Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes—Focus on Toll-like Receptors Expression
Abstract
1. Introduction
2. Results
2.1. Assessment of the Subpopulation of Peripheral Blood Lymphocytes in the Study and Control Group
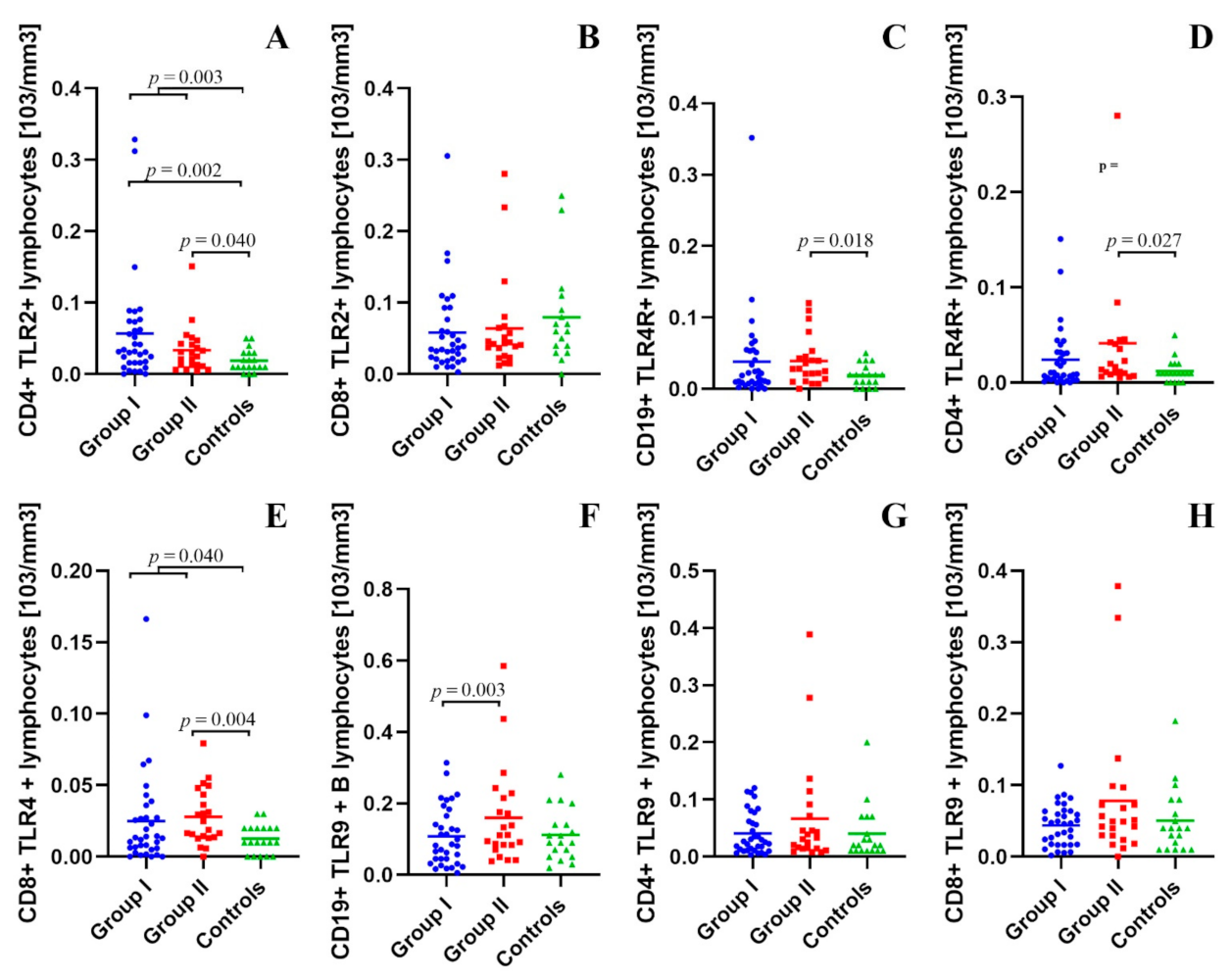
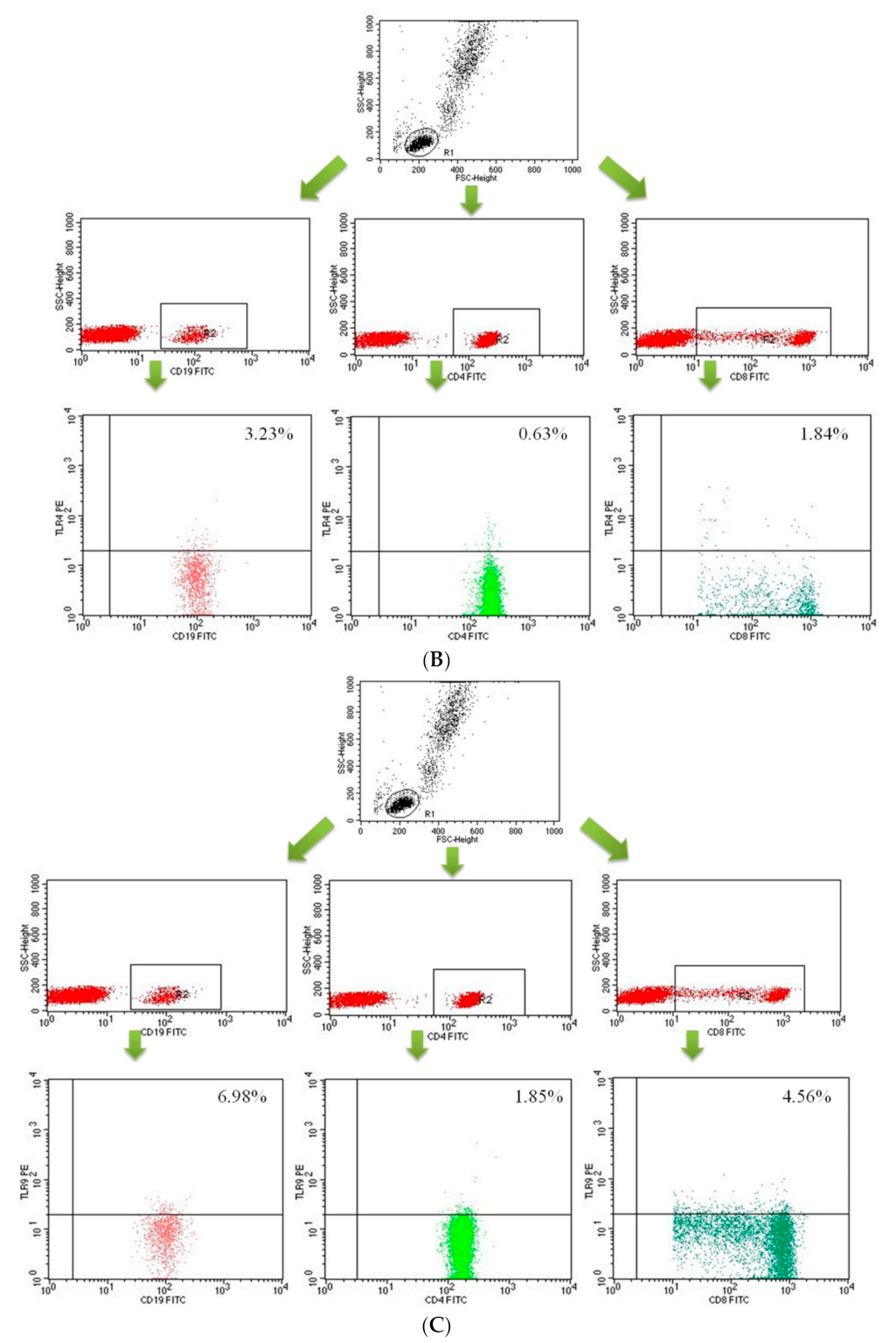
2.2. Assessment of B CD19+, T CD4+ and T CD8+ Lymphocytes with TLR2, TLR4, TLR9 Expression in the Study and Control Groups
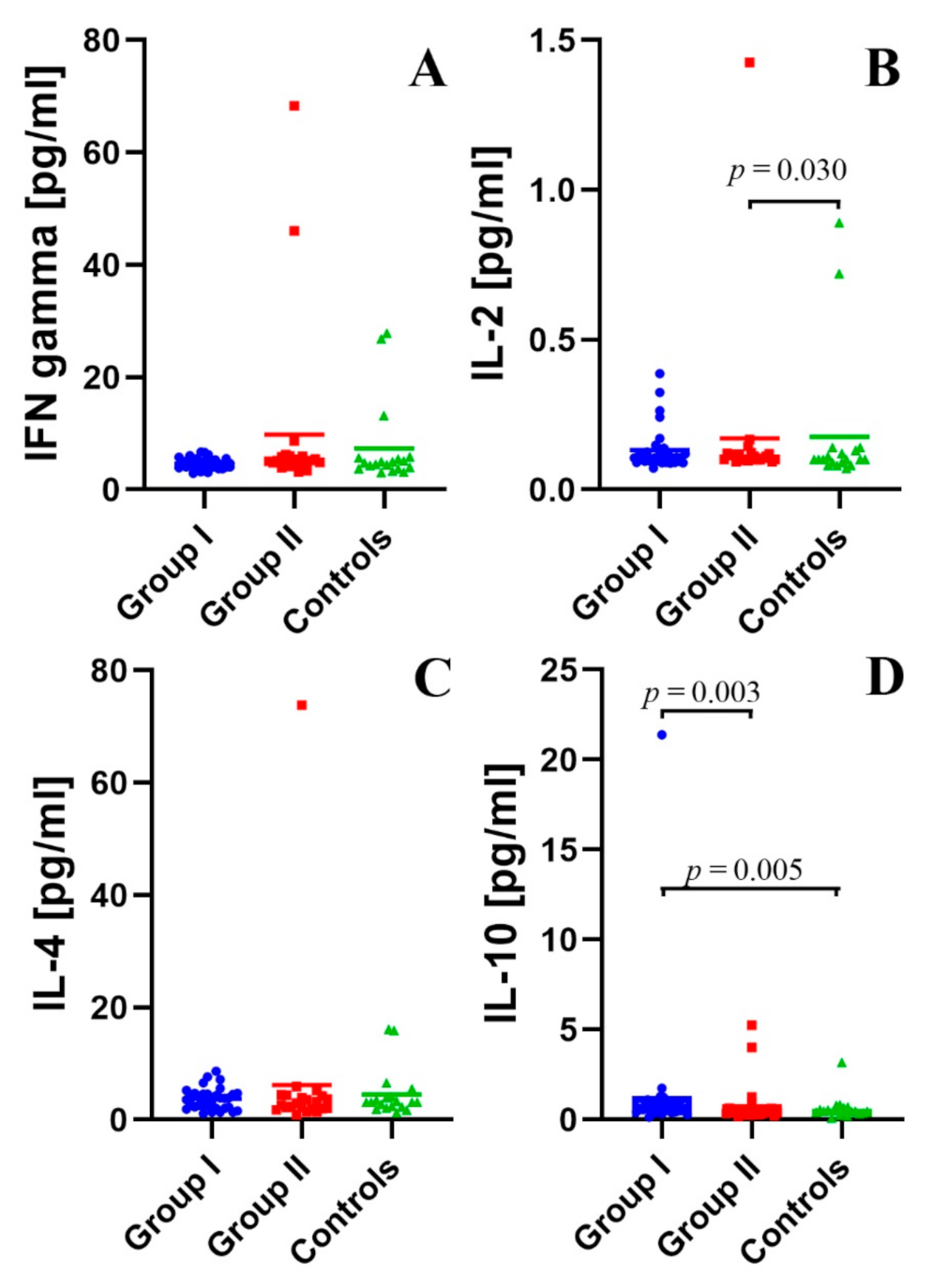
2.3. Assessment of the Concentration of IFN-γ, IL-2, IL-4, and IL-10 in the Peripheral Blood Plasma in the Study and Control Groups
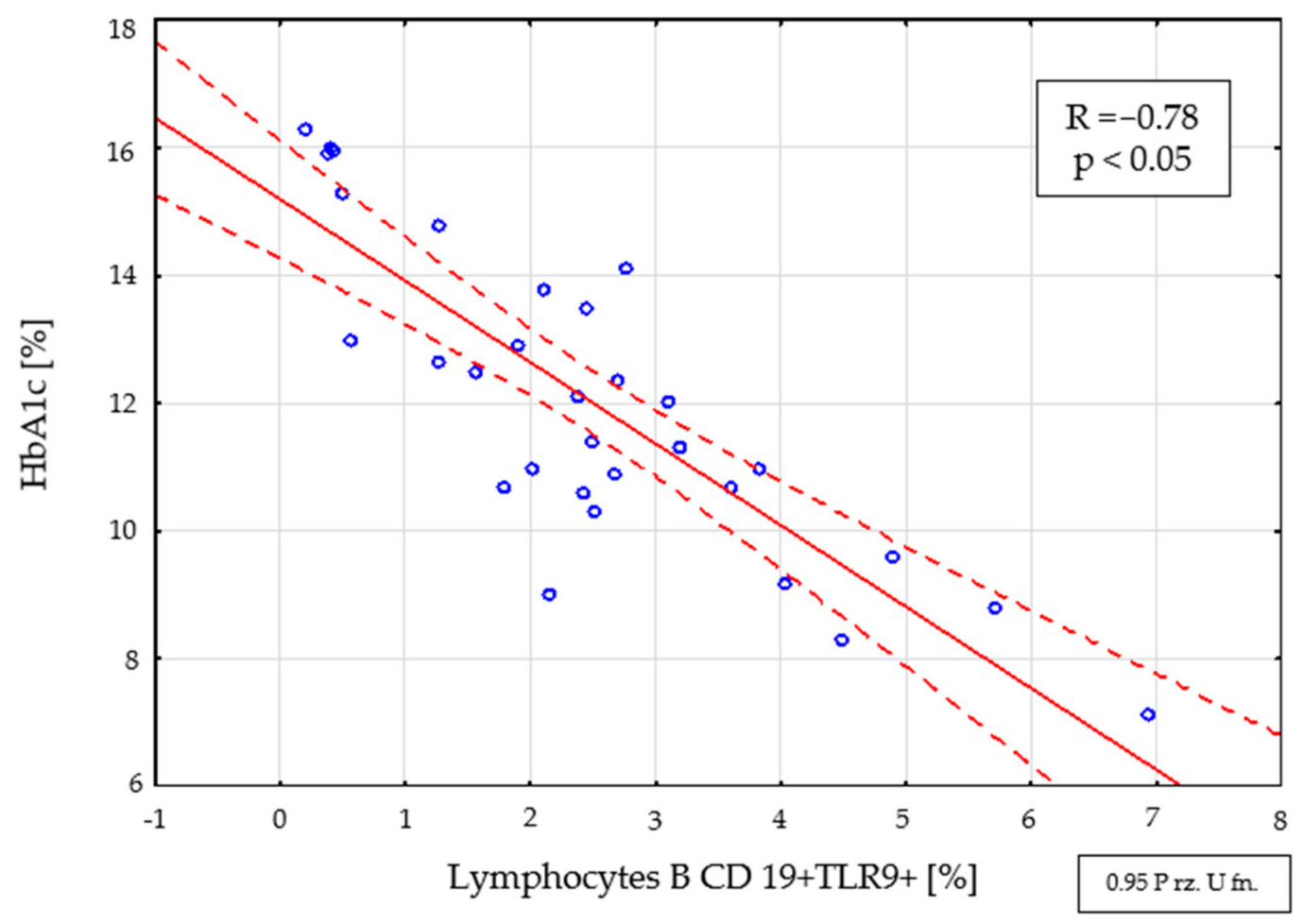
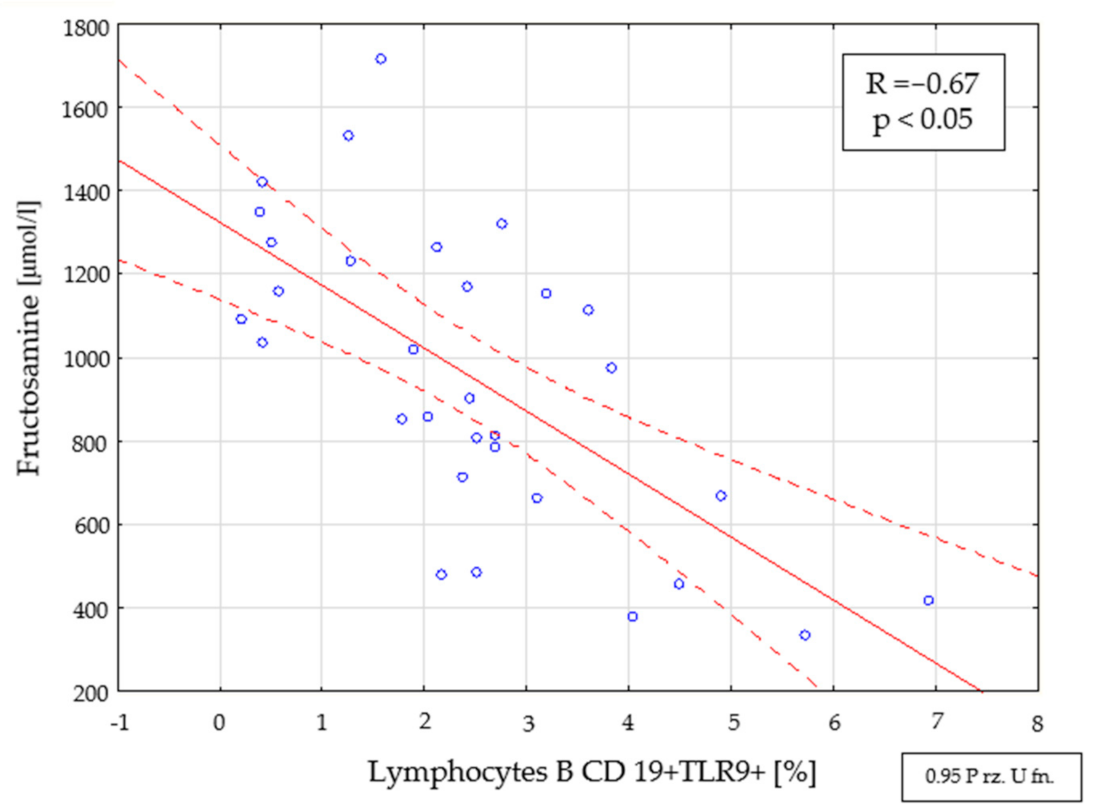
2.4. Assessment of Interdependencies between the Investigated Immunological and Clinical Parameters in the Study Group
2.5. Assessment of the Relationships between B CD19+, T CD4+, and T CD8+ Lymphocytes with TLR2, TLR4, TLR9 Expression, and Duration Time after the Onset of DM1
3. Discussion
4. Materials and Methods
4.1. Patients and Control
4.2. Preparation of the Material
4.3. Assessment of Peripheral Blood Lymphocyte Subpopulation by Flow Cytometry
4.4. Assessment of the Concentration of IFN-γ, IL-2, IL-4 and IL-10 in the Plasma
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginter, E.; Simko, V. Global prevalence and future of diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 35–41. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2019, 42, 13–28. [Google Scholar]
- IDF. Diabetes Atlas—Eighth Edition. Available online: https://www.idf.org/ (accessed on 1 October 2021).
- EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000, 355, 873–876. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis Mellitus and Intermediate Hyperglycemia: Report of WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef]
- Ting, C.; Bansal, V.; Batal, I.; Mounayar, M.; Chabtini, L.; El Akikki, G.; Azzi, J. Impairment of immune systems in diabetes. Adv. Exp. Med. Biol. 2012, 771, 62–75. [Google Scholar]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Damo, X.; Haiying, L.; Mousa, K.K. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cell Mol. Immunol. 2004, 1, 239–246. [Google Scholar]
- Majewska, M.; Szczepanik, M. The role of Toll-Like Receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Post Hig. Med. Dośw. 2006, 60, 52–63. [Google Scholar]
- Zipris, D. Innate immunity and its role in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 326–331. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Xiao, F.; Ma, L.; Zhao, M.; Huang, G.; Mirenda, V.; Dorling, A.; Lechler, R.; Lombardi, G. Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rγnull mice. PLoS ONE 2014, 9, e90387. [Google Scholar] [CrossRef]
- Salomon, B.; Lenschow, D.J.; Rhee, L.; Ashourian, N.; Singh, B.; Sharpe, A.; Bluestone, J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000, 12, 431–440. [Google Scholar] [CrossRef]
- Pop, S.M.; Wong, C.P.; Culton, D.A.; Clarke, S.H.; Tisch, R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J. Exp. Med. 2005, 201, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Shimizu, J.; Yamazaki, S.; Sakihama, T.; Itoh, M.; Kuniyasu, Y.; Nomura, T.; Toda, M.; Takahashi, T. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001, 182, 18–32. [Google Scholar] [CrossRef]
- Papadimitraki, E.D.; Bertsias, G.K.; Boumpas, D.T. Toll like receptors and autoimmunity: A critical appraisal. J. Autoimmun. 2007, 29, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, M.; Ravetch, J.V. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007, 28, 74–79. [Google Scholar] [CrossRef]
- Nündel, K.; Green, N.M.; Shaffer, A.L.; Moody, K.L.; Busto, P.; Eilat, D.; Miyake, K.; Oropallo, M.A.; Cancro, M.P.; Marshak-Rothstein, A. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J. Immunol. 2015, 194, 2504–2512. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Park, S.H.; Jialal, I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia 2009, 52, 1665–1668. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine 2009, 47, 6–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.; Maratha, A.; Siednienko, J.; Natarajan, A.; Gajanayake, T.; Hoashi, S.; Miggin, S. Analysis of inflammatory cytokine and TLR expression levels in Type 2 diabetes with complications. Sci. Rep. 2017, 7, 7633. [Google Scholar] [CrossRef] [PubMed]
- Tisch, R.; McDevitt, H. Insulin-dependent diabetes mellitus. Cell 1996, 85, 291–297. [Google Scholar] [CrossRef]
- Yoon, J.W.; Jun, H.S. Autoimmune destruction of pancreatic beta cells. Am. J. Ther. 2005, 12, 580–591. [Google Scholar] [CrossRef]
- Faustman, D.L.; Davis, M. The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J. Mol. Med. 2009, 87, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P. CD8+ T-Cell deficiency, epstein-barr virus infection, vitamin D deficiency and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Menart-Houtermans, B.; Ruetter, R.; Nowotny, B.; Gehrmann, U.; Markgraf, D.; Szendroedi, J.; Schloot, N.C.; Roden, M. Characterization of circulating leukocytes and correlation of leukocyte subsets with metabolic parameters 1 and 5 years after diabetes diagnosis. Acta Diabetol. 2018, 55, 723–731. [Google Scholar] [CrossRef]
- Sarikonda, G.; Pettus, J.; Phatak, S.; Sachithanantham, S.; Miller, J.F.; Wesley, J.D.; Cadag, E.; Chae, J.; Ganesan, L.; Mallios, R.; et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J. Autoimmun. 2014, 50, 77–82. [Google Scholar] [CrossRef]
- Filippi, C.; Ehrhardt, K.; Estes, E.; Larsson, P.; Oldham, J.; Herrath, M. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur. J. Immunol. 2011, 41, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Fraker, C.; Bayer, A.L. The expanding role of natural killer cells in type 1 diabetes and immunotherapy. Curr. Diabetes Rep. 2016, 16, 109. [Google Scholar] [CrossRef]
- Gur, C.; Porgador, A.; Elboim, M.; Gazit, R.; Mizrahi, S.; Stern-Ginossar, N.; Achdout, H.; Ghadially, H.; Dor, Y.; Nir, T.; et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat. Immunol. 2010, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; Planas, R.; Clemente, X.; Carrillo, J.; Ampudia, R.; Puertas, M.-C.; Pastor, X.; Tolosa, E.; Pujol-Borrell, R.; Verdaguer, J.; et al. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-beta. Clin. Exp. Immunol. 2008, 151, 467–475. [Google Scholar] [CrossRef]
- Enk, J.; Mandelboim, O. The role of natural cytotoxicity receptors in various pathologies: Emphasis on type I diabetes. Front. Immunol. 2014, 5, 4. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Wang, H.; Lou, X.; Qu, X.; Gao, L.; Liu, X.; Li, M.; Guo, H.; Jiang, Y. Decreased percentage of NKG2D+NK cells in patients with incident onset of Type 1 Diabetes. Clin. Exp. Pharmacol. Physiol. 2017, 44, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Kopf, M. Toll-like receptors: Paving the path to T cell-driven autoimmunity? Curr. Opin. Immunol. 2007, 19, 611–614. [Google Scholar] [CrossRef]
- Alkanani, A.K.; Rewers, M.; Dong, F.; Waugh, K.; Gottlieb, P.A.; Zipris, D. Dysregulated Toll-like receptor-induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes 2012, 61, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Rydén, A.; Faresjö, M. Altered immune profile from pre-diabetes to manifestation of type 1 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 74–84. [Google Scholar] [CrossRef]
- He, J.S.; Xie, P.S.; Luo, D.S.; Sun, C.J.; Zhang, Y.G.; Liu, F.X. Role of immune dysfunction in pathogenesis of type 1 diabetes mellitus in children. Asian Pac. J. Trop. Med. 2014, 7, 823–826. [Google Scholar] [CrossRef]
- Anders, H.J.; Zecher, D.; Pawar, R.D.; Patole, P.S. Molecular mechanisms of autoimmunity triggered by microbial infection. Arthritis Res. Ther. 2005, 7, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mortezagholi, S.; Babaloo, Z.; Rahimzadeh, P.; Namdari, H.; Ghaedi, M.; Gharibdost, F.; Mirzaei, R.; Bidad, K.; Salehi, E. Evaluation of TLR9 expression on PBMCs and CpG ODN-TLR9 ligation on IFN-α production in SLE patients. Immunopharmacol. Immunotoxicol. 2017, 39, 11–18. [Google Scholar] [CrossRef]
- Kikodze, N.; Pantsulaia, I.; Rekhviashvili, K.; Iobadze, M.; Dzhakhutashvili, N.; Pantsulaia, N.; Kukuladze, N.; Bikashvili, N.; Metreveli, D.; Chikovani, T. Cytokines and T regulatory cells in the pathogenesis of type 1 diabetes. Georgian Med. News 2013, 29–35. [Google Scholar]
- Esposito, K.; Pontillo, A.; Giugliano, F.; Giugliano, G.; Marfella, R.; Nicoletti, G.; Giugliano, D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J. Endocronol. Metab. 2003, 88, 1055–1058. [Google Scholar] [CrossRef]
- Kulshrestha, H.; Gupta, V.; Mishra, S.; Mahdi, A.A.; Awasthi, S.; Kumar, S. Interleukin-10 as a novel biomarker of metabolic risk factors. Diabetes Metab. Syndr. 2018, 12, 543–547. [Google Scholar] [CrossRef]
- Pestana, R.M.C.; Domingueti, C.P.; Duarte, R.C.F.; Fóscolo, R.B.; Reis, J.S.; Rodrigues, A.M.S.; Martins, L.B.; Sousa, L.P.; Lage, D.P.; Ferreira, C.N.; et al. Cytokines profile and its correlation with endothelial damage and oxidative stress in patients with type 1 diabetes mellitus and nephropathy. Immunol. Res. 2016, 64, 951–960. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M. Serum IL-1β, IL-2, and IL-6 in insulin-dependent diabetic children. Mediat. Inflamm. 2006, 2006, 59206. [Google Scholar] [CrossRef]
- Pérol, L.; Lindner, J.M.; Caudana, P.; Nunez, N.G.; Baeyens, A.; Valle, A.; Sedlik, C.; Loirat, D.; Boyer, O.; Créange, A.; et al. Loss of immune tolerance to IL-2 in type 1 diabetes. Nat. Commun. 2016, 7, 13027. [Google Scholar] [CrossRef]
- Gitelman, S.E.; Bluestone, J.A. Regulatory T cell therapy for type 1 diabetes: May the force be with you. J. Autoimmun. 2016, 71, 78–87. [Google Scholar] [CrossRef]
- Rosenzwajg, M.; Churlaud, G.; Hartemann, A.; Klatzmann, D. Interleukin 2 in the pathogenesis and therapy of type 1 diabetes. Curr. Diab. Rep. 2014, 14, 553. [Google Scholar] [CrossRef]
- Long, S.A.; Rieck, M.; Sanda, S.; Bollyky, J.B.; Samuels, P.L.; Goland, R.; Ahmann, A.; Rabinovitch, A.; Aggarwal, S.; Phippard, D.; et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes 2012, 61, 2340–2348. [Google Scholar] [CrossRef]
- Russell, M.A.; Morgan, N.G. The impact of anti-inflammatory cytokines on the pancreatic β cell. Islets 2014, 6, e950547. [Google Scholar] [CrossRef]
- Kaminski, A.; Kaminski, E.R.; Morgan, N.G. Pre-incubation with interleukin-4 mediates a direct protective effect against the loss of pancreatic β-cell viability induced by proinflammatory cytokines. Clin. Exp. Immunol. 2007, 148, 583–588. [Google Scholar] [CrossRef]
- Wong, H.L.; Costa, G.L.; Lotze, M.T.; Wahl, S.M. Interleukin-4 diferentially regulates monocyte IL-1 family gene expression and synthesis in vitro and in vivo. J. Exp. Med. 1993, 177, 775–781. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ho, K.T.; Lu, S.H. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Shiau, M.Y.; Lai, Y.R.; Ho, K.T.; Hsiao, C.W.; Chen, C.-J.; Lo, Y.-L.; Chang, Y.-H. Interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. Oxid. Med. Cell Longev. 2018, 2018, 6923187. [Google Scholar] [CrossRef] [PubMed]

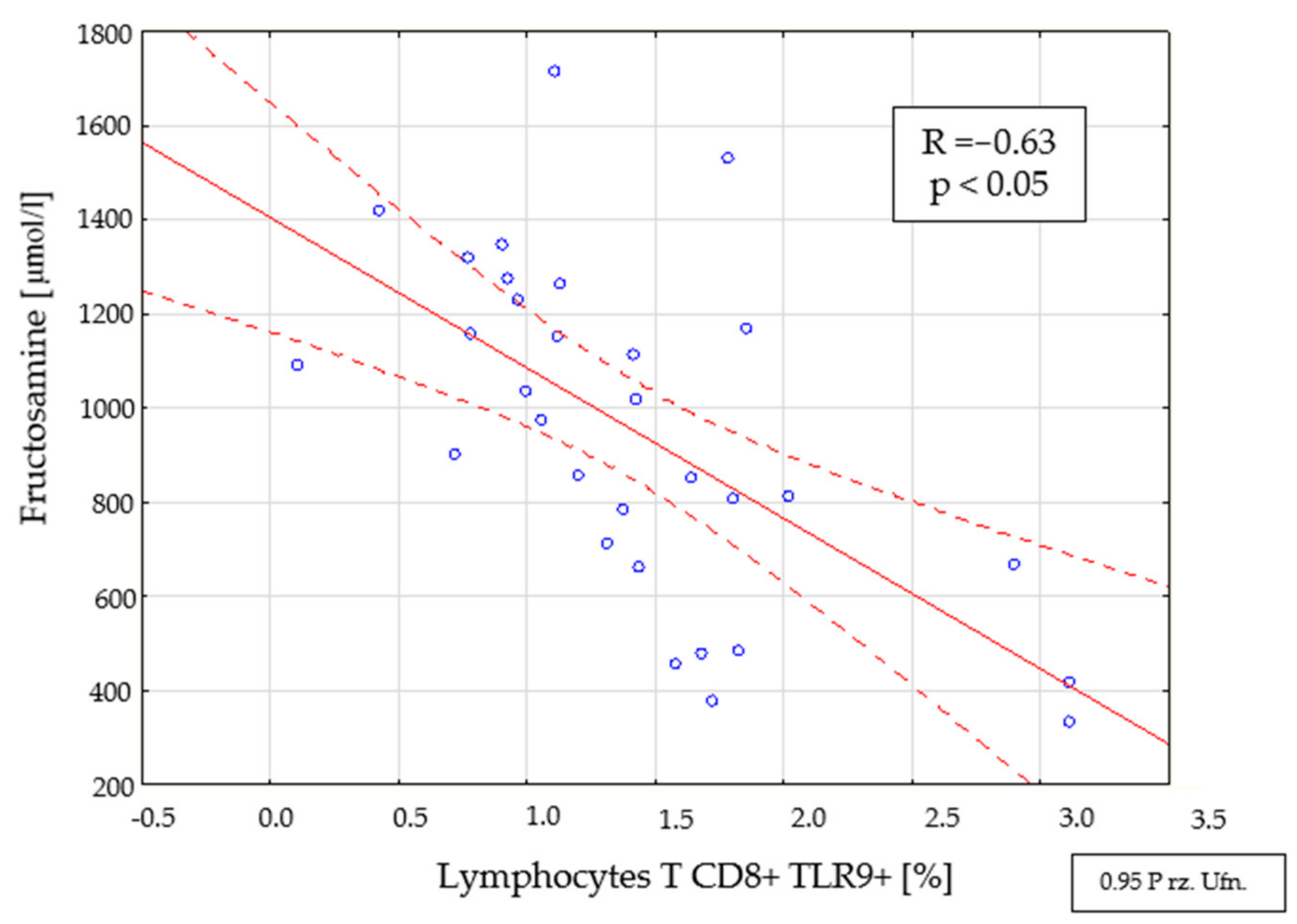
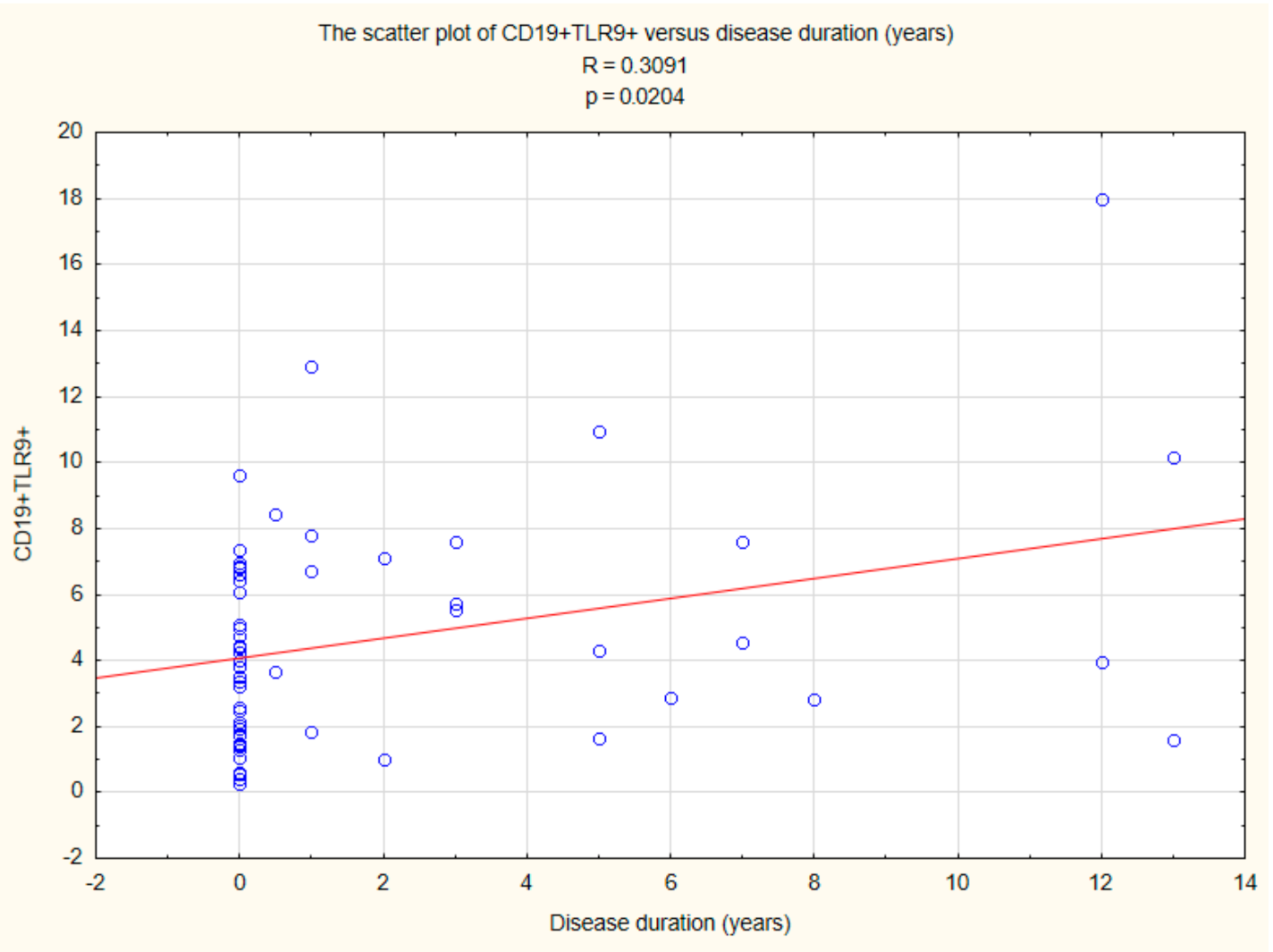
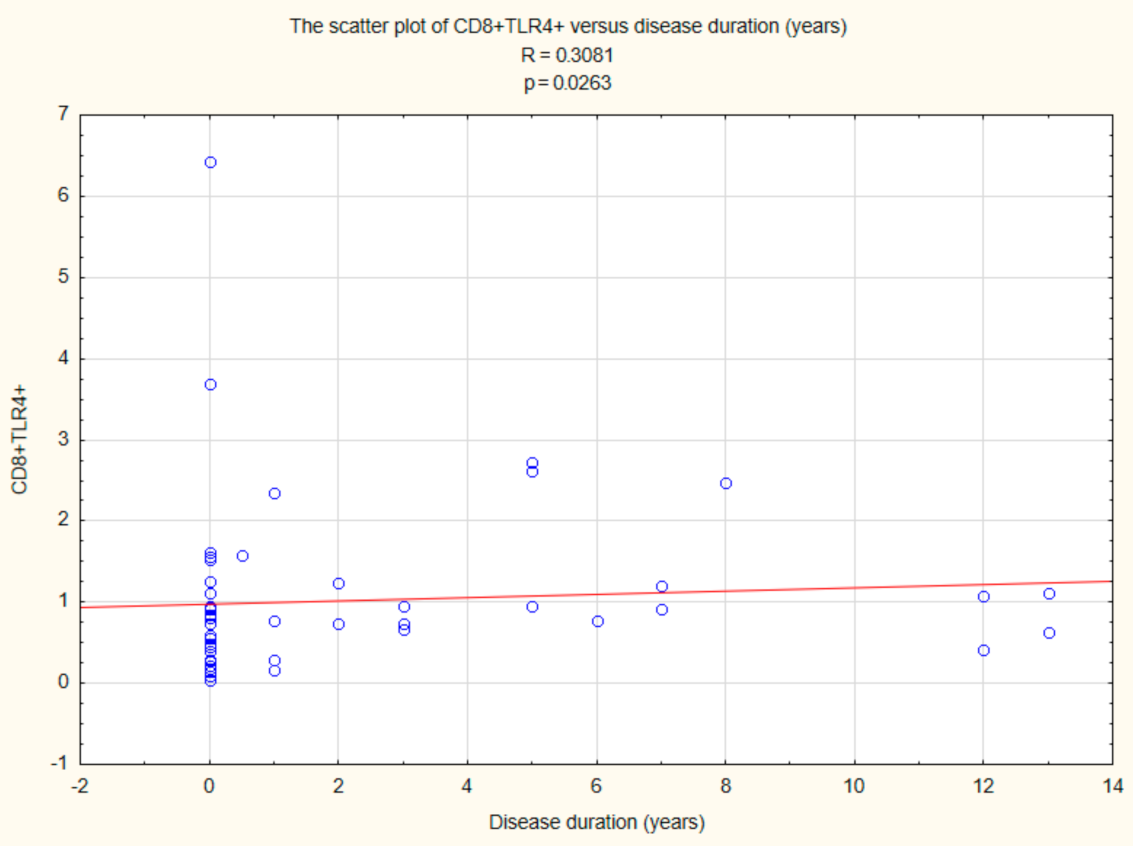

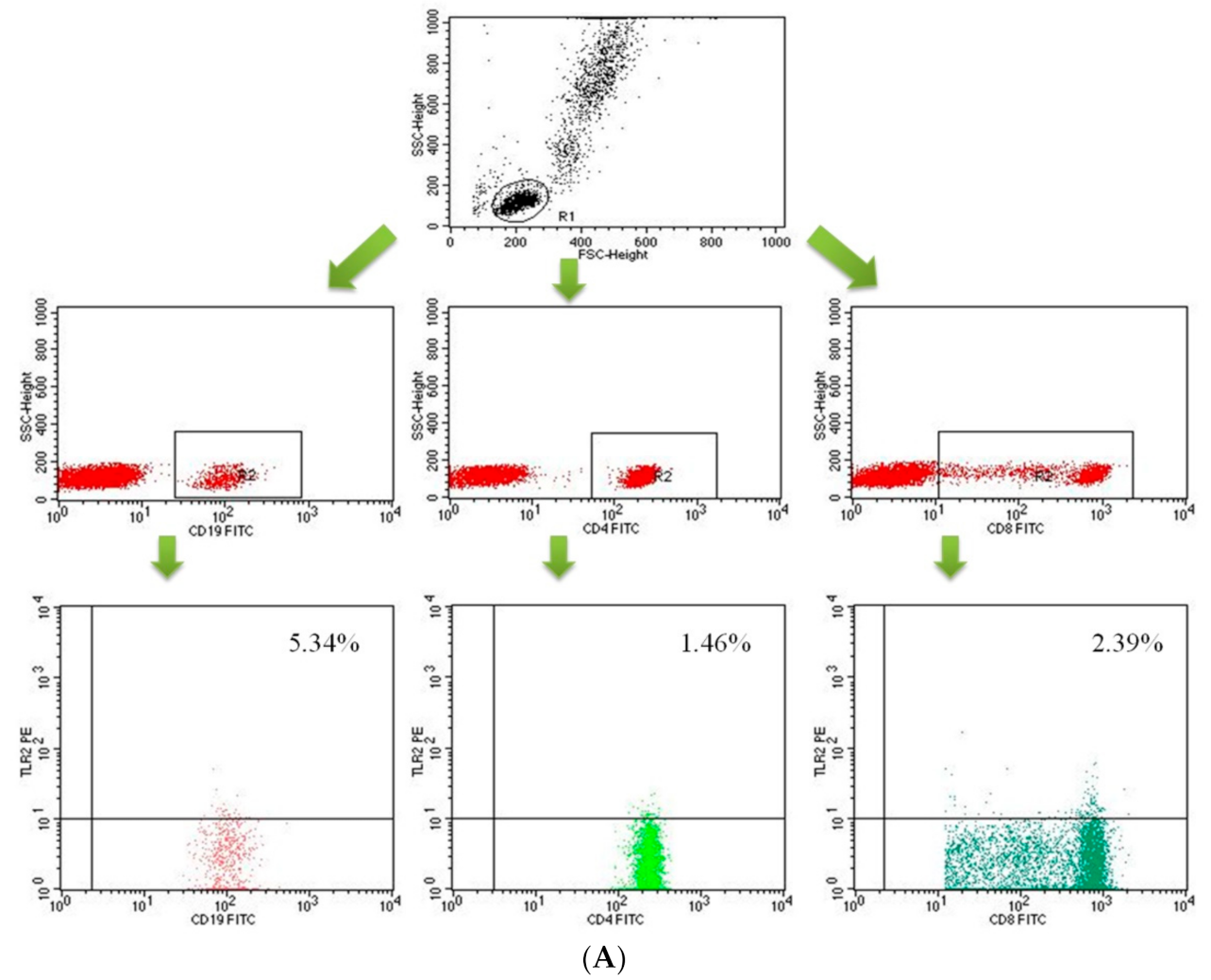
| Parameter | Mean | Median | Minimum | Maximum | SD | |
|---|---|---|---|---|---|---|
| Glycated hemoglobin [%] | Group I | 12 | 12.01 | 7.14 | 16 | 2.40 |
| Group II | 8.8 | 9.20 | 5.70 | 16 | 2 | |
| Fructosamine [μmol/L] | Group I | 950.04 | 974 | 333 | 1714 | 360.34 |
| Group II | 513.10 | 520.50 | 286 | 7000 | 134.58 | |
| C-peptide [ng/mL] | Group I | 0.46 | 0.34 | 0.10 | 1.89 | 0.39 |
| Group II | 0.53 | 0.53 | 0.20 | 0.82 | 0.21 | |
| White blood cells [103/μL] | Group I | 9.63 | 8.40 | 4.18 | 24.32 | 4.28 |
| Group II | 6.94 | 6.71 | 4.04 | 12.99 | 1.89 | |
| Controls | 6.43 | 5.91 | 4.05 | 8.99 | 1.38 | |
| Neutrocytes [103/μL] | Group I | 5.86 | 4.77 | 1.50 | 17.41 | 3.64 |
| Group II | 3.55 | 3.35 | 1.60 | 6.90 | 1.22 | |
| Controls | 3.33 | 2.98 | 2.06 | 5.55 | 1.11 | |
| Lymphocytes [103/μL] | Group I | 2.88 | 2.80 | 0.72 | 4.53 | 0.95 |
| Group II | 2.58 | 2.26 | 1.66 | 5.06 | 0.85 | |
| Controls | 2.33 | 2.53 | 1.23 | 3.48 | 0.67 | |
| Red blood cells [106/μL] | Group I | 5.06 | 4.84 | 4.14 | 6.68 | 0.59 |
| Group II | 5.08 | 4.93 | 4.10 | 6.10 | 0.51 | |
| Controls | 4.79 | 4.79 | 4.04 | 5.28 | 0.34 | |
| Hemoglobin [g/dL] | Group I | 14.04 | 14.20 | 11.60 | 16.20 | 1.25 |
| Group II | 14.58 | 14.20 | 12.70 | 16.90 | 1.51 | |
| Controls | 13.66 | 13.85 | 11.70 | 16.00 | 0.97 | |
| Platelets [103/μL] | Group I | 274.39 | 262 | 99 | 534 | 82.35 |
| Group II | 278.82 | 282.50 | 203 | 382 | 49.52 | |
| Controls | 291.69 | 289.50 | 221 | 400 | 50.05 | |
| Lymphocytes T CD3+ [%] | Study group | 72.71 | 74.02 | 58.96 | 83.70 | 5.70 |
| Group I | 73.00 | 74.02 | 58.96 | 83.70 | 6.01 | |
| Group II | 72.31 | 73.94 | 62.75 | 81.14 | 5.34 | |
| Controls | 68.13 | 70.94 | 52.19 | 75.22 | 6.89 | |
| Lymphocytes T CD3+ [103/mm3] | Study group | 2.017 | 1.851 | 0.537 | 3.860 | 0.740 |
| Group I | 2.116 | 1.946 | 0.537 | 3.600 | 0.770 | |
| Group II | 1.879 | 1.598 | 1.148 | 3.860 | 0.670 | |
| Controls | 1.596 | 1.752 | 0.783 | 2.448 | 0.507 | |
| Lymphocytes B CD19+ [%] | Study group | 14.61 | 14.28 | 4.64 | 30.76 | 5.23 |
| Group I | 16.02 | 15.48 | 4.64 | 30.76 | 5.77 | |
| Group II | 12.63 | 12.79 | 6.43 | 20.53 | 3.64 | |
| Controls | 11.76 | 10.55 | 5.27 | 27.51 | 5.02 | |
| Lymphocytes B CD19+ [103/mm3] | Study group | 0.401 | 0.333 | 0.118 | 1.050 | 0.200 |
| Group I | 0.454 | 0.461 | 0.118 | 1.050 | 0.212 | |
| Group II | 0.327 | 0.309 | 0.126 | 0.740 | 0.150 | |
| Controls | 0.271 | 0.252 | 0.092 | 0.527 | 0.121 | |
| Lymphocytes T CD3+CD4+ [%] | Study group | 41.09 | 42.27 | 28.83 | 53.07 | 5.73 |
| Group I | 40.91 | 42.41 | 28.83 | 53.07 | 6.27 | |
| Group II | 41.33 | 41.64 | 30.31 | 48.93 | 5.03 | |
| Controls | 37.37 | 36.07 | 28.56 | 51.68 | 7.35 | |
| Lymphocytes T CD3+CD4+ [103/mm3] | Study group | 1.131 | 1.043 | 0.382 | 2.090 | 0.420 |
| Group I | 1.182 | 1.139 | 0.382 | 2.090 | 0.459 | |
| Group II | 1.063 | 0.947 | 0.667 | 2.080 | 0.360 | |
| Controls | 0.877 | 0.906 | 0.359 | 1.387 | 0.310 | |
| Lymphocytes T CD3+CD8+ [%] | Study group | 27.30 | 27.91 | 15.30 | 42.06 | 4.93 |
| Group I | 28.00 | 28.56 | 17.18 | 42.06 | 5.21 | |
| Group II | 26.35 | 26.81 | 15.30 | 32.92 | 4.47 | |
| Controls | 24.97 | 23.60 | 19.06 | 34.75 | 4.43 | |
| Lymphocytes T CD3+CD8+ [103/mm3] | Study group | 0.767 | 0.684 | 0.143 | 1.470 | 0.320 |
| Group I | 0.825 | 0.827 | 0.143 | 1.470 | 0.340 | |
| Group II | 0.688 | 0.640 | 0.372 | 1.350 | 0.270 | |
| Controls | 0.587 | 0.581 | 0.285 | 0.998 | 0.212 | |
| CD3+CD4+/CD3+CD8+ ratio | Study group | 1.57 | 1.46 | 0.80 | 3.10 | 0.46 |
| Group I | 1.53 | 1.45 | 0.80 | 2.67 | 0.45 | |
| Group II | 1.63 | 1.52 | 0.97 | 3.10 | 0.46 | |
| Controls | 1.56 | 1.49 | 0.90 | 2.56 | 0.48 | |
| NK cells [%] | Study group | 9.23 | 8.45 | 2.25 | 22.21 | 4.99 |
| Group I | 7.43 | 7.06 | 2.25 | 14.07 | 3.49 | |
| Group II | 11.77 | 11.25 | 2.68 | 22.21 | 5.72 | |
| Controls | 13.34 | 12.56 | 4.05 | 22.62 | 5.47 | |
| NK cells [103/mm3] | Study group | 0.248 | 0.230 | 0.021 | 0.720 | 0.140 |
| Group I | 0.214 | 0.188 | 0.021 | 0.570 | 0.124 | |
| Group II | 0.295 | 0.256 | 0.056 | 0.720 | 0.160 | |
| Controls | 0.309 | 0.247 | 0.110 | 0.654 | 0.165 | |
| The level of significance of differences between the studied groups in the percentage and number of parameter. | ||||||
| Parameter | Study Group/Controls | Group I/Controls | Group II/Controls | Group I/Group II | ||
| Lymphocytes T CD3+ [%] | 0.009 | 0.016 | 0.042 | NS | ||
| Lymphocytes T CD3+ [103/mm3] | 0.036 | 0.019 | NS | NS | ||
| Lymphocytes B CD19+ [%] | NS | 0.016 | NS | 0.019 | ||
| Lymphocytes B CD19+ [103/mm3] | 0.015 | 0.003 | NS | 0.019 | ||
| Lymphocytes T CD3+CD4+ [%] | 0.038 | NS | NS | NS | ||
| Lymphocytes T CD3+CD4+ [103/mm3] | 0.028 | 0.022 | NS | NS | ||
| Lymphocytes T CD3+CD8+ [%] | NS | 0.015 | NS | NS | ||
| Lymphocytes T CD3+CD8+ [103/mm3] | 0.038 | 0.015 | NS | NS | ||
| CD3+CD4+/CD3+CD8+ ratio | NS | NS | NS | NS | ||
| NK cells [%] | 0.007 | <0.001 | NS | 0.001 | ||
| NK cells [103/mm3] | NS | 0.035 | NS | 0.041 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurianowicz, K.; Klatka, M.; Polak, A.; Hymos, A.; Bębnowska, D.; Podgajna, M.; Hrynkiewicz, R.; Sierawska, O.; Niedźwiedzka-Rystwej, P. Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes—Focus on Toll-like Receptors Expression. Int. J. Mol. Sci. 2021, 22, 12135. https://doi.org/10.3390/ijms222212135
Kurianowicz K, Klatka M, Polak A, Hymos A, Bębnowska D, Podgajna M, Hrynkiewicz R, Sierawska O, Niedźwiedzka-Rystwej P. Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes—Focus on Toll-like Receptors Expression. International Journal of Molecular Sciences. 2021; 22(22):12135. https://doi.org/10.3390/ijms222212135
Chicago/Turabian StyleKurianowicz, Katarzyna, Maria Klatka, Agnieszka Polak, Anna Hymos, Dominika Bębnowska, Martyna Podgajna, Rafał Hrynkiewicz, Olga Sierawska, and Paulina Niedźwiedzka-Rystwej. 2021. "Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes—Focus on Toll-like Receptors Expression" International Journal of Molecular Sciences 22, no. 22: 12135. https://doi.org/10.3390/ijms222212135
APA StyleKurianowicz, K., Klatka, M., Polak, A., Hymos, A., Bębnowska, D., Podgajna, M., Hrynkiewicz, R., Sierawska, O., & Niedźwiedzka-Rystwej, P. (2021). Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes—Focus on Toll-like Receptors Expression. International Journal of Molecular Sciences, 22(22), 12135. https://doi.org/10.3390/ijms222212135








