, M.; Santo, N.; Idilli, A. Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 2007, 120, 3804–3819. [Google Scholar] [CrossRef] [Green Version]
- Yim, A.K.Y.; Wong, J.W.H.; Ku, Y.S.; Qin, H.; Chan, T.F.; Lam, H.M. Using RNA-seq data to evaluate reference genes suitable for gene expression studies in soybean. PLoS ONE 2015, 10, e0136343. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, M.-S.; Ku, Y.-S.; Yung, W.-S.; Cheng, S.-S.; Man, C.-K.; Yang, L.; Song, S.; Chung, G.; Lam, H.-M. MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds. Int. J. Mol. Sci. 2021, 22, 12017. https://doi.org/10.3390/ijms222112017
Ng M-S, Ku Y-S, Yung W-S, Cheng S-S, Man C-K, Yang L, Song S, Chung G, Lam H-M. MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds. International Journal of Molecular Sciences. 2021; 22(21):12017. https://doi.org/10.3390/ijms222112017
Chicago/Turabian StyleNg, Ming-Sin, Yee-Shan Ku, Wai-Shing Yung, Sau-Shan Cheng, Chun-Kuen Man, Liu Yang, Shikui Song, Gyuhwa Chung, and Hon-Ming Lam. 2021. "MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds" International Journal of Molecular Sciences 22, no. 21: 12017. https://doi.org/10.3390/ijms222112017






