Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells
Abstract
:1. Introduction
2. Results
2.1. Detection of Wnt (Co-)Receptor Interactions
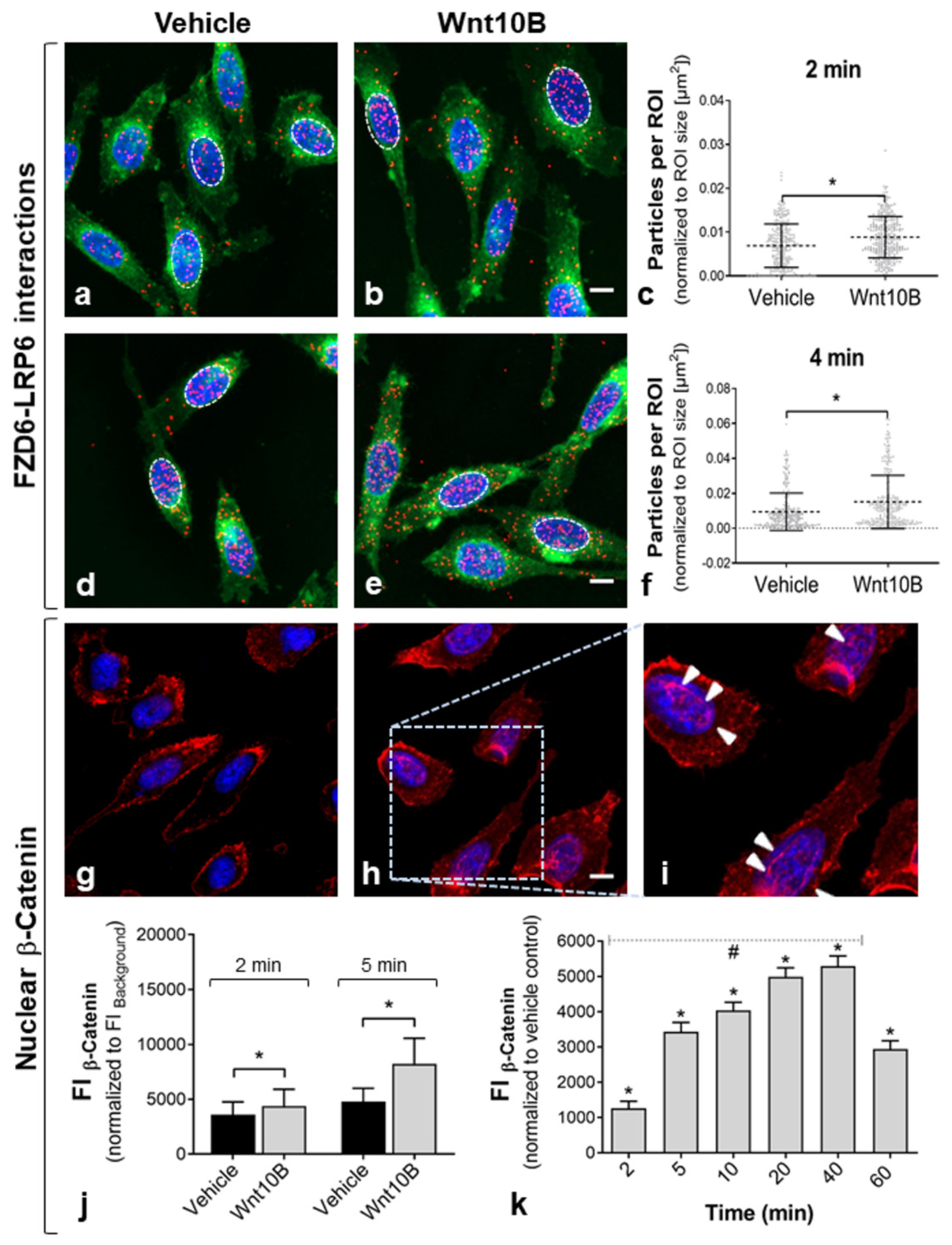
2.2. Rapid Activation of the Pathway
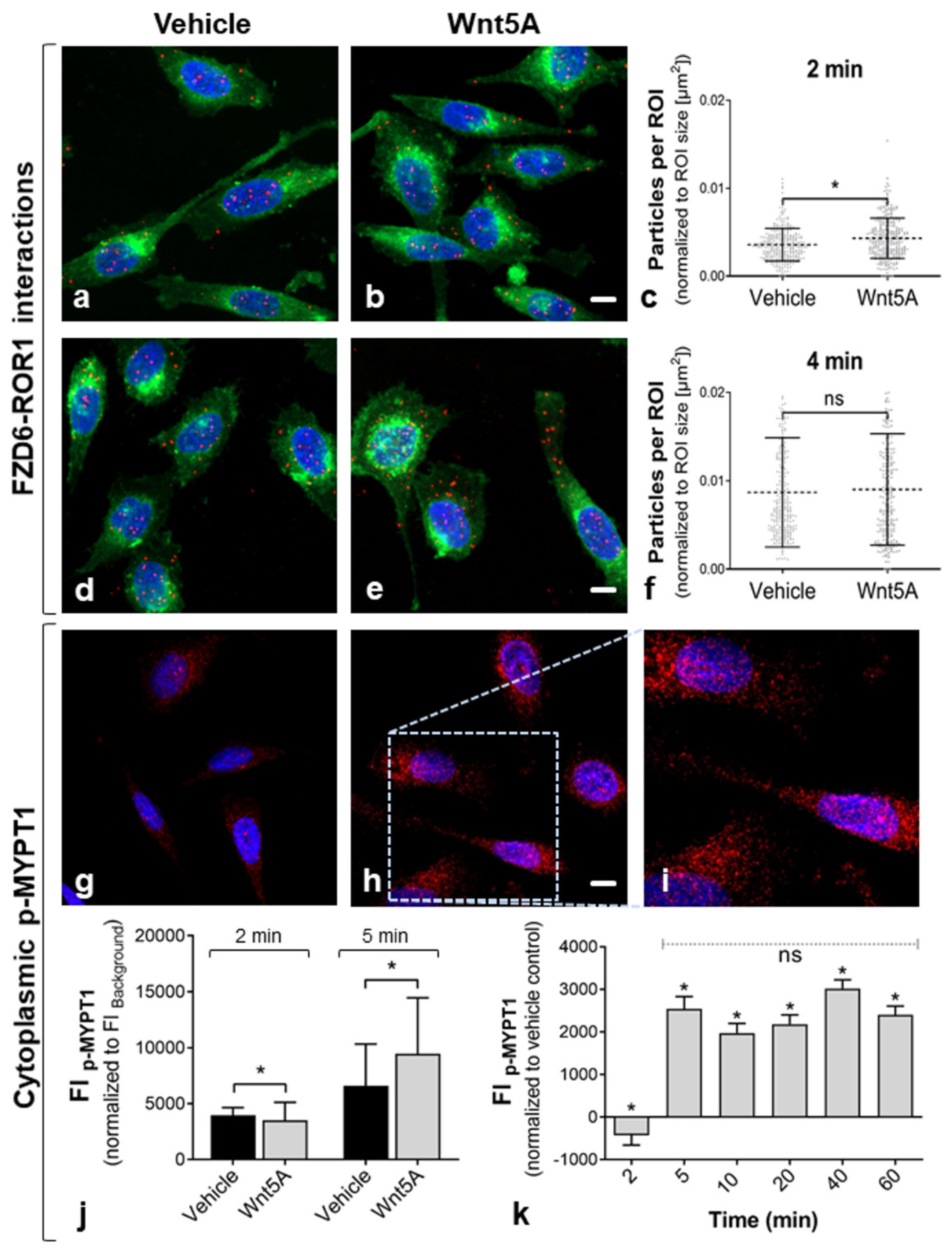
2.3. Activation of Non-Canonical Wnt/PCP Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Transfection
4.3. RNA Extraction and qRT-PCR
4.4. Wnt Ligand Treatment
4.5. Immunofluorescence
4.6. In Situ PLA
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [Green Version]
- Klaus, A.; Birchmeier, W. Wnt signaling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Nigam, P.; Tektas, S.S.; Selva, E. microRNA regulation of Wnt signaling pathways in development and disease. Cell. Signal. 2015, 27, 1380–1391. [Google Scholar] [CrossRef] [Green Version]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The Class Frizzled Receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, B.T.; Semenov, M.V.; He, X. SnapShot: Wnt/beta-catenin signaling. Cell 2007, 131, 1204. [Google Scholar] [CrossRef] [Green Version]
- Semenov, M.V.; Habas, R.; Macdonald, B.T.; He, X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell 2007, 131, 1378.e1–1378.e2. [Google Scholar] [CrossRef] [Green Version]
- Kühl, M.; Sheldahl, L.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000, 16, 279–283. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Baljinnyam, B.; Stanger, K.; Sercan, H.O.; Ji, Y.; Andres, O.; Rubin, J.S.; Hannoush, R.N.; Schulte, G. Systematic Mapping of WNT-FZD Protein Interactions Reveals Functional Selectivity by Distinct WNT-FZD Pairs. J. Biol. Chem. 2015, 290, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- van Amerongen, R.; Mikels, A.; Nusse, R. Alternative Wnt Signaling Is Initiated by Distinct Receptors. Sci. Signal. 2008, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Kypta, R.; Waxman, J. Wnt/β-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012, 9, 418–428. [Google Scholar] [CrossRef]
- Yamamoto, H.; Oue, N.; Sato, A.; Hasegawa, Y.; Matsubara, A.; Yasui, W.; Kikuchi, A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 2010, 29, 2036–2046. [Google Scholar] [CrossRef] [Green Version]
- Thiele, S.; Göbel, A.; Rachner, T.D.; Fuessel, S.; Froehner, M.; Muders, M.H.; Baretton, G.B.; Bernhardt, R.; Jakob, F.; Glüer, C.C.; et al. WNT5A Has Anti-Prostate Cancer Effects In Vitro and Reduces Tumor Growth in the Skeleton In Vivo. J. Bone Miner. Res. 2015, 30, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Sandsmark, E.; Hansen, A.F.; Selnæs, K.M.; Bertilsson, H.; Bofin, A.M.; Wright, A.J.; Viset, T.; Richardsen, E.; Drabløs, F.; Bathen, T.F.; et al. A novel non-canonical Wnt signature for prostate cancer aggressiveness. Oncotarget 2016, 8, 9572–9586. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Iljin, K.; Sara, H.; Mpindi, J.P.; Mirtti, T.; Vainio, P.; Rantala, J.; Alanen, K.; Nees, M.; Kallioniemi, O. FZD4 as a Mediator of ERG Oncogene–Induced WNT Signaling and Epithelial-to-Mesenchymal Transition in Human Prostate Cancer Cells. Cancer Res. 2010, 70, 6735–6745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, S.; Rauner, M.; Goettsch, C.; Rachner, T.D.; Benad, P.; Fuessel, S.; Erdmann, K.; Hamann, C.; Baretton, G.B.; Wirth, M.P.; et al. Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J. Cell. Biochem. 2011, 112, 1593–1600. [Google Scholar] [CrossRef]
- Wissmann, C.; Wild, P.J.; Kaiser, S.; Roepcke, S.; Stoehr, R.; Woenckhaus, M.; Kristiansen, G.; Hsieh, J.-C.; Hofstaedter, F.; Hartmann, A.; et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J. Pathol. 2003, 201, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pascal, L.E.; Vêncio, R.Z.; Page, L.S.; Liebeskind, E.S.; Shadle, C.P.; Troisch, P.; Marzolf, B.; True, L.D.; Hood, L.E.; Liu, A.Y. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer 2009, 9, 452. [Google Scholar] [CrossRef]
- Thiele, S.; Zimmer, A.; Gobel, A.; Rachner, T.D.; Rother, S.; Fuessel, S.; Froehner, M.; Wirth, M.P.; Muders, M.H.; Baretton, G.B.; et al. Role of WNT5A receptors FZD5 and RYK in prostate cancer cells. Oncotarget 2018, 9, 27293–27304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wend, P.; Wend, K.; Krum, S.A.; Miranda-Carboni, G.A. The role of WNT10B in physiology and disease. Acta Physiol. 2011, 204, 34–51. [Google Scholar] [CrossRef]
- Rocque, B.; Torban, E. Planar Cell Polarity Pathway in Kidney Development and Function. Adv. Nephrol. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- van Amerongen, R.; Fuerer, C.; Mizutani, M.; Nusse, R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev. Biol. 2012, 369, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo-Garzón, V.; Gorroño-Etxebarria, I.; Akerfelt, M.; Puustinen, M.C.; Sistonen, L.; Nees, M.; Carton, J.; Waxman, J.; Kypta, R.M. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat. Commun. 2018, 9, 1747. [Google Scholar] [CrossRef] [Green Version]
- Thrasivoulou, C.; Millar, M.; Ahmed, A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: A convergent model of Wnt/Ca2+ and Wnt/β-catenin pathways. J. Biol. Chem. 2013, 288, 35651–35659. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Chen, M.; Kang, H.; Steinhart, Z.; Angers, S.; He, X.; Kirschner, M.W. Single-molecule dynamics of Dishevelled at the plasma membrane and Wnt pathway activation. Proc. Natl. Acad. Sci. USA 2020, 117, 16690–16701. [Google Scholar] [CrossRef] [PubMed]
- Beagle, B.; Johnson, G.V.W. Differential Modulation of TCF/LEF-1 Activity by the Soluble LRP6-ICD. PLoS ONE 2010, 5, e11821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Wang, S.; Hu, C.; Wu, Y.; Liang, D.; Li, L.; Liu, Y.; Li, J.; Chen, Y.-H. Low-density lipoprotein receptor-related protein 6 regulates alternative pre-mRNA splicing. J. Cell. Mol. Med. 2018, 22, 4653–4663. [Google Scholar] [CrossRef] [Green Version]
- Di Liddo, R.; Bertalot, T.; Schuster, A.; Schrenk, S.; Tasso, A.; Zanusso, I.; Conconi, M.T.; Schäfer, K.H. Anti-inflammatory activity of Wnt signaling in enteric nervous system: In vitro preliminary evidences in rat primary cultures. J. Neuroinflamm. 2015, 12, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolben, T.; Peröbner, I.; Fernsebner, K.; Lechner, F.; Geissler, C.; Ruiz-Heinrich, L.; Capovilla, S.; Jochum, M.; Neth, P. Dissecting the impact of Frizzled receptors in Wnt/β-catenin signaling of human mesenchymal stem cells. Biol. Chem. 2012, 393, 1433–1447. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chang, H.; Rattner, A.; Nathans, J. Frizzled Receptors in Development and Disease. Curr. Top. Dev. Biol. 2016, 117, 113–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Symes, A.J.; Kane, C.A.; Freeman, A.; Nariculam, J.; Munson, P.; Thrasivoulou, C.; Masters, J.R.W.; Ahmed, A. A Novel Role for Wnt/Ca2+ Signaling in Actin Cytoskeleton Remodeling and Cell Motility in Prostate Cancer. PLoS ONE 2010, 5, e10456. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Wang-Rodriguez, J.; Zhang, L.; Cui, B.; Frankel, W.; Wu, R.; Kipps, T.J. The Onco-Embryonic Antigen ROR1 Is Expressed by a Variety of Human Cancers. Am. J. Pathol. 2012, 181, 1903–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Corda, G.; Sala, A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis 2017, 6, e364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
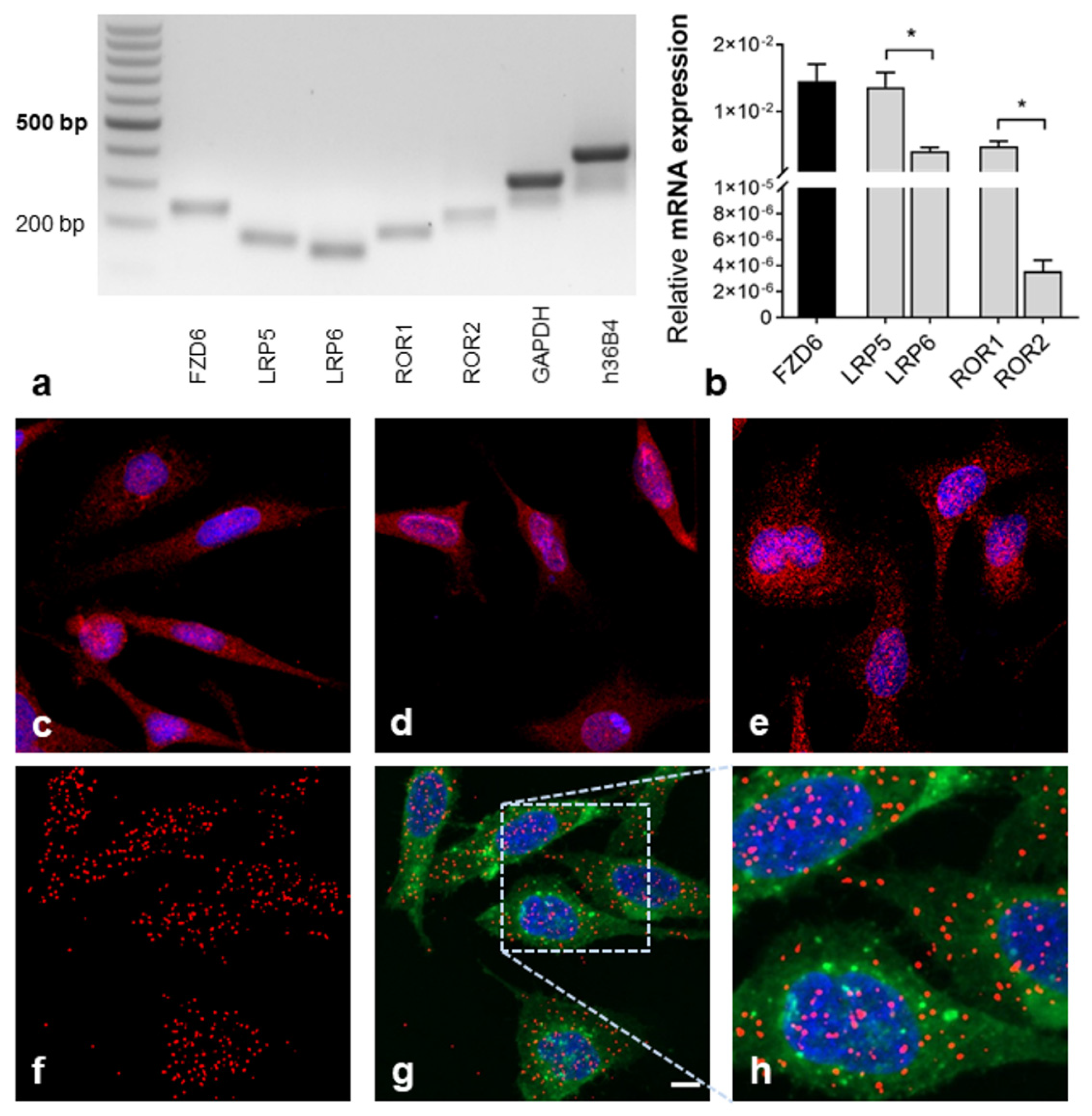
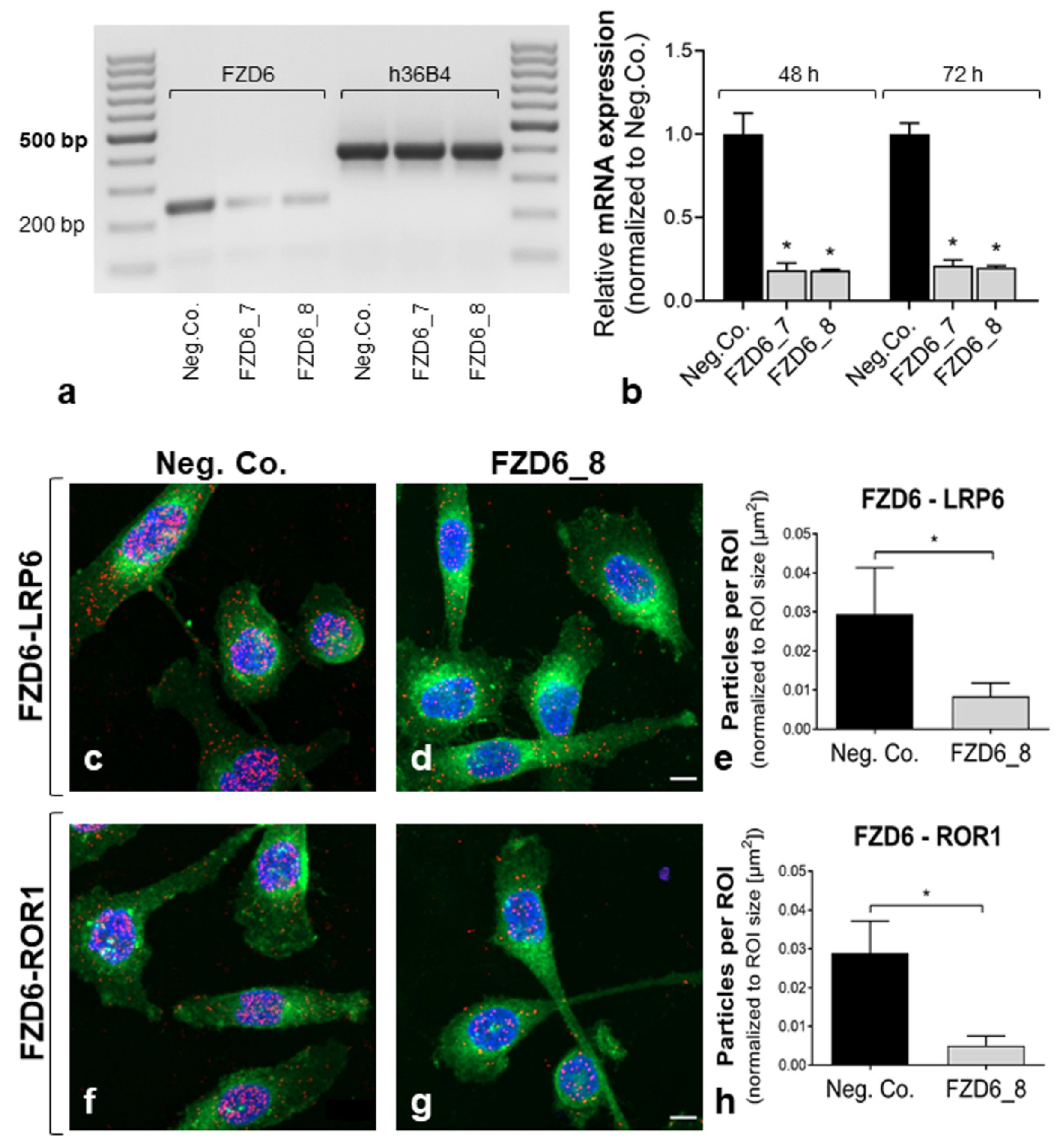
| Marker | Gene | Acc.-No. | Sequence (5′–3′) | Binding Site | Product Size (bp) |
|---|---|---|---|---|---|
| Frizzled class receptor 6 | FZD6 | NM_003506.4 | f-CGAATTGGAGTCTTCAGCGG r-TTCCAACCCAGAAGACAGCA | Exon 3 Exon 4 | 241 |
| LDL receptor-related protein 5 | LRP5 | NM_002335.4 | F-TCAGCCCTGGACTTTGATGT R-CCAGTAGAGGTTCTTGCCCA | Exon 9 Exon 10 | 171 |
| LDL receptor-related protein 6 | LRP6 | NM_002336.3 | f-AGAGTCCCAGTTCCAGTGTG r-GCTTTCCAATGCACTGACCA | Exon 18 Exon 19 | 158 |
| Receptor tyrosine kinase-like orphan receptor 1 | ROR1 | NM_005012.4 | F-AATGATGCTCCTGTGGTCCA R-TATCCTGGACTTGCAGTGGG | Exon 3 Exon 4 | 200 |
| Receptor tyrosine kinase-like orphan receptor 1 | ROR2 | NM_004560.4 | F-GCAGCAAGATGGGGATTCTG R-AGCTCCTCCATGAACCTCAC | Exon 8 Exon 9 | 241 |
| Pathway | Name | Specificity | Host | Source | Dilution |
|---|---|---|---|---|---|
| Wnt pathway | FZD6 | Frizzled-6 receptor; associated with PCa | Rb | Life Span Biosciences, Seattle, WA | 1:200 |
| Canonical Wnt/b-Catenin pathway (receptor) | LRP6 | Co-receptor function in Wnt/b-Catenin signaling (major) | Go | Novus Biologicals, Abingdon, UK | 1:100 |
| Non-canonical Wnt pathway (receptor) | ROR1 | Tyrosine kinase receptor associated with co-receptor function in Wnt/PCP signaling | Go | Abcam, Cambridge, UK | 1:100 |
| Canonical Wnt/b-Catenin pathway (effector) | β-Catenin | Total β-Catenin; key downstream activator of canonical Wnt signaling | Rb | Cell Signaling Technology, Frankfurt/Main, Germany | 1:100 |
| Non-canonical Wnt pathway (effector) | p-MYPT1 | Phospho-Myosin phosphatase target subunit 1; regulates actin–myosin interactions | Go | Acris Antibodies, Herford, Germany | 1:200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuhaus, J.; Weimann, A.; Berndt-Paetz, M. Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells. Int. J. Mol. Sci. 2021, 22, 12057. https://doi.org/10.3390/ijms222112057
Neuhaus J, Weimann A, Berndt-Paetz M. Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells. International Journal of Molecular Sciences. 2021; 22(21):12057. https://doi.org/10.3390/ijms222112057
Chicago/Turabian StyleNeuhaus, Jochen, Annett Weimann, and Mandy Berndt-Paetz. 2021. "Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells" International Journal of Molecular Sciences 22, no. 21: 12057. https://doi.org/10.3390/ijms222112057
APA StyleNeuhaus, J., Weimann, A., & Berndt-Paetz, M. (2021). Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells. International Journal of Molecular Sciences, 22(21), 12057. https://doi.org/10.3390/ijms222112057







