Abstract
Coronaviruses cause diseases in humans and livestock. The SARS-CoV-2 is infecting millions of human beings, with high morbidity and mortality worldwide. The main protease (Mpro) of coronavirus plays a pivotal role in viral replication and transcription, which, in theory, is an attractive drug target for antiviral drug development. It has been extensively discussed whether Xanthohumol is able to help COVID-19 patients. Here, we report that Xanthohumol, a small molecule in clinical trials from hops (Humulus lupulus), was a potent pan-inhibitor for various coronaviruses by targeting Mpro, for example, betacoronavirus SARS-CoV-2 (IC50 value of 1.53 μM), and alphacoronavirus PEDV (IC50 value of 7.51 μM). Xanthohumol inhibited Mpro activities in the enzymatical assays, while pretreatment with Xanthohumol restricted the SARS-CoV-2 and PEDV replication in Vero-E6 cells. Therefore, Xanthohumol is a potent pan-inhibitor of coronaviruses and an excellent lead compound for further drug development.
1. Introduction
Coronaviruses (CoVs) infect mammals and birds, leading to severe infectious diseases in rare cases. Most human coronavirus (hCoV) infections cause no, or mild, symptoms, but some become fatal, such as SARS-CoV-2, SARS-CoV, and MERS-CoV [1,2]. Animal coronaviruses lead to gross economic losses, with examples including the porcine epidemic diarrhea virus (PEDV), and the transmissible gastroenteritis virus (TGEV) [3]. To September 2021, SARS-CoV-2 has caused over 200 million infections and 4.58 million deaths worldwide. Vaccines have largely controlled viral infections, but various emerging variants are escaping the neutralization by vaccines. On the other hand, some coronaviruses infecting livestock have been reported to be zoonotic [4,5], which may be the origin for the next breakout. It is highly desirable to screen pan-inhibitors for emerging coronaviruses.
Coronaviruses are large positive-strand RNA viruses, with a unique life cycle, belonging to the Coronaviridae family [6]. After entry, viruses immediately translate two polyproteins, pp1a (~450 kDa) and pp1ab (~750 kDa), using their genomic RNA, and generate 12–16 individual nonstructural proteins (NSPs) by the cleavages of the main protease (Mpro or 3CLpro) and papain-like protease (PLpro). NSPs assemble into viral replication machinery, taking part in various critical processes, such as genome duplication, protein translation, and immune evasion [6]. To be noted, these viral proteins are highly conserved in the Coronaviridae family, which means chemicals targeting these proteins might inhibit multiple coronaviruses. Thus, people initially expected that the RdRp (RNA-dependent RNA polymerase) inhibitor, Remdesivir (GS-5734), could cure COVID-19 patients because its parent chemical, GS-441524, displayed antiviral activities against other CoVs [7,8]. Unfortunately, the unsuccessful clinical trials of Remdesivir for COVID-19 remind us that screening novel antiviral agents remains attractive, especially for pan-inhibitors against coronaviruses [9].
Mpro is a viral-encoding cysteine protease [10]. Protease inhibitors are attractive candidates for antiviral drug development. Many protease inhibitors have been marketed as antiviral agents (i.e., Boceprevir for HCV, Darunavir for HIV). Because coronaviral Mpro is distinct from all human proteases [10], and critical in the viral life cycle, compounds that suppress Mpro activities may be pan-inhibitors of coronaviruses without side effects. The first generation of Mpro is the covalent inhibitor, N3, which mimics the native substrate peptides with additional Michael acceptors as warheads [11]. More and more peptidic inhibitors/peptidomimetics have been developed with amazing activities and low toxicities, for example, GC-376, 11a, and 11b [12,13]. Moreover, Mpro inhibitors have also been found via high-throughput screening approaches. Ebselen, PX-12, and carmofur may covalently interact with the C145 residue of Mpro, which is critical to its catalytical activities [14]. Pfizer has announced the launch of a phase I clinical trial with PF-07321332 [15]. Until now, it is the first Mpro inhibitor in a clinical trial, and we are expecting the coming clinical results.
Plants are excellent resources for protease inhibitor discovery. Shikonin, a natural product from herbs, is a potential Mpro inhibitor against SARS-CoV-2 [14]. Phenolic compounds (PCs) from plants and their derivatives present antiviral activities against HSV-1, HIV, HCV, and others [16]. Phenolic compounds (PCs) are generally found in fruits, vegetables, herbs, flowers, and seeds, including phenolic acids and flavonoids (flavonols, catechins, flavones, chalcones, etc.). Xanthohumol is a prenylated chalcone from the hop plant (Humulus lupulus) that contributes to the bitterness in beer. It has been highly analyzed by researchers as an antiviral agent or antioxidant [17]. It has been reported to inhibit cervical cancer, colorectal cancer, and prostate cancer [18,19,20,21]. Our previous works found that Xanthohumol regulated the Th1/Th2 balance in a tumor model [22]. Xanthohumol inhibits HSV-1 with an IC50 value of 2.7 μg/mL, HIV with an IC50 value of ≈20.74 μg/mL, and CMV with an IC50 value of 2.5 μg/mL [23,24]. Because of its potent utilization of its antioxidant and anti-inflammatory properties, Xanthohumol has been approved in clinical studies for the safety and subjective tolerability in healthy adults (NCT03735420) [25]. After the breakout of COVID-19, many researchers keep discussing the potent roles of Xanthohumol against SARS-CoV-2. In this study, we found that Xanthohumol inhibited Mpro, and that it was a potent pan-inhibitor against various coronaviruses. Xanthohumol restricted SARS-CoV-2 and PEDV in vitro. This suggests the potential of Xanthohumol as a coronavirus Mpro inhibitor.
2. Results
2.1. Coronaviral Mpro Is Highly Conserved
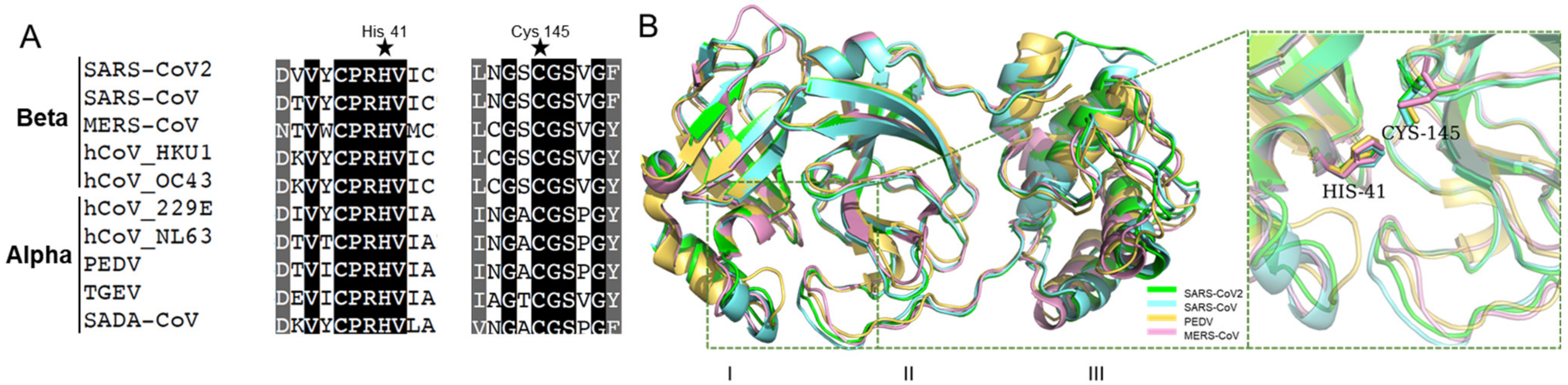
Mpro contains three conserved domains: I, II, and III. Domain I and II are closely interacted to form a cleft, forming a catalytic core and substrate binding sites. His-41 in Domain I, and Cys-145 in Domain II, are catalytic dyads [10,26]. As shown in Figure 1A, His-41, Cys-145, and their neighbor residues were highly conserved in alpha-coronaviruses (i.e., PEDV and TGEV) and beta-coronaviruses (i.e., SARS-CoV and SARS-CoV-2). Moreover, the three-dimensional (3D) structures were similar for various Mpro (Figure 1B), and the amino acid sequences of Domain I and II were consistent in pathogenic coronaviruses (i.e., SARS-CoV-2, SARS-CoV, PEDV, and MERS-CoV) (Figure S1). This suggests that Mpro is a promising drug target for pan-inhibitor screening against coronaviruses. Interestingly, the cysteine protease inhibitor, GC376, is a potent Mpro inhibitor that shows antiviral activities against SARS-CoV-2, SARS-CoV, and feline coronavirus (FCoV) [27,28]. A recent study developed a pan-inhibitor of Mpro restricting multiple coronaviruses in vitro [29]. It suggests that Mpro inhibitors might be pan-inhibitors against coronaviruses.
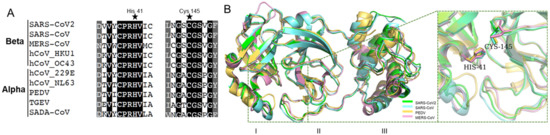
Figure 1.
The conserved amino acid sequences and 3D structures of the Mpro catalytic domains for different coronaviruses. (A) Alignment of neighbor residues on both flanks of the pivotal residue His41, Cys145. (B) The three-dimensional (3D) structures of Mpro are highly conserved.
2.2. SARS-CoV-2 Mpro Inhibitor Screening
To screen Mpro inhibitors, recombinant GST-tagged Mpro was generated from the E. coli BL21/DE3 strain (CodonPlus, Agilent Technologies Inc., Santa Clara, CA, USA;) using pGEX4 vectors. Since any additional amino acid residues in the N-terminus of Mpro could reduce its activity [11,27], tags were removed by Factor Xa (P8010L, New England Biolab, Ipswich, MA, USA) to generate native Mpro proteins (Supplementary Figure S2A). The Michaelis constant (Km) was measured to verify the hydrolase reaction. The Km value was 1.10 ± 0.22 μM (Supplementary Figure S2C), referring to a high affinity between the substrates and the enzyme.
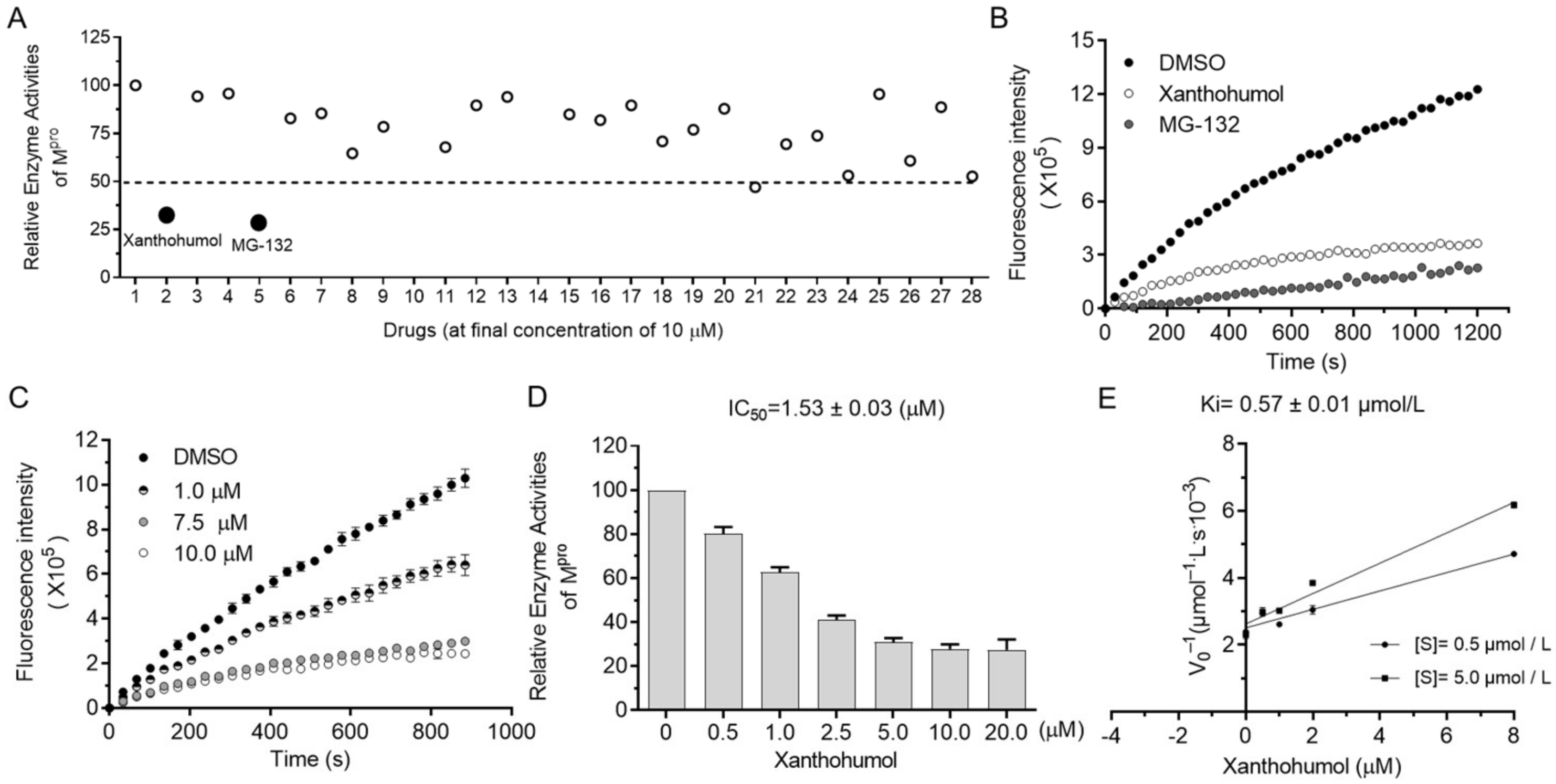
The orchestrated protease system of cells is essential to many biological processes (i.e., misfolded protein degradation, inflammation, antimicrobe invasion, and digestion) that require the low toxicity of protease inhibitors as drug candidates. Since protease inhibitors are abundant in animals and plants, we decided to screen Mpro inhibitors from natural products, especially focusing on natural products from foods, food additives, and herbs, which were safe and easily accessible in theory. As shown in Supplementary Figure S2D, more than a hundred natural products were randomly picked from the compound bank and briefly screened at the final concentration of 50 μM. Hits were further tested in the presence of 10 μM compounds, and candidates with an inhibition rate of more than 50% were considered as active. Surprisingly, Xanthohumol and MG132 almost abolished the enzyme activities of Mpro at the final concentration of 10 μM (Figure 2A,B). MG132 is a well-established broad-spectrum proteasome inhibitor, used as a spy compound here.
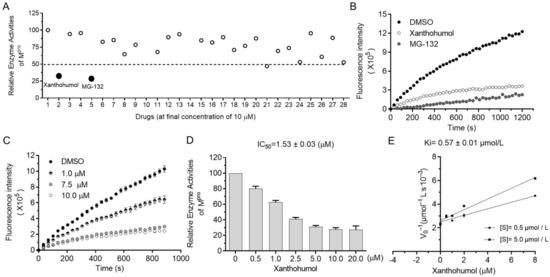
Figure 2.
Xanthohumol inhibited hydrolase activities of SARS-CoV-2 Mpro in a dose-dependent manner. (A) Relative enzyme activities of SARS-CoV-2 Mpro in the presence of 10 μM compounds. (B) The kinetic curves in the presence of Xanthohumol, MG132, and the solvent, DMSO. Xanthohumol and MG132 were added to the final concentration of 20 μM. (C) Xanthohumol inhibited Mpro dose-dependently. (D) The IC50 value of Xanthohumol. (E) The Ki value of Xanthohumol on SARS-CoV-2 Mpro. Data are shown as means ± standard error of mean (SEM) from three independent experiments.
It was found that Xanthohumol reduced Mpro hydrolase activities at low concentrations in vitro in a dose-dependent manner (Figure 2C,D). The half inhibition concentration of Xanthohumol on SARS-CoV-2 Mpro was 1.53 ± 0.03 μM (Figure 2D). As discussed in our early publications, the IC50 value of enzyme inhibitors is closely related to the enzymatical assay. To further analyze the inhibition efficiency of Xanthohumol on SARS-CoV-2 Mpro, the Ki (the inhibition constant for the inhibitor) value was also measured, which was 0.57 ± 0.01 μM (Figure 2E). It indicated that Xanthohumol reduced SARS-CoV-2 Mpro enzymatic activities efficiently in vitro.
2.3. Xanthohumol Potentially Inhibits Various Coronaviral Mpro
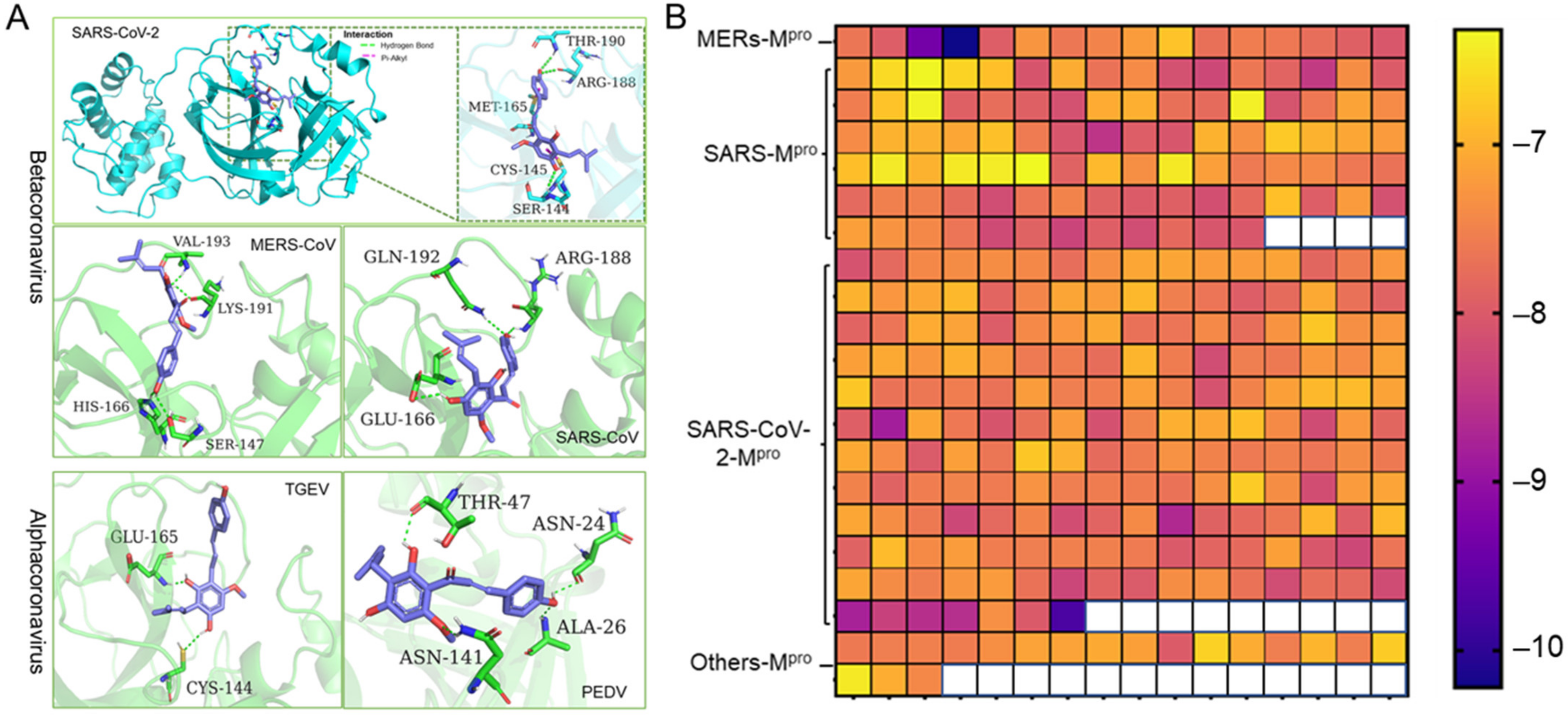
Coronaviruses are members of the Coronaviridae family. They are further subdivided into four genera: alpha-, beta-, gamma-, and delta- coronaviruses. The alpha- and beta- coronaviruses infect only mammals [1]. Noting that the 3D structure and the catalytic dyads of Mpro are conserved in alpha- and beta- coronaviruses, we employed molecular docking to predict the potent inhibition activities of Xanthohumol against various coronaviruses. A high-quality crystal structure of SARS-CoV-2 Mpro has been reported earlier [10], and the crystal structures of SARS-CoV, MERS-CoV, PEDV, and TGEV Mpro have been explored as well [30,31,32,33]. As shown in Figure 3A, Xanthohumol could be docked into the active pocket of a series of coronaviruses, Mpro, and form hydrogen bonds and other molecular interactions. Most docking scores are between −6 to −8, which indicate that Xanthohumol presents excellent affinities with various Mpro structures (Figure 3B and Supplementary Data). To be noted, a hydrogen bond was found between Xanthohumol and the Cys-145 of SARS-CoV-2 Mpro that was essential to Mpro catalytical activities, explaining that Xanthohumol inhibited SARS-CoV-2 efficiently. It indicated that Xanthohumol is a potent drug candidate for further drug development.
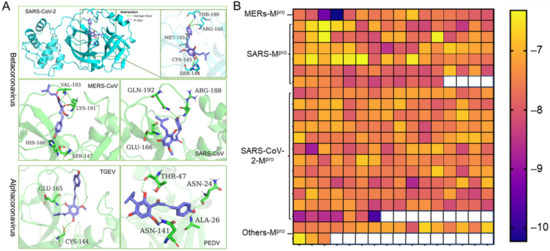
Figure 3.
Xanthohumol is a potent pan-inhibitor of coronaviral Mpro. (A) The interaction pattern between Xanthohumol and indicated Mpro. The Mpro was shown in cyan and green. The Xanthohumol was presented in purple. (B) The affinity diagrams between Xanthohumol and different Mpro. Mpro structures were downloaded from PDB, and the PDB ID and detailed docking scores are shown in Supplementary Data.
2.4. Xanthohumol Restricts SARS-CoV-2 and PEDV Replication in the Cell-Based Assay
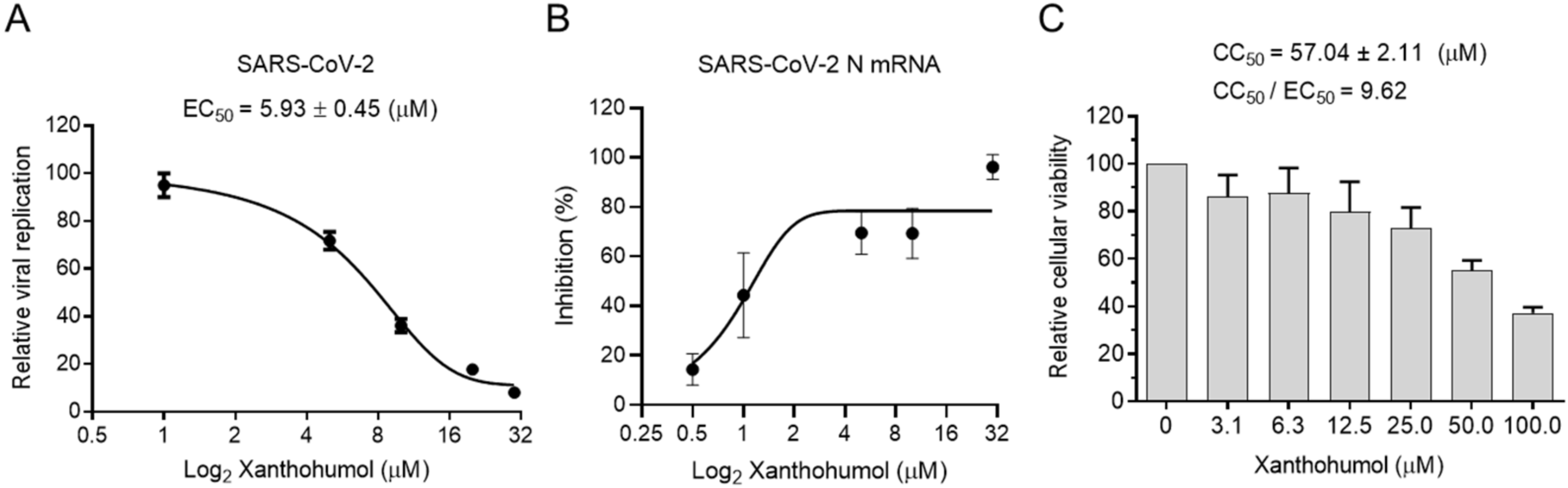
Many chemicals presented significant inhibition efficiency in the enzymatic assay but failed in cell models because of their poor permeability, improper cellular metabolism, and other issues. We were wondering if Xanthohumol were able to hamper SARS-CoV-2 replication in cells. Calpeptin, a well-established cell-permeable cysteine protease inhibitor, was used as the positive control. Calpeptin significantly restricted SARS-CoV-2 infection (IC50 = 0.38 ± 0.01 μM), indicating that the infection model could properly reveal the inhibition activity of candidate compounds (Supplementary Figure S3A). To be noted, Xanthohumol dose-dependently inhibited SARS-CoV-2 in cells (Figure 4A,B), while it did not slow cell growth, and only negligibly reduced cellular viability at the high concentration (Figure 4C). In a plague assay, the IC50 value of Xanthohumol on SARS-CoV-2 was 5.93 ± 0.45 μM (Figure 4A). Mpro cleaves viral polyprotein to assemble viral replication machinery, which is critical for viral RNA duplication. This suggests that Mpro inhibitors could directly inhibit viral RNA replication by hampering the replication machinery assembly. To monitor whether Xanthohumol could inhibit SARS-CoV-2 RNA duplication by targeting its Mpro, viral RNA loads of infected cells were also measured by qRT-PCR. This indicated that Xanthohumol could eliminate the viral genome effectively, which is highly consistent with the results from the plague assays mentioned above (Figure 4B). Moreover, the CC50 (the half cytotoxic concentration) value of Xanthohumol in Vero-E6 cells was 57.04 ± 2.11 μM, and the selection index was more than 9.5. This suggests that Xanthohumol is an outstanding lead compound for further developments.
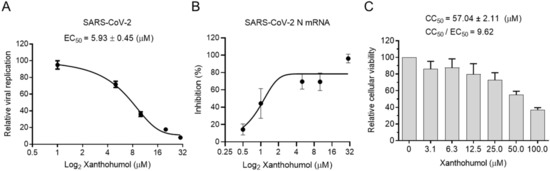
Figure 4.
Xanthohumol inhibited SARS-CoV-2 replication in Vero-E6 cells. (A) Xanthohumol-inhibited SARS-CoV-2. Vero-E6 cells were pretreated with compounds at indicated concentrations for 1 h, and then infected with recombinant SARS-CoV-2 mNeonGreen virus (MOI = 0.5) for 24 h. The green fluorescence was scanned as described. The EC50 values of Xanthohumol were calculated and are shown. (B) Xanthohumol reduced viral RNA loads. SARS-CoV-2 N mRNA was measured with qRT-PCR and normalized to GAPDH. (C) Cytotoxicities of Xanthohumol. Data are shown as means ± SEM from three independent experiments.
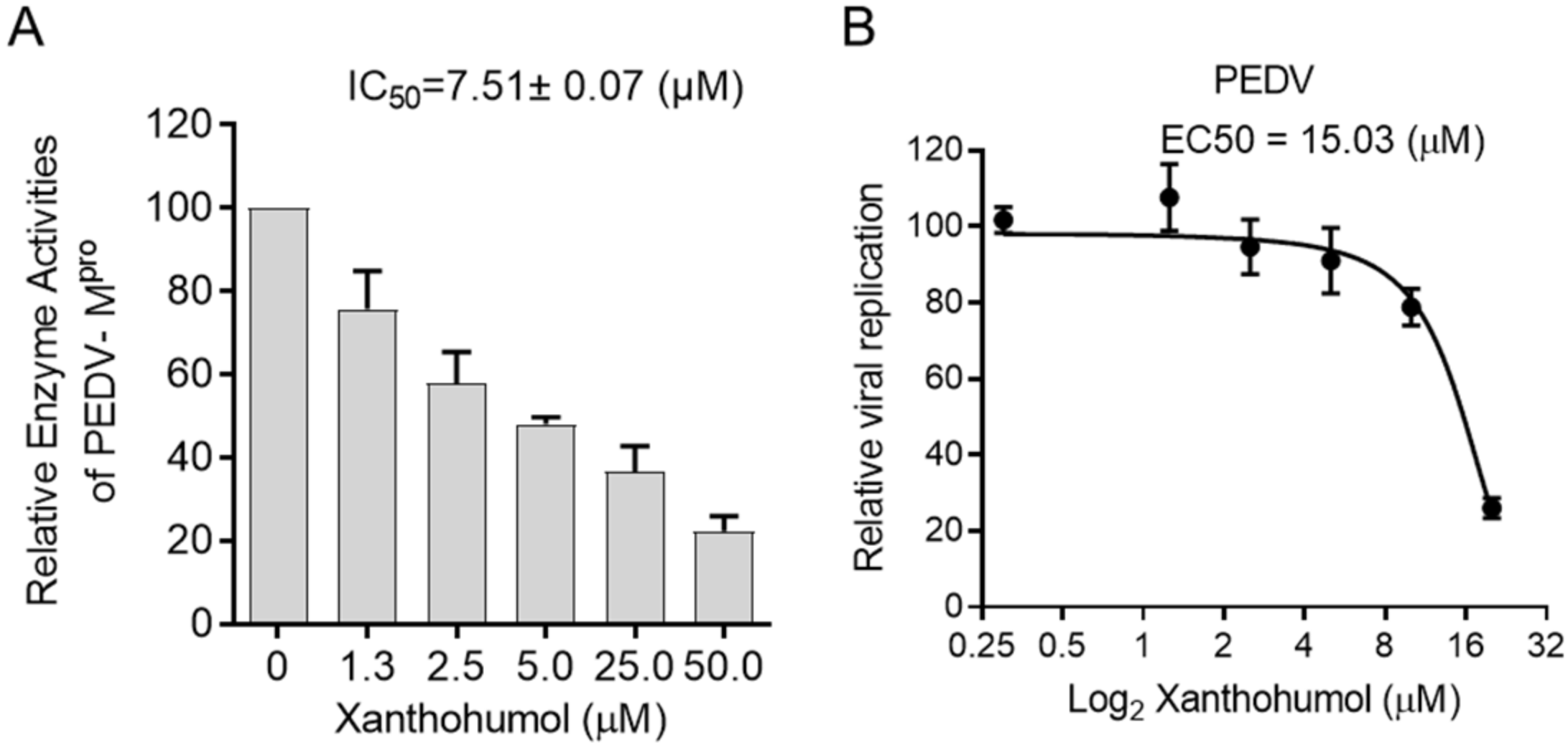
Considering the structural similarity between PEDV and SARS-CoV-2 Mpro, we were wondering if Xanthohumol would inhibit PEDV Mpro enzymatic activity and then restrict PEDV replication. The enzymatic assay was performed as SARS-CoV-2, in which the PEDV Mpro was used instead of SARS-CoV-2 Mpro. It indicated that Xanthohumol inhibited PEDV Mpro efficiently with an IC50 value of 7.51 ± 0.07 μM (Figure 5A). Moreover, Xanthohumol dose-dependently inhibited PEDV in Vero-E6 cells (Figure 5B).
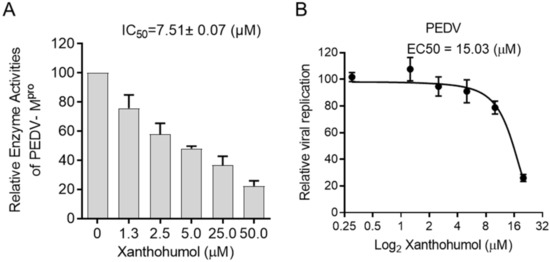
Figure 5.
Xanthohumol inhibited PEDV Mpro and PEDV in vitro. (A) Xanthohumol inhibited PEDV Mpro. (B) Xanthohumol inhibited PEDV replication in vitro. Vero-E6 cells were pretreated with the indicated concentration of Xanthohumol for 1 h and infected with PEDV (MOI = 1). Data are shown as means ± SEM from three independent experiments.
3. Discussion
COVID-19 might recede soon. However, as the predicted Disease X by the WHO after the outbreak of SARS and MERS, coronaviral infections might routinely break out as the seasonal flu in the future. Moreover, coronaviruses are threatening livestock. It is still meaningful to screen safe, cheap, and broad-spectrum antiviral agents for coronaviruses. As shown in our study, Xanthohumol inhibited alpha- and beta- coronaviruses (Figure 4 and Figure 5), which contain all fatal pathogenic coronaviruses: PEDV, SARS-CoV, MERS-CoV, and the recent SRAS-CoV-2 [34]. As previously reported, Xanthohumol modulated inflammation and oxidative stresses [35], which might benefit infected individuals, rather than an antiviral agent. At this moment, Xanthohumol is an excellent lead compound for drug optimization, and more detailed studies are required for further developments.
The safety and tolerability in healthy adults of Xanthohumol has been well-established, and Xanthohumol is available as dietary supplements and ingredients in medical foods. Although it is impossible to get enough Xanthohumol from beers before being severely hurt by alcohol, because of its relatively high IC50 value against coronaviruses, it is hoped that Xanthumol might guard healthy individuals against the initiation of infection at a low concentration. Of course, more detailed systematic studies should be performed before the usage of Xanthohumol for any medicinal purpose. In this case, using Xanthohumol in nonalcohol foods and drinks may be an available strategy. Hops are cheap and easily available in some areas. Coronaviruses are heavily damaging our livestock industry and using hops as feed additives may also be suggested.
Xanthohumol had been found to be a broad-spectrum antiviral agent, presenting inhibition activities against many viruses, which include human cytomegalovirus (CMV), herpes simplex virus (HSV), human immunodeficiency virus (HIV), hepatitis C virus (HCV), and porcine reproductive and respiratory syndrome viruses (PRRSV) [36,37,38]. Recently, many researchers keep discussing the potent role of Xanthohumol or the crude extracts of hops in COVID-19 treatments [39,40]. However, it is still unknown whether Xanthohumol can inhibit coronaviruses, and the antiviral mechanism of Xanthohumol is elusive. It has been shown that its antioxidative activities could reduce viral-induced tissue damage, and Xanthohumol could also boost the antiviral activities of type I interferon [41,42]. In our study, we demonstrate that Xanthohumol target viral Mpro as a protease inhibitor. Interestingly, many protease inhibitors were clinically used for HIV treatments [43], of which Lopinavir, Indinavir, and Darunavir have been repurposed to inhibit SARS-CoV-2 Mpro [44]. The HCV NS3–4A protease drug, boceprevir, could inhibit SARS-CoV-2 replication by targeting Mpro in our and other groups’ independent studies [45,46]. To be noted, PRRSV belongs to Arteriviridae. Both Arteriviridae and Coronaviridea are members of Nidovirales. PRRSV has a similar assembly process of replicase as coronaviruses. It encodes a polyprotein that is be cleaved by viral proteins (also called main protease and Papain-like protease as SARS-CoV-2). This suggests that Xanthohumol might inhibit HIV, HCV, PRRSV, and coronaviruses via a similar mechanism, being a protease inhibitor.
As mentioned above, because of the large number of infected individuals, the high mutation rates, and the potent zoonotic feature of coronaviruses [4], the war against coronaviruses will be a long one. It is necessary to screen potent pan-inhibitors for the next unexpected outbreak. Herein, identifying a promised drug target becomes critical. Xanthohumol showed a pan-inhibitor activity against alpha- and beta- coronaviruses targeting Mpro, and the GC-376 could inhibit SARS-CoV, SARS-CoV-2, and the Feline coronavirus (FcoV) via inhibiting Mpro [10,27,28]. A recently designed Mpro inhibitor also presented pan-inhibitor activities [10]. It will be interesting to screen Mpro inhibitors against coronaviral infection and to clarify whether Mpro is a promised drug target for the development of pan-inhibitors against various coronaviruses.
4. Materials and Methods
4.1. Cell Culture and Reagents
Vero-E6 cells (ATCC, VERO C1008 [Vero 76, clone E6, Vero E6] CRL-1586™) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplement, with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 mg/mL). Cells were maintained in the incubator at 37 °C with 5% CO2. Candidate compounds were obtained from a chemical bank in the lab, which was originally purchased from TargetMol (Shanghai, China), or were synthesized by us. Xanthohumol, Calpeptin, Remdesivir, and MG132 were purchased from Selleck (Shanghai, China). We purchased 96-well black plates with transparent glass-bottoms from Cellvis (Shanghai, China).
4.2. Drug Treatment and Viral Infection
For cytotoxicity analysis, Vero-E6 cells (3000 cells/well) were seeded into 96-well plates. After 12 h, the cells were treated with the indicated chemicals at the final concentration, as shown. At 48-h post-treatment, 10 µL of resazurin (1 mg/mL) was added to each well and incubated for 3 h. The absorbance was measured on a SpectraMax i3 (Molecular Devices, San Jose, CA, USA).
For antiviral analysis, Vero-E6 cells were pretreated with Xanthohumol and Calpeptin for 1 h and infected with SARS-CoV-2 mNeonGreen virus (MOI = 0.5) [47]. At 24-h post-infection (hpi), the cells were fixed and scanned by Amersham Typhoon 5 (GE, Laurel, MD, USA). The fluorescence intensities were quantified by ImageJ (NIH). The mNeonGreen signals and the mRNA of the SARS-CoV-2 N gene were measured, as previously described [48]. For the PEDV analysis, cells were pretreated with Xanthohumol or Remdesivir for 1 h and then infected with PEDV (MOI = 1) in the presence of drugs for 24 h. Cells were harvested and fixed with 2% paraformaldehyde, permeabilized, and intracellularly stained with house-made mouse anti-PEDV nucleocapsid serum. Goat anti-mouse IgG, conjugated with Alexa Fluor 647 (Thermo Fisher, Waltham, MA, USA), was used as the secondary antibody. After two additional washes, cells were subjected to flow cytometry analysis (Thermo, Waltham, MA, USA) and data processing (FlowJo, Becton, Dickinson & Company, Franklin Lakes, NJ, USA). Porcine epidemic diarrhea virus (PEDV) was propagated in Vero-E6 cells [49,50].
4.3. Protein Expression, Purification, and Enzymatic Assay
Protein expression and enzymatical assays were performed as previously described, with minor modifications [51]. SARS-CoV-2 and PEDV Mpro were generated as described previously [51]. Protein purity was identified by Coomassie Brilliant Blue staining. The enzymatic activity of Mpro was measured by continuous kinetic assays using an identical fluorogenic substrate, MCA-AVLQ/SGFR-Lys (DNP)-Lys-NH2 (Apetide Co., Ltd., Shanghai, China), as previously described [51] (Supplementary Figure S2B). The fluorescence intensities were monitored by a microplate reader (SpectraMax i3x, Molecular Devices). The excitation and emission wavelengths were 320 and 405 nm, respectively. Experiments were performed in 100 μL buffer (50 mM Tris-HCl, 1 mM EDTA, pH 7.3) containing 100 nM Mpro, 2 µM substrate, and 1 µL of the desired concentration of drugs. The compounds and the Mpro were incubated at RT for 15 min. Reaction velocities were analyzed by SoftMax Pro. EC50, Km, and Ki were calculated by GraphPad Prism6.
4.4. Molecular Docking
The Mpro structure information was obtained from the PDB databank (SARS-CoV-2: 6lu7; SARS-CoV: 3sn8; MERS-CoV: 4rsp; PEDV: 5gwz; TGEV: 1p9u). In addition, 378 of the Mpro structure information, analyzed by different research groups, was also obtained from the PDB database. The details are available in SI1. The structures of the proteins were prepared via the Protein Preparation Wizard task from Schröndinger for structure optimization. The structures of the compounds were prepared by LigPrep of Schrödinger (Schrödinger), and docked into the Mpro substrate-binding domain by AutoDock Vina. The docking score was the top score of the different docked modes. The Mpro structural superposition was analyzed by PyMol alignment.
Supplementary Materials
The Supplementary Materials are available online at https://www.mdpi.com/article/10.3390/ijms222212134/s1.
Author Contributions
X.W. and Y.L. designed and supervised the study, and wrote the manuscript; Y.L. performed the data analysis, with significant contributions from Y.L., R.Z. (Ruochen Zang), L.L., Y.M. and Z.W.(Zhuoya Wang); S.D., R.Z. (Rong Zhang) and Z.W. (Zhiqiang Wei) helped with methodology; J.Y. and X.W. contributed to with conceptualization, writing and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the special scientific research fund for COVID-19 from the Pilot National Laboratory for Marine Science and Technology (Qingdao) (QNLM202001), the National Natural Science Foundation of China (81991525, 31700755), the Taishan Scholars Program (tsqn201909170), the Fundamental Research Funds for the Central Universities to X.W., the Innovative Leader of the Qingdao Program (19-3-2-26-zhc), and the National Natural Science Foundation of China (32041005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
We acknowledge Xin Li for providing the expression vector, pGEX4T1-Mpro. Furthermore, the authors give thanks for the financial support from Washington University (St. Louis) and Shanghai Medical College.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. Evolution. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Vaccination against coronaviruses in domestic animals. Vaccine 2020, 38, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.E.; Yount, B.L.; Graham, R.L.; Leist, S.R.; Hou, Y.J.; Dinnon, K.H.; Sims, A.C.; Swanstrom, J.; Gully, K.; Scobey, T.D.; et al. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc. Natl. Acad. Sci. USA 2020, 117, 26915–26925. [Google Scholar] [CrossRef]
- Lee, P.I.; Hsueh, P.R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J. Microbiol. Immunol. Infect. 2020, 53, 365–367. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Wit, E.D.; Feldmann, F.; Cronin, J.; Jordan, R.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, E.S.; Levy, J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health 2020, 9, 100128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Yang, H.; Shen, W.; Zhao, Q.; Li, J.; Yang, K.; Chen, C.; Jin, Y.; Bartlam, M.; Rao, Z. Production of authentic SARS-CoV M(pro) with enhanced activity: Application as a novel tag-cleavage endopeptidase for protein overproduction. J. Mol. Biol. 2007, 366, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, C.B. Progress in Developing Inhibitors of SARS-CoV-2 3C-Like Protease. Microorganisms 2020, 8, 1250. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Liu, H. Structure-Based Design, Synthesis and Biological Evaluation of Peptidomimetic Aldehydes as a Novel Series of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hu, Y.; Townsend, J.A.; Lagarias, P.I.; Marty, M.T.; Kolocouris, A.; Wang, J. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are non-specific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharm. Transl. Sci. 2020, 3, 1265–1277. [Google Scholar] [CrossRef]
- Vandyck, K.; Deval, J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr. Opin. Virol. 2021, 49, 36–40. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Golonko, A.; Świsłocka, R.; Kalinowska, M.; Parcheta, M.; Swiergiel, A.; Lewandowski, W.J. Drug Design Strategies for the Treatment of Viral Disease. Plant Phenolic Compounds and Their Derivatives. Front. Pharm. 2021, 12, 709104. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Z.; Bai, J.; Nauwynck, H.; Zhao, Y.; Jiang, P. Xanthohumol inhibits PRRSV proliferation and alleviates oxidative stress induced by PRRSV via the Nrf2–HMOX1 axis. Vet. Res. 2019, 50, 61. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, W.; Liu, H.; Yu, X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol protects against Azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.K.; Malek, S.N.A. Xanthohumol induces growth inhibition and apoptosis in ca ski human cervical cancer cells. Evid. Based Complement. Altern. Med. 2015, 2015, 921306. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Arczewska, M.; Sławińska-Brych, A.; Rzeski, W.; Stepulak, A.; Gagoś, M. Prostate and breast cancer cells death induced by xanthohumol investigated with Fourier transform infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 231, 118112. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.; Gou, P.; Zhou, C.; Ma, L.; Liu, Q.; Du, Y.; Yang, J.; Wang, Q. Effect of xanthohumol on Th1/Th2 balance in a breast cancer mouse model. Oncol. Rep. 2018, 39, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Buckwold, V.E.; Wilson, R.J.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W., III; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Z.H.; Liu, J.K.; Zheng, Y.T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Bradley, R.; Langley, B.O.; Ryan, J.J.; Phipps, J.; Hanes, D.A.; Stack, E.; Jansson, J.K.; Metz, T.O.; Stevens, J.F. Xanthohumol microbiome and signature in healthy adults (the XMaS trial): A phase I triple-masked, placebo-controlled clinical trial. Trials 2020, 21, 835. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, M.; Ding, Y.; Liu, Y.; Lou, Z.; Zhou, Z.; Sun, L.; Mo, L.; Ye, S.; Pang, H.; et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA 2003, 100, 13190–13195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Ye, F.; Feng, Y.; Yu, F.; Wang, Q.; Wu, Y.; Zhao, C.; Sun, H.; Huang, B.; Niu, P.; et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020, 11, 4417. [Google Scholar] [CrossRef] [PubMed]
- Vuong, W.; Khan, M.B.; Fischer, C.; Arutyunova, E.; Lamer, T.; Shields, J.; Saffran, H.A.; McKay, R.T.; van Belkum, M.J.; Joyce, M.A.; et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020, 11, 4282. [Google Scholar] [CrossRef]
- Qiao, J.; Li, Y.S.; Zeng, R.; Liu, F.L.; Luo, R.-H.; Huang, C.; Wang, Y.-F.; Zhang, J.; Quan, B.; Shen, C.; et al. SARS-CoV-2 M pro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374–1378. [Google Scholar] [CrossRef]
- Zhu, L.; George, S.; Schmidt, M.F.; Al-Gharabli, S.I.; Rademann, J.; Hilgenfeld, R. Peptide aldehyde inhibitors challenge the substrate specificity of the SARS-coronavirus main protease. Antivir. Res. 2011, 92, 204–212. [Google Scholar] [CrossRef]
- Tomar, S.; Johnston, M.L.; John, S.; Osswald, H.L.; Nyalapatla, P.R.; Paul, L.N.; Ghosh, A.K.; Denison, M.; Mesecar, A.D. Ligand-induced Dimerization of Middle East Respiratory Syndrome (MERS) Coronavirus nsp5 Protease (3CLpro): Implications for Nsp5 Regulation and the Development of Antivirals. J. Biol. Chem. 2015, 290, 19403–19422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chen, C.; Yang, K.; Xu, Y.; Liu, X.; Gao, F.; Liu, H.; Chen, X.; Zhao, Q.; Liu, X.J.; et al. Michael Acceptor-Based Peptidomimetic Inhibitor of Main Protease from Porcine Epidemic Diarrhea Virus. J. Med. Chem. 2017, 60, 3212–3216. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Costa, R.; Negrão, R.; Valente, I.; Castela, A.; Duarte, D.; Guardão, L.; Magalhães, P.J.; Rodrigues, J.A.; Guimarães, J.T.; Gomes, P.J.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, J.; Jiang, C.; Song, Z.; Zhao, Y.; Nauwynck, H.; Jiang, P. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2019, 238, 108431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Li, N.; Li, F.; Zhu, Q.; Liu, X.; Han, Q.; Wang, Y.; Chen, Y.; Zeng, X.; Lv, Y.; et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int. Immunopharmacol. 2013, 16, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.; Chen, L. The Potential Role of Xanthohumol in SARS-CoV-2 Treatment—Globally Accessible and Economically Viable. Nutr. Diet. Suppl. 2020, 12, 201–204. [Google Scholar] [CrossRef]
- Lucas, K.; Frhlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases. Front. Plant. Sci. 2021, 12, 589783. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Han, Q.; Chen, J.; Lv, Y. Xanthohumol enhances antiviral effect of interferon α-2b against bovine viral diarrhea virus, a surrogate of hepatitis C virus. Phytomedicine 2010, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, Z.; Han, Q.; Chen, J.; Lou, S.; Qiu, J.; Zhang, G. Inhibition of bovine viral diarrhea virus in vitro by xanthohumol: Comparisons with ribavirin and interferon-α and implications for the development of anti-hepatitis C virus agents. Eur. J. Pharm. Sci. 2009, 38, 332–340. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Osswald, H.L.; Prato, G.J. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, T.S.; Okimoto, N.; Koyama, Y.M.; Hirano, Y.; Taiji, M. Drug binding dynamics of the dimeric SARS-CoV-2 main protease, determined by molecular dynamics simulation. Sci. Rep. 2020, 10, 16986. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, A.; Blass, B.E.; Colussi, D.J.; Almi, I.; Elokely, K.M. Targeting SARS-CoV-2 M3CLpro by HCV NS3/4a Inhibitors: In Silico Modeling and In Vitro Screening. J. Chem. Inf. Model. 2021, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.S.; Amanlou, M. Anti-HCV and anti-malaria agent, potential candidates to repurpose for coronavirus infection: Virtual screening, molecular docking, and molecular dynamics simulation study. Life Sci. 2020, 258, 118205. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Muruato, A.; Lokugamage, K.G.; Narayanan, K.; Zhang, X.; Zou, J.; Liu, J.; Schindewolf, C.; Bopp, N.E.; Aguilar, P.V.; et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe 2020, 27, 841–848.e3. [Google Scholar] [CrossRef]
- Zang, R.; Case, J.B.; Yutuc, E.; Ma, X.; Shen, S.; Gomez Castro, M.F.; Liu, Z.; Zeng, Q.; Zhao, H.; Son, J.; et al. Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion. Proc. Natl. Acad. Sci. USA 2020, 117, 32105–32113. [Google Scholar] [CrossRef]
- Hofmann, M.; Wyler, R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988, 26, 2235–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses. J. Virol. 2019, 93, 2000–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, X.; Huang, Y.Y.; Wu, Y.; Liu, R.; Zhou, L.; Lin, Y.; Wu, D.; Zhang, L.; Liu, H.; et al. Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs. Proc. Natl. Acad. Sci. USA 2020, 117, 27381–27387. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).