Immune Tolerance in the Oral Mucosa
Abstract
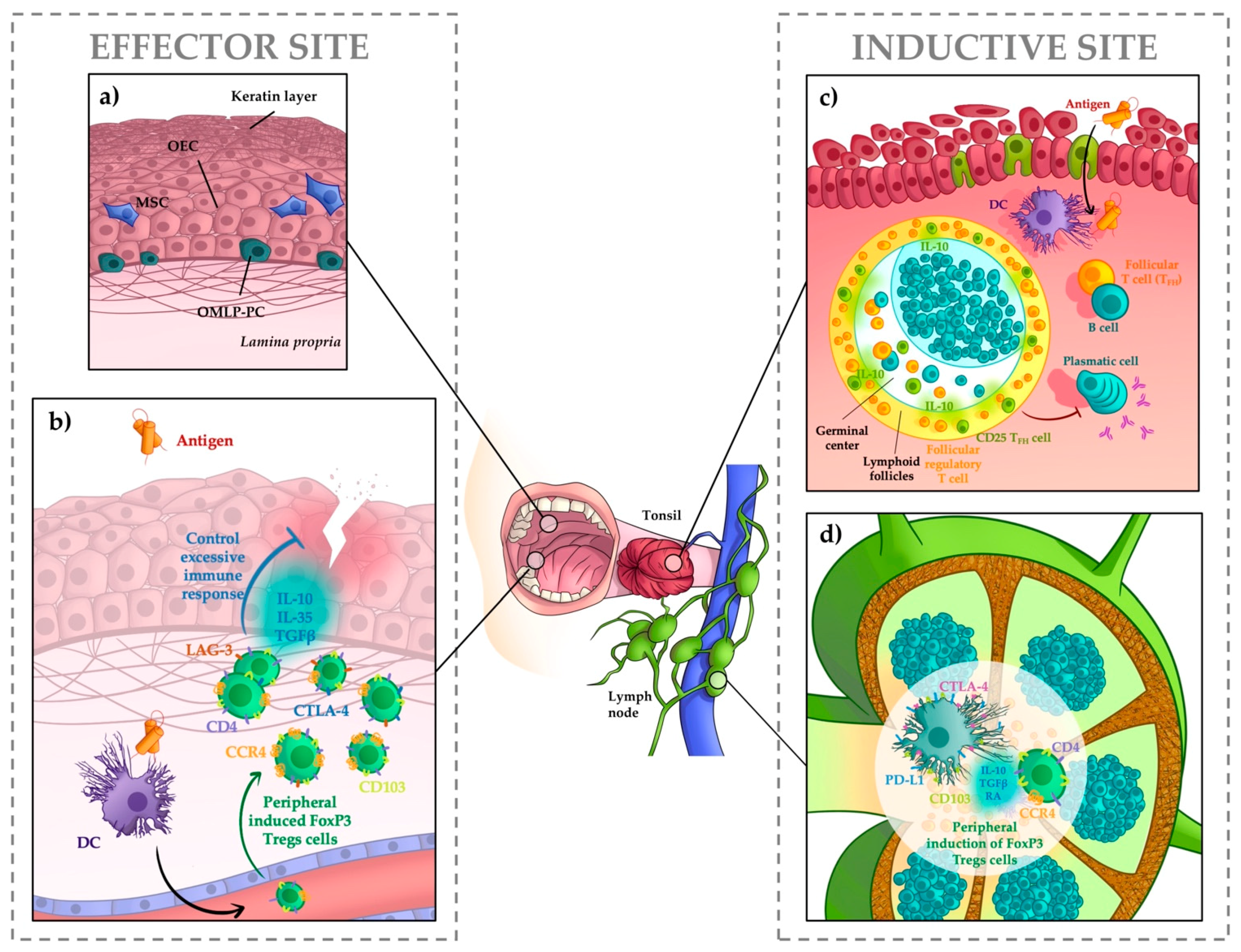
:1. Anatomy of Oral Mucosa and Associated Lymphoid Tissues
2. Immune Responses in the Oral Mucosa
3. Tolerance Mediated by Regulatory T Cells
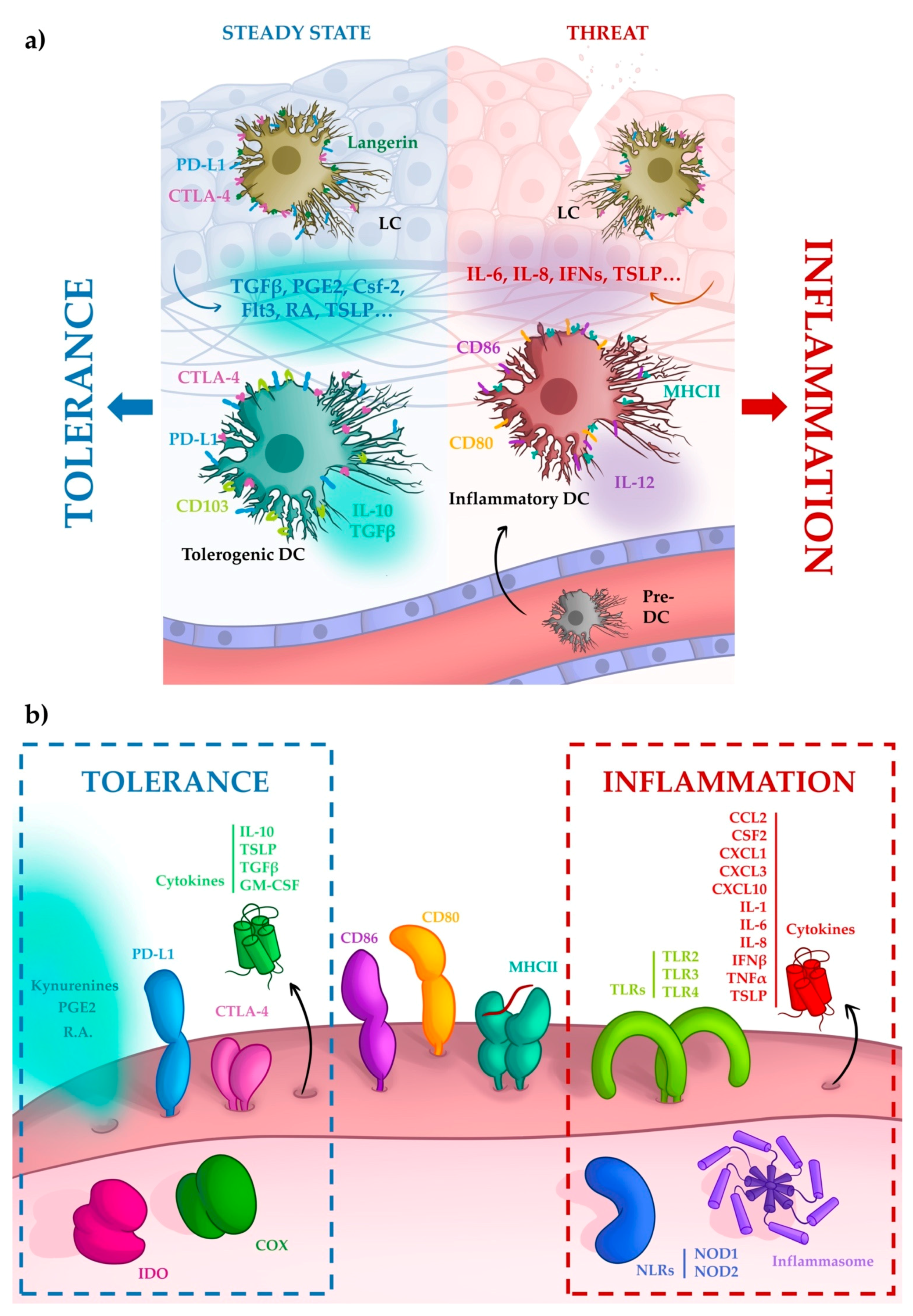
4. Tolerance Mediated by Dendritic Cells
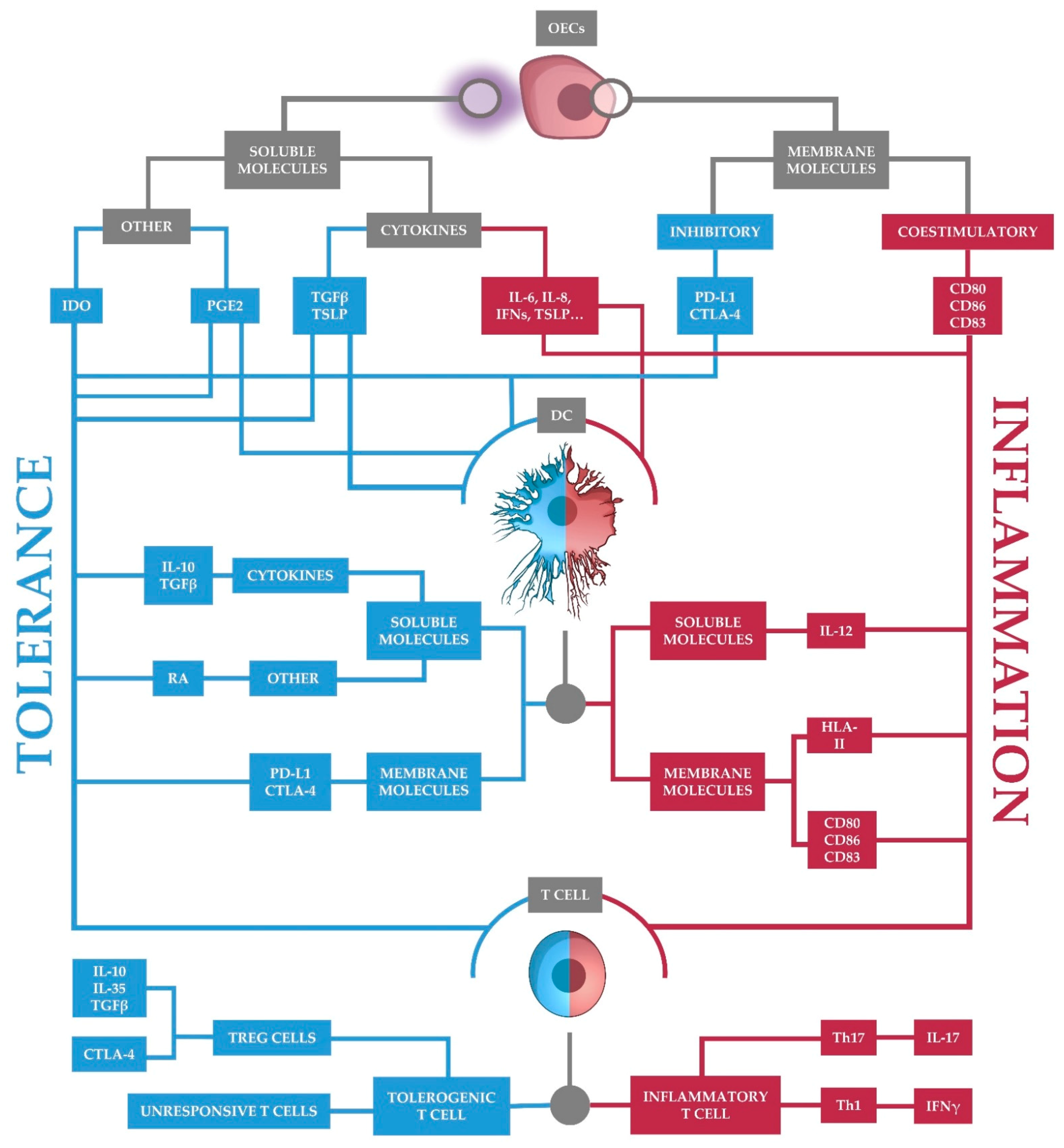
5. Tolerance Begins with Oral Epithelial Cells
5.1. Soluble Molecules
5.2. Surface Molecules
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.P. Oral anatomy and tissue types. Semin. Dermatol. 1994, 13, 68–73. [Google Scholar] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Elsevier: Montreal, QC, Canada, 2012. [Google Scholar]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling. Annu. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef] [Green Version]
- van der Bilt, A.; Engelen, L.; Pereira, L.J.; van der Glas, H.W.; Abbink, J.H. Oral physiology and mastication. Physiol. Behav. 2006, 89, 22–27. [Google Scholar] [CrossRef]
- Hellings, P.; Jorissen, M.; Ceuppens, J.L. The Waldeyer’s ring. Acta Otorhinolaryngol. Belg. 2000, 54, 237–241. [Google Scholar] [PubMed]
- Sakr, M. (Ed.) Head and Neck and Endocrine Surgery; Springer: Cham, Switzerland, 2016; p. XIII, 393. [Google Scholar]
- Kiyono, H.; Bienenstock, J.; McGhee, J.R.; Ernst, P.B. The mucosal immune system: Features of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg. Immunol. 1992, 4, 54–62. [Google Scholar]
- Scadding, G.K. Immunology of the tonsil: A review. J. R. Soc. Med. 1990, 83, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Van Kempen, M.J.; Rijkers, G.T.; Van Cauwenberge, P.B. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 2000, 122, 8–19. [Google Scholar] [CrossRef]
- Novak, N.; Haberstok, J.; Bieber, T.; Allam, J.P. The immune privilege of the oral mucosa. Trends Mol. Med. 2008, 14, 191–198. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, H.; DiPalma, D.T.; Tai, X.; Park, J.H. Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3(+) regulatory T cells. Mucosal Immunol. 2018, 11, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Bluestone, J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerwenka, A.; Swain, S.L. TGF-beta1: Immunosuppressant and viability factor for T lymphocytes. Microbes Infect. 1999, 1, 1291–1296. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Oderup, C.; Cederbom, L.; Makowska, A.; Cilio, C.M.; Ivars, F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006, 118, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Lee, G.R. Transcriptional regulation and development of regulatory T cells. Exp. Mol. Med. 2018, 50, e456. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Louvet, C.; Davini, D.; Gardner, J.M.; Martinez-Llordella, M.; Bailey-Bucktrout, S.; Anthony, B.A.; Sverdrup, F.M.; Head, R.; Kuster, D.J.; et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012, 209, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.M.; Bilate, A.M.; Gobert, M.; Ding, Y.; Curotto de Lafaille, M.A.; Parkhurst, C.N.; Xiong, H.; Dolpady, J.; Frey, A.B.; Ruocco, M.G.; et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012, 209, 1723–1742. [Google Scholar] [CrossRef]
- Lehmann, J.; Huehn, J.; de la Rosa, M.; Maszyna, F.; Kretschmer, U.; Krenn, V.; Brunner, M.; Scheffold, A.; Hamann, A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 13031–13036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Xu, J.; Bromberg, J.S. Regulatory T cell migration during an immune response. Trends Immunol. 2012, 33, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, J.; Jia, R. Treg: A Promising Immunotherapeutic Target in Oral Diseases. Front Immunol. 2021, 12, 2195. [Google Scholar] [CrossRef]
- Francisconi, C.F.; Vieira, A.E.; Biguetti, C.C.; Glowacki, A.J.; Trombone, A.P.; Letra, A.; Menezes Silva, R.; Sfeir, C.S.; Little, S.R.; Garlet, G.P. Characterization of the Protective Role of Regulatory T Cells in Experimental Periapical Lesion Development and Their Chemoattraction Manipulation as a Therapeutic Tool. J. Endod. 2016, 42, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Fonseca, V.R.; Graca, L. Contribution of FoxP3(+) Tfr cells to overall human blood CXCR5(+) T cells. Clin. Exp. Immunol. 2019, 195, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Canete, P.F.; Sweet, R.A.; Gonzalez-Figueroa, P.; Papa, I.; Ohkura, N.; Bolton, H.; Roco, J.A.; Cuenca, M.; Bassett, K.J.; Sayin, I.; et al. Regulatory roles of IL-10-producing human follicular T cells. J. Exp. Med. 2019, 216, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Carrington, E.M.; van Nieuwenhuijze, A.; Bedoui, S.; Seah, S.; Xu, Y.; Wang, N.; Mintern, J.D.; Villadangos, J.A.; Wicks, I.P.; et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur. J. Immunol. 2011, 41, 2585–2595. [Google Scholar] [CrossRef]
- Edelson, B.T.; Bradstreet, T.R.; Kc, W.; Hildner, K.; Herzog, J.W.; Sim, J.; Russell, J.H.; Murphy, T.L.; Unanue, E.R.; Murphy, K.M. Batf3-dependent CD11b(low/-) peripheral dendritic cells are GM-CSF-independent and are not required for Th cell priming after subcutaneous immunization. PLoS ONE 2011, 6, e25660. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Patil, R.S.; Pradhan, T.N.; Chaukar, D.A.; D’Cruz, A.K.; Chiplunkar, S.V. Myeloid-derived suppressor cells impede T cell functionality and promote Th17 differentiation in oral squamous cell carcinoma. Cancer Immunol. Immunother. 2020, 69, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fan, H.Y.; Tang, Y.L.; Wang, S.S.; Cao, M.X.; Wang, H.F.; Dai, L.L.; Wang, K.; Yu, X.H.; Wu, J.B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.M.; Liu, Z.G.; Zhou, X.; Song, S.H.; Weng, G.Y.; Wen, Y.; Liu, F.B.; Cao, D.L.; Liu, Y.F. Expansion of PMN-myeloid derived suppressor cells and their clinical relevance in patients with oral squamous cell carcinoma. Oral Oncol. 2019, 95, 157–163. [Google Scholar] [CrossRef]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef]
- Hume, D.A. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008, 181, 5829–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 2013, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef]
- Lee, H.K.; Zamora, M.; Linehan, M.M.; Iijima, N.; Gonzalez, D.; Haberman, A.; Iwasaki, A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 2009, 206, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam, J.P.; Peng, W.M.; Appel, T.; Wenghoefer, M.; Niederhagen, B.; Bieber, T.; Berge, S.; Novak, N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J. Allergy Clin. Immunol. 2008, 121, 368–374.el. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nagashima, H.; Bando, K.; Lu, L.; Ozaki, A.; Morita, Y.; Fukumoto, S.; Ishii, N.; Sugawara, S. Oral CD103(-)CD11b(+) classical dendritic cells present sublingual antigen and induce Foxp3(+) regulatory T cells in draining lymph nodes. Mucosal Immunol. 2017, 10, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [Green Version]
- Sasai, M.; Yamamoto, M. Pathogen recognition receptors: Ligands and signaling pathways by Toll-like receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Uehara, A.; Fujimoto, Y.; Kusumoto, S.; Fukase, K.; Shibata, K.; Sugawara, S.; Sasano, T.; Takada, H. Toll-like receptors, NOD1, and NOD2 in oral epithelial cells. J. Dent. Res. 2006, 85, 524–529. [Google Scholar] [CrossRef]
- He, Z.; Huang, X.; Ni, Y.; Shi, P.; Wang, Z.; Han, W.; Hu, Q. Functional toll-like receptor 3 expressed by oral squamous cell carcinoma induced cell apoptosis and decreased migration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Johnson, L.; Roberts, J.; Hung, S.C.; Lee, J.; Atanasova, K.R.; Huang, P.R.; Yilmaz, O.; Ojcius, D.M. Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1beta and the danger signals ASC and HMGB1. Cell. Microbiol. 2016, 18, 970–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molero-Abraham, M.; Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Torres-Gomez, A.; Subiza, J.L.; Lafuente, E.M.; Reche, P.A. Human Oral Epithelial Cells Impair Bacteria-Mediated Maturation of Dendritic Cells and Render T Cells Unresponsive to Stimulation. Front. Immunol. 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Lappin, D.F.; Millhouse, E.; Malcolm, J.; Jose, A.; Yang, J.; Bradshaw, D.J.; Pratten, J.R.; Culshaw, S. The epithelial cell response to health and disease associated oral biofilm models. J. Periodontal Res. 2017, 52, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Uehara, A.; Sugawara, S.; Tamai, R.; Takada, H. Contrasting responses of human gingival and colonic epithelial cells to lipopolysaccharides, lipoteichoic acids and peptidoglycans in the presence of soluble CD14. Med. Microbiol. Immunol. 2001, 189, 185–192. [Google Scholar] [CrossRef]
- Iliev, I.D.; Matteoli, G.; Rescigno, M. The yin and yang of intestinal epithelial cells in controlling dendritic cell function. J. Exp. Med. 2007, 204, 2253–2257. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.; Jamora, C.; Havran, W.; Hayday, A. Epithelial decision makers: In search of the ‘epimmunome’. Nat. Immunol. 2010, 11, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Rimoldi, M.; Chieppa, M.; Salucci, V.; Avogadri, F.; Sonzogni, A.; Sampietro, G.M.; Nespoli, A.; Viale, G.; Allavena, P.; Rescigno, M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005, 6, 507–514. [Google Scholar] [CrossRef]
- Iliev, I.D.; Spadoni, I.; Mileti, E.; Matteoli, G.; Sonzogni, A.; Sampietro, G.M.; Foschi, D.; Caprioli, F.; Viale, G.; Rescigno, M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 2009, 58, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J. Exp. Med. 2006, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.J.; Spits, H.; et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Wechsler, M.E.; Brightling, C.E. Unmet need in severe, uncontrolled asthma: Can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir. Res. 2020, 21, 268. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Nguyen, A.L.; Yu, W.H.; Le, A.D. Human oral mucosa and gingiva: A unique reservoir for mesenchymal stem cells. J. Dent. Res. 2012, 91, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Li, W.; Meng, H. A double-edged sword: Role of butyrate in the oral cavity and the gut. Mol. Oral Microbiol. 2021, 36, 121–131. [Google Scholar] [CrossRef]
- Lee, J.S.; Chowdhury, N.; Roberts, J.S.; Yilmaz, O. Host surface ectonucleotidase-CD73 and the opportunistic pathogen, Porphyromonas gingivalis, cross-modulation underlies a new homeostatic mechanism for chronic bacterial survival in human epithelial cells. Virulence 2020, 11, 414–429. [Google Scholar] [CrossRef]
- Board-Davies, E.; Moses, R.; Sloan, A.; Stephens, P.; Davies, L.C. Oral Mucosal Lamina Propria-Progenitor Cells Exert Antibacterial Properties via the Secretion of Osteoprotegerin and Haptoglobin. Stem Cells Transl. Med. 2015, 4, 1283–1293. [Google Scholar] [CrossRef]
- Zhong, M.; Lin, B.; Pathak, J.L.; Gao, H.; Young, A.J.; Wang, X.; Liu, C.; Wu, K.; Liu, M.; Chen, J.M.; et al. ACE2 and Furin Expressions in Oral Epithelial Cells Possibly Facilitate COVID-19 Infection via Respiratory and Fecal-Oral Routes. Front. Med. (Lausanne) 2020, 7, 580796. [Google Scholar] [CrossRef]
- Solis, N.V.; Swidergall, M.; Bruno, V.M.; Gaffen, S.L.; Filler, S.G. The Aryl Hydrocarbon Receptor Governs Epithelial Cell Invasion during Oropharyngeal Candidiasis. mBio 2017, 8, e00025-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maan, A.S.; Patil, A.K. Assessment of salivary interleukin-1beta (IL-1beta), prostaglandin E2 (PGE2) levels and pain intensity in children and adults during initial orthodontic treatment. J. Orthod. Sci. 2019, 8, 16. [Google Scholar] [CrossRef]
- Schmalz, G.; Schweikl, H.; Hiller, K.A. Release of prostaglandin E2, IL-6 and IL-8 from human oral epithelial culture models after exposure to compounds of dental materials. Eur. J. Oral Sci. 2000, 108, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Warner, T.D.; Giuliano, F.; Vojnovic, I.; Bukasa, A.; Mitchell, J.A.; Vane, J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 7563–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.Y.; Chang, Y.; Sun, X.J.; Dai, X.; Wei, W. The role of prostaglandin E2 receptor signaling of dendritic cells in rheumatoid arthritis. Int. Immunopharmacol. 2014, 23, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Harizi, H.; Norbert, G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell. Immunol. 2004, 228, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Aldajani, W.A.; Salazar, F.; Sewell, H.F.; Knox, A.; Ghaemmaghami, A.M. Expression and regulation of immune-modulatory enzyme indoleamine 2,3-dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget 2016, 7, 57606–57617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor, A.L.; Lemos, H.; Huang, L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front. Immunol. 2017, 8, 1360. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Lonnies, H.; Locke, M.; Sundberg, B.; Rosendahl, K.; Gotherstrom, C.; Le Blanc, K.; Stephens, P. Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev. 2012, 21, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The immunosuppressive capacity of human mesenchymal stromal cells derived from amnion and bone marrow. Biochem. Biophys. Rep. 2016, 8, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Hattori, T.; Kezuka, T.; Usui, Y.; Okunuki, Y.; Takeuchi, M.; Maruyama, K.; Haneda, M.; Shirato, S.; Goto, H. Human iris pigment epithelial cells suppress T-cell activation via direct cell contact. Exp. Eye Res. 2009, 89, 358–364. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Gao, W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin. Immunol. 2005, 115, 184–191. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Groeger, S.; Howaldt, H.P.; Raifer, H.; Gattenloehner, S.; Chakraborty, T.; Meyle, J. Oral Squamous Carcinoma Cells Express B7-H1 and B7-DC Receptors in Vivo. Pathol. Oncol. Res. 2017, 23, 99–110. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemann, M.; Atiakshin, D.; Samoilova, V.; Buchwalow, I. Identification of CTLA-4-Positive Cells in the Human Tonsil. Cells 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Laubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern. Med. 2016, 279, 541–562. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Strome, S.E. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef]
- Itakura, E.; Huang, R.R.; Wen, D.R.; Paul, E.; Wunsch, P.H.; Cochran, A.J. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011, 24, 801–809. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mulder, D.J.; Pooni, A.; Mak, N.; Hurlbut, D.J.; Basta, S.; Justinich, C.J. Antigen presentation and MHC class II expression by human esophageal epithelial cells: Role in eosinophilic esophagitis. Am. J. Pathol. 2011, 178, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Telega, G.W.; Baumgart, D.C.; Carding, S.R. Uptake and presentation of antigen to T cells by primary colonic epithelial cells in normal and diseased states. Gastroenterology 2000, 119, 1548–1559. [Google Scholar] [CrossRef]
- Thelemann, C.; Eren, R.O.; Coutaz, M.; Brasseit, J.; Bouzourene, H.; Rosa, M.; Duval, A.; Lavanchy, C.; Mack, V.; Mueller, C.; et al. Interferon-gamma induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS ONE 2014, 9, e86844. [Google Scholar] [CrossRef] [Green Version]
- Cruickshank, S.M.; Southgate, J.; Selby, P.J.; Trejdosiewicz, L.K. Inhibition of T cell activation by normal human biliary epithelial cells. J. Hepatol. 1999, 31, 1026–1033. [Google Scholar] [CrossRef]
- Cruickshank, S.M.; McVay, L.D.; Baumgart, D.C.; Felsburg, P.J.; Carding, S.R. Colonic epithelial cell mediated suppression of CD4 T cell activation. Gut 2004, 53, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.C.; Huang, G.T.; Lin, L.M.; Warner, N.A.; Gim, J.S.; Jewett, A. Expression of MHC Class II, CD70, CD80, CD86 and pro-inflammatory cytokines is differentially regulated in oral epithelial cells following bacterial challenge. Oral Microbiol. Immunol. 2003, 18, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Matthews, J.B.; Midda, M.; Scully, C.; Prime, S.S. MHC antigen expression in human oral squamous carcinoma cell lines. J. Pathol. 1991, 165, 129–136. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 12149. https://doi.org/10.3390/ijms222212149
Pelaez-Prestel HF, Sanchez-Trincado JL, Lafuente EM, Reche PA. Immune Tolerance in the Oral Mucosa. International Journal of Molecular Sciences. 2021; 22(22):12149. https://doi.org/10.3390/ijms222212149
Chicago/Turabian StylePelaez-Prestel, Hector F., Jose L. Sanchez-Trincado, Esther M. Lafuente, and Pedro A. Reche. 2021. "Immune Tolerance in the Oral Mucosa" International Journal of Molecular Sciences 22, no. 22: 12149. https://doi.org/10.3390/ijms222212149
APA StylePelaez-Prestel, H. F., Sanchez-Trincado, J. L., Lafuente, E. M., & Reche, P. A. (2021). Immune Tolerance in the Oral Mucosa. International Journal of Molecular Sciences, 22(22), 12149. https://doi.org/10.3390/ijms222212149







