MALDI-MS Analysis of Peptide Libraries Expands the Scope of Substrates for Farnesyltransferase
Abstract
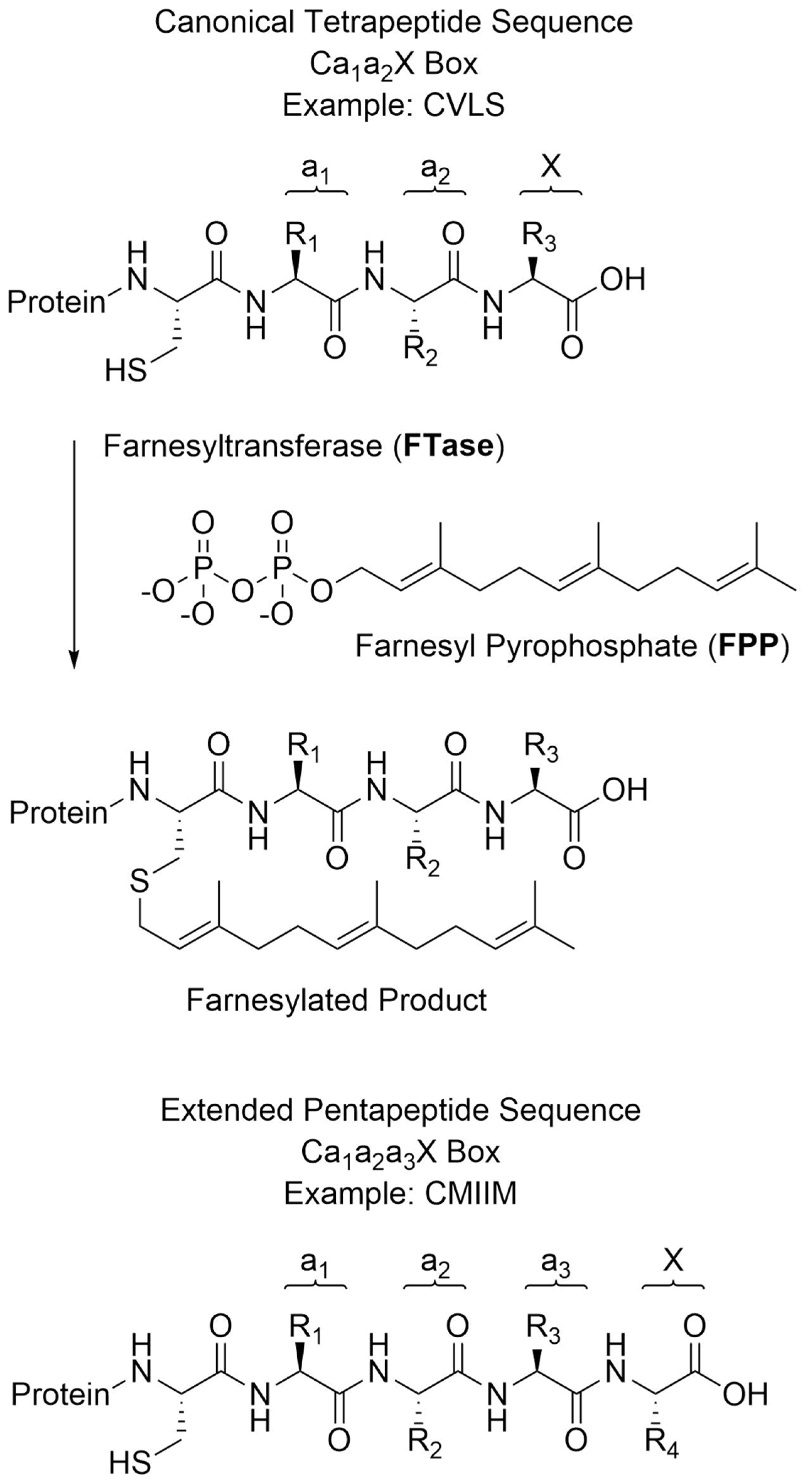
:1. Introduction
2. Results
2.1. Validation and Optimization of MALDI Method with Known Substrates
2.2. Identification of Novel Substrates from the CMIIM Motif Using MALDI Analysis
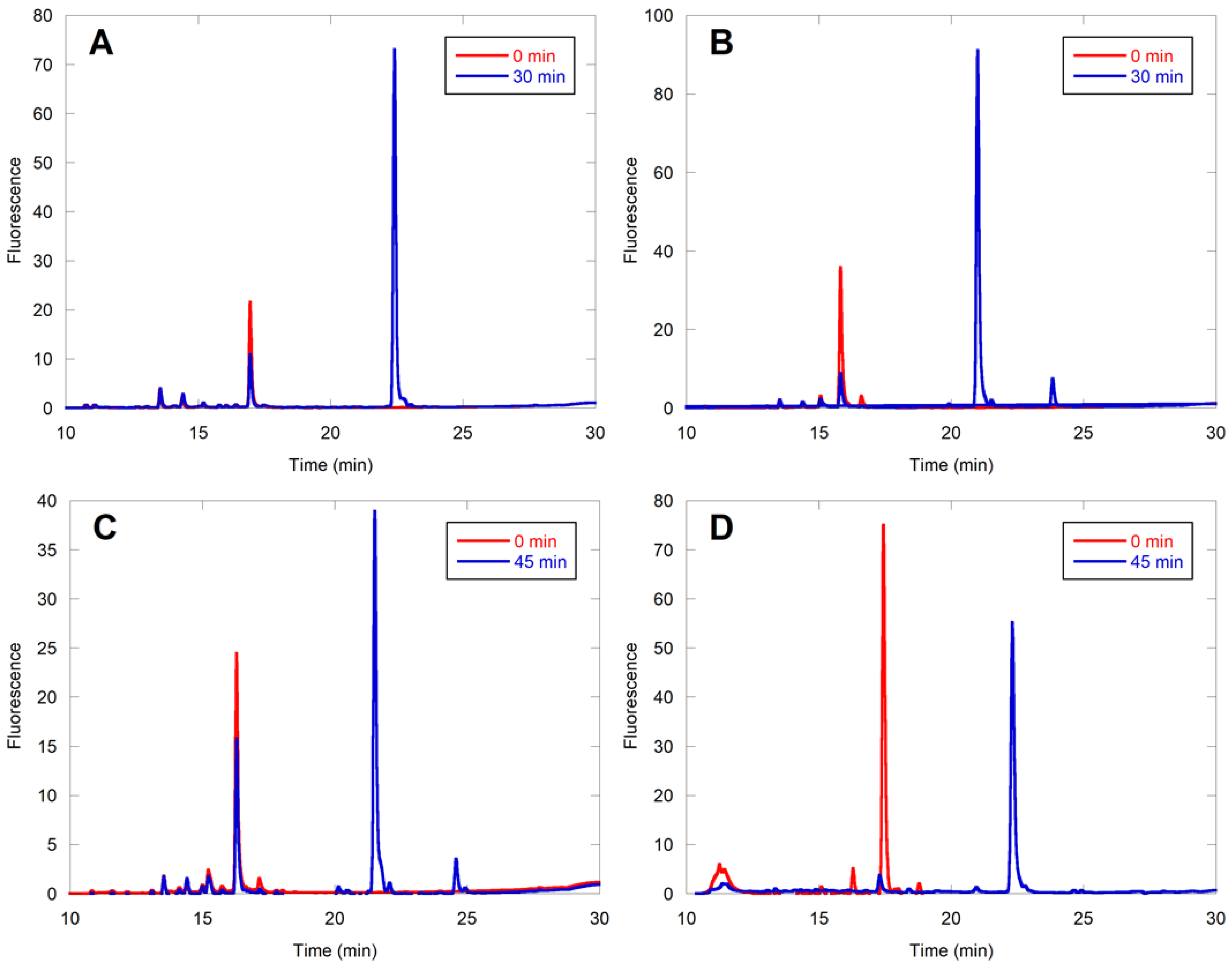
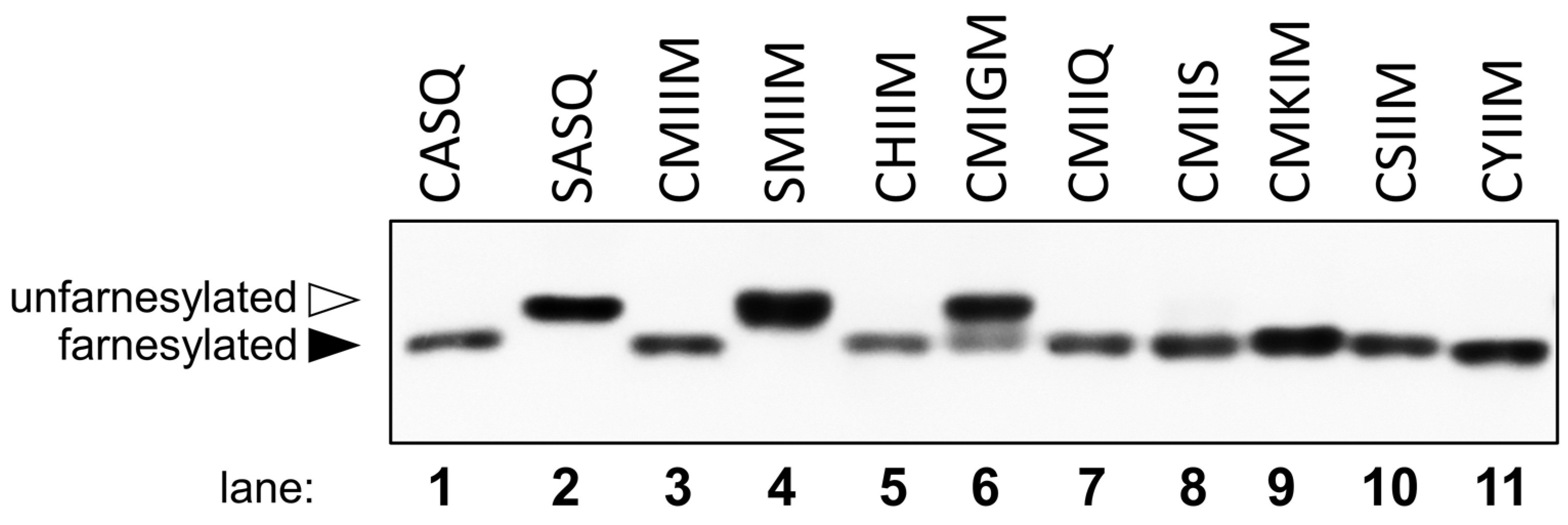
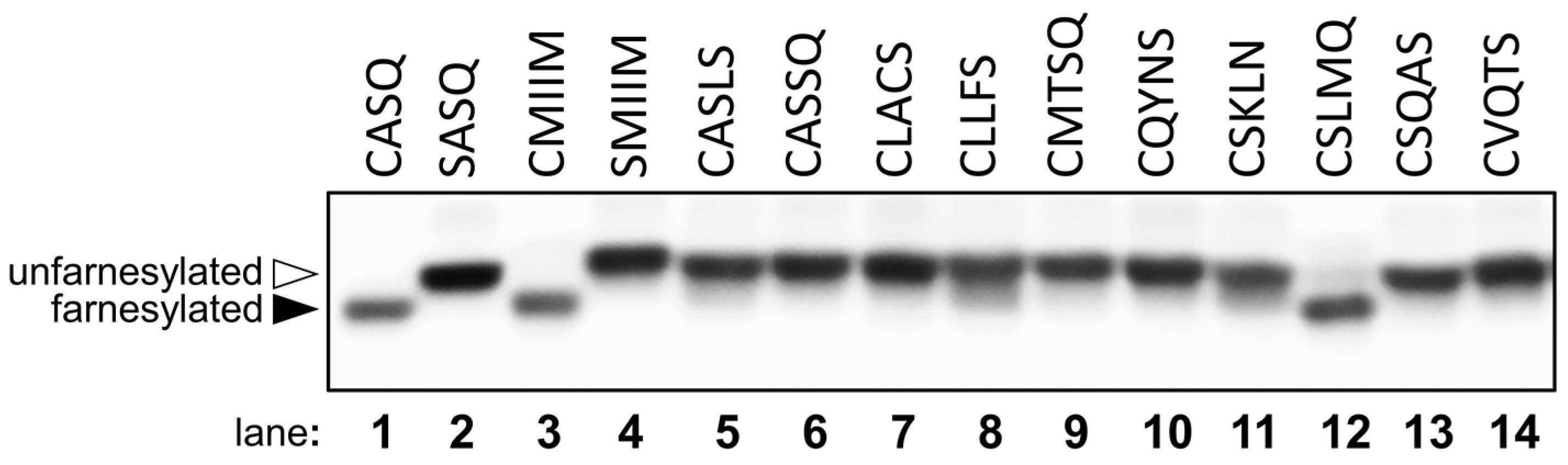
2.3. Evaluation of Individual Peptide Hits by HPLC
2.4. CaaaX Hits in the Mammalian Genome
2.5. Farnesylation of CaaaX Sequences Can Occur Efficiently in Cells
3. Discussion
4. Materials and Methods
4.1. Library Synthesis
4.2. Enzymatic Farnesylation of Peptide Libraries
4.3. MALDI-TOF MS of Farnesylated Peptide libraries
4.4. HPLC-Based Enzymatic Farnesylation Assay
4.5. Peptide Search of the Human Proteome
4.6. Yeast Strains and Plasmids
4.7. Mobility Shift Analysis of Ydj1p Farnesylation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.L.; Casey, P.J. Protein Prenylation: Molecular Mechanisms and Functional Consequences. Annu. Rev. Biochem. 1996, 65, 241–269. [Google Scholar] [CrossRef]
- Palsuledesai, C.C.; Distefano, M.D. Protein Prenylation: Enzymes, Therapeutics, and Biotechnology Applications. ACS Chem. Biol. 2015, 10, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghomashchi, F.; Zhang, X.; Liu, L.; Gelb, M.H. Binding of Prenylated and Polybasic Peptides to Membranes: Affinities and Intervesicle Exchange. Biochemistry 1995, 34, 11910–11918. [Google Scholar] [CrossRef]
- Resh, M.D. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006, 2, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Hottman, D.A.; Li, L. Protein Prenylation and Synaptic Plasticity: Implications for Alzheimer’s Disease. Mol. Neurobiol. 2014, 50, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Capell, B.C.; Erdos, M.R.; Madigan, J.P.; Fiordalisi, J.J.; Varga, R.; Conneely, K.N.; Gordon, L.B.; Der, C.J.; Cox, A.D.; Collins, F.S. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 12879–12884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suazo, K.F.; Schaber, C.; Palsuledesai, C.C.; Odom John, A.R.; Distefano, M.D. Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Bordier, B.B.; Marion, P.L.; Ohashi, K.; Kay, M.A.; Greenberg, H.B.; Casey, J.L.; Glenn, J.S. A Prenylation Inhibitor Prevents Production of Infectious Hepatitis Delta Virus Particles. J. Virol. 2002, 76, 10465–10472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaya, M.; Baranova, A.; Van Hoek, M.L. Protein prenylation: A new mode of host-pathogen interaction. Biochem. Biophys. Res. Commun. 2011, 416, 1–6. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B.; Shappell, H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children with Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, L.B.; Shappell, H.; Massaro, J.; D’Agostino, R.B.; Brazier, J.; Campbell, S.E.; Kleinman, M.E.; Kieran, M.W. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson-Gilford progeria syndrome. JAMA J. Am. Med. Assoc. 2018, 319, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Distefano, M.D. Synthesis and screening of peptide libraries with free C-termini. Curr. Top. Pept. Protein Res. 2014, 15, 1–23. [Google Scholar]
- Reiss, Y.; Stradley, S.J.; Gierasch, L.M.; Brown, M.S.; Goldstein, J.L. Sequence requirement for peptide recognition by rat brain p21(ras) protein farnesyltransferase. Proc. Natl. Acad. Sci. USA 1991, 88, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hougland, J.L.; Gangopadhyay, S.A.; Fierke, C.A. Expansion of protein farnesyltransferase specificity using “tunable” active site interactions: Development of bioengineered prenylation pathways. J. Biol. Chem. 2012, 287, 38090–38100. [Google Scholar] [CrossRef] [Green Version]
- Blanden, M.J.; Suazo, K.F.; Hildebrandt, E.R.; Hardgrove, D.S.; Patel, M.; Saunders, W.P.; Distefano, M.D.; Schmidt, W.K.; Hougland, J.L. Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome. J. Biol. Chem. 2018, 293, 2770–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.C.; Dozier, J.K.; Beese, L.S.; Distefano, M.D. Rapid analysis of protein farnesyltransferase substrate specificity using peptide libraries and isoprenoid diphosphate analogues. ACS Chem. Biol. 2014, 9, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Nadler, W.M.; Waidelich, D.; Kerner, A.; Hanke, S.; Berg, R.; Trumpp, A.; Rösli, C. MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. J. Proteome Res. 2017, 16, 1207–1215. [Google Scholar] [CrossRef]
- Sandoval, W. Matrix-assisted laser desorption/ionization time-of-flight mass analysis of peptides. Curr. Protoc. Protein Sci. 2014, 2014, 16.2.1–16.2.11. [Google Scholar] [CrossRef] [PubMed]
- Scott Youngquist, R.; Fuentes, G.R.; Lacey, M.P.; Keough, T.; Baillie, T.A. Matrix-assisted laser desorption ionization for rapid determination of the sequences of biologically active peptides isolated from support-bound combinatorial peptide libraries. Rapid Commun. Mass Spectrom. 1994, 8, 77–81. [Google Scholar] [CrossRef]
- Schlosser, G.; Pocsfalvi, G.; Huszár, E.; Malorni, A.; Hudecz, F. MALDI-TOF mass spectrometry of a combinatorial peptide library: Effect of matrix composition on signal suppression. J. Mass Spectrom. 2005, 40, 1590–1594. [Google Scholar] [CrossRef]
- Park, S.J.; Song, J.S.; Kim, H.J. Dansylation of tryptic peptides for increased sequence coverage in protein identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometric peptide mass fingerprinting. Rapid Commun. Mass Spectrom. 2005, 19, 3089–3096. [Google Scholar] [CrossRef]
- Krause, E.; Wenschuh, H.; Jungblut, P.R. The dominance of arginine-containing peptides in MALDI-derived tryptic mass fingerprints of proteins. Anal. Chem. 1999, 71, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Distefano, M.D. Solid-phase synthesis of C-terminal peptide libraries for studying the specificity of enzymatic protein prenylation. Chem. Commun. 2012, 48, 8228–8230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.B.; Hancock, P.J.; Kral, A.M.; Hellinga, H.W.; Beese, L.S. The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics. Proc. Natl. Acad. Sci. USA 2001, 98, 12948–12953. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, E.R.; Cheng, M.; Zhao, P.; Kim, J.H.; Wells, L.; Schmidt, W.K. A shunt pathway limits the CaaX processing of Hsp40 Ydj1p and regulates Ydj1p-dependent phenotypes. Elife 2016, 5, 1–22. [Google Scholar] [CrossRef]
- Berger, B.M.; Kim, J.H.; Hildebrandt, E.R.; Davis, I.C.; Morgan, M.C.; Hougland, J.L.; Schmidt, W.K. Protein isoprenylation in yeast targets COOH-terminal sequences not adhering to the CaaX consensus. Genetics 2018, 210, 1301–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee Herman, W.; Tarr, G.; Kates, S.A. Optimization of the synthesis of peptide combinatorial libraries using a one-pot method. Mol. Divers. 1997, 2, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Khatwani, S.L.; Kang, J.S.; Mullen, D.G.; Hast, M.A.; Beese, L.S.; Distefano, M.D.; Taton, T.A. Covalent protein-oligonucleotide conjugates by copper-free click reaction. Bioorganic Med. Chem. 2012, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGraw, A.J.; Hast, M.A.; Xu, J.; Mullen, D.; Beese, L.S.; Barany, G.; Distefano, M.D. Caged protein prenyltransferase substrates: Tools for understanding protein prenylation. Chem. Biol. Drug Des. 2008, 72, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashok, S.; Hildebrandt, E.R.; Ruiz, C.S.; Hardgrove, D.S.; Coreno, D.W.; Schmidt, W.K.; Hougland, J.L. Protein Farnesyltransferase Catalyzes Unanticipated Farnesylation and Geranylgeranylation of Shortened Target Sequences. Biochemistry 2020, 59, 1149–1162. [Google Scholar] [CrossRef]
- Elble, R.C. A simple and efficient procedure for transformation of yeasts. Biotechniques 1992, 13, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lapham, A.N.; Freedman, C.G.K.; Reed, T.L.; Schmidt, W.K. Yeast as a tractable genetic system for functional studies of the insulin-degrading enzyme. J. Biol. Chem. 2005, 280, 27481–27490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
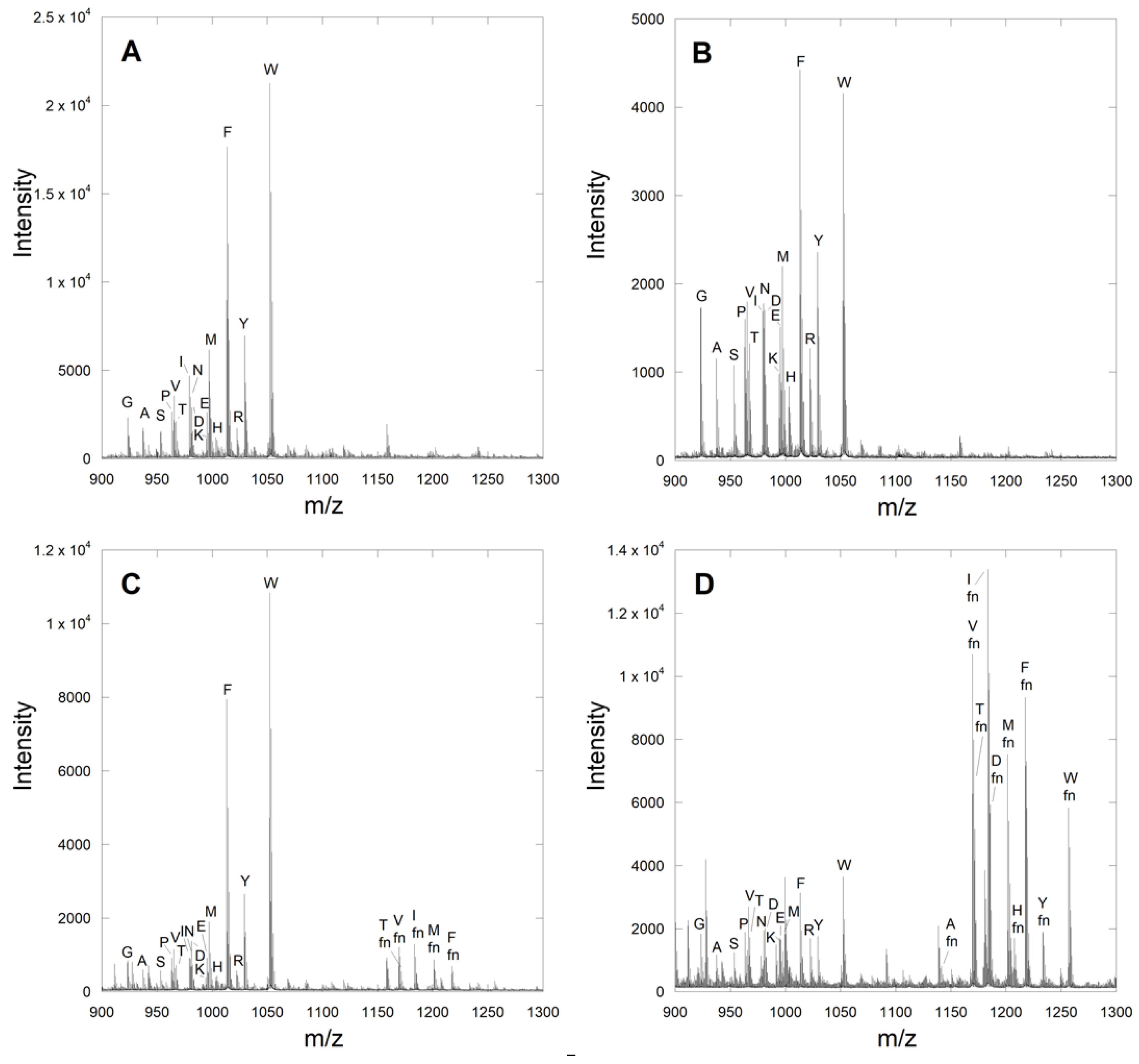
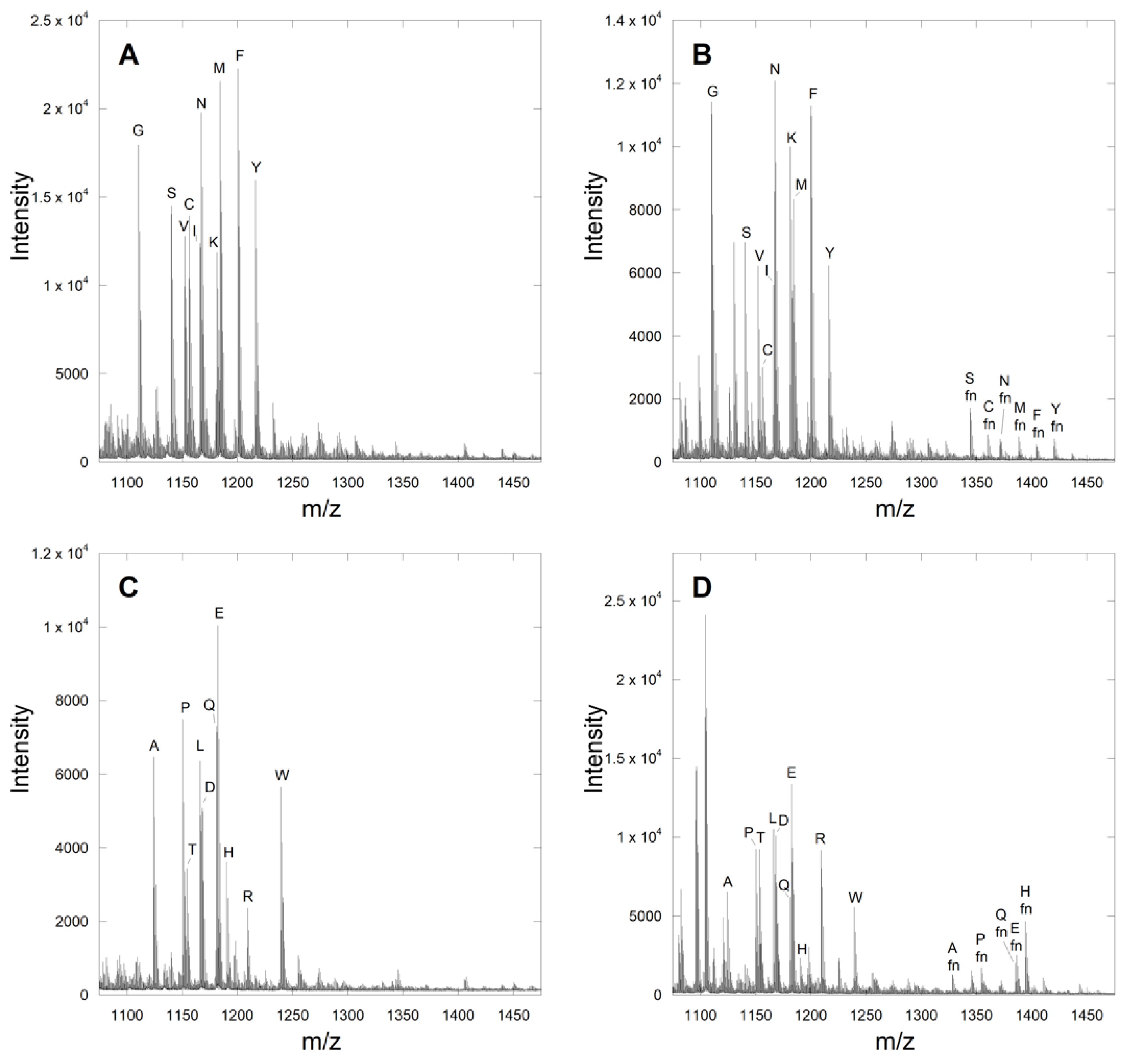

| Library Sequence | Observed Amino Acid Hits |
|---|---|
| Ca1IIM | S, C, M, F, Y, A, P, Q, E, H |
| CMa2IM a | G, S, N, K, Q, E, H, R |
| CMIa3M a | G, N, M, A, T, L, Q, E, H |
| CMIIX | S, C, K, A, Q, M |
| Extent of Conversion at | Extent of Conversion at | |
|---|---|---|
| Sequence | 25 nM yFTase, rt (%) | 200 nM rFTase, 35 °C (%) |
| CMIIM | 56 ± 10 | 50 ± 3 |
| CMIIS | 61 ± 14 | 25 ± 3 |
| CMIIQ | 76 ± 3 | 80 ± 2 |
| CSIIM | 64 ± 5 | <1 |
| CMKIM | 54 ± 10 | <1 |
| CYIIM | 95 ± 1 | 49 ± 1 |
| CHIIM | 15 ± 6 | <1 |
| CMIGM | <1 | <1 |
| CMIIK | <1 | <1 |
| Extent of Conversion at | Extent of Conversion at | Extent of Conversion at | Extent of Conversion at | |
|---|---|---|---|---|
| Sequence | 25 nM yFTase (%) | 100 nM yFTase (%) | 25 nM rFTase (%) | 100 nM rFTase(%) |
| CSLMQ | 95 ± 4 | >99 | 79 ± 2 | >99 |
| CSQAS | 43 ± 3 | >99 | ND | 66 ± 1 |
| CMTSQ | ND | 62 ± 1 | ND | 9 ± 1 |
| CASSQ | ND | 33 ± 3 | ND | <1 |
| CQYNS | ND | <1 | ND | <1 |
| CLACS | ND | <1 | ND | <1 |
| CVQTS | ND | <1 | ND | <1 |
| CASLS | ND | <1 | ND | <1 |
| CSKLN | ND | <1 | ND | <1 |
| CLLFS | ND | <1 | ND | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schey, G.L.; Buttery, P.H.; Hildebrandt, E.R.; Novak, S.X.; Schmidt, W.K.; Hougland, J.L.; Distefano, M.D. MALDI-MS Analysis of Peptide Libraries Expands the Scope of Substrates for Farnesyltransferase. Int. J. Mol. Sci. 2021, 22, 12042. https://doi.org/10.3390/ijms222112042
Schey GL, Buttery PH, Hildebrandt ER, Novak SX, Schmidt WK, Hougland JL, Distefano MD. MALDI-MS Analysis of Peptide Libraries Expands the Scope of Substrates for Farnesyltransferase. International Journal of Molecular Sciences. 2021; 22(21):12042. https://doi.org/10.3390/ijms222112042
Chicago/Turabian StyleSchey, Garrett L., Peter H. Buttery, Emily R. Hildebrandt, Sadie X. Novak, Walter K. Schmidt, James L. Hougland, and Mark D. Distefano. 2021. "MALDI-MS Analysis of Peptide Libraries Expands the Scope of Substrates for Farnesyltransferase" International Journal of Molecular Sciences 22, no. 21: 12042. https://doi.org/10.3390/ijms222112042






