Comparison and Optimization of Quantification Methods for Shigella flexneri Serotype 6 O-antigen Containing Galacturonic Acid and Methyl-Pentose
Abstract
1. Introduction
2. Results
2.1. Dische Colorimetric Method
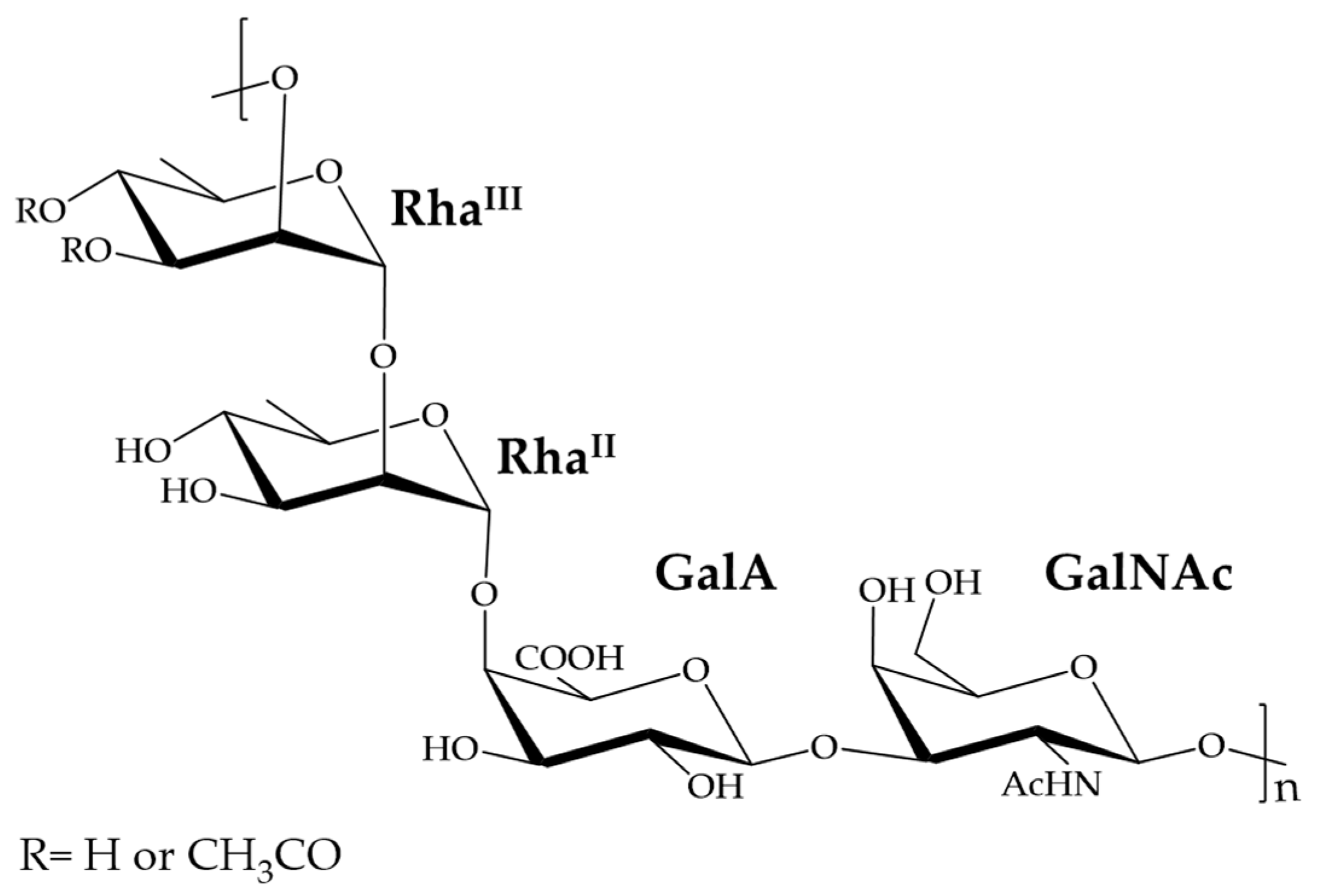
2.2. Dische Applied to S. flexneri 6 Serotype OAg
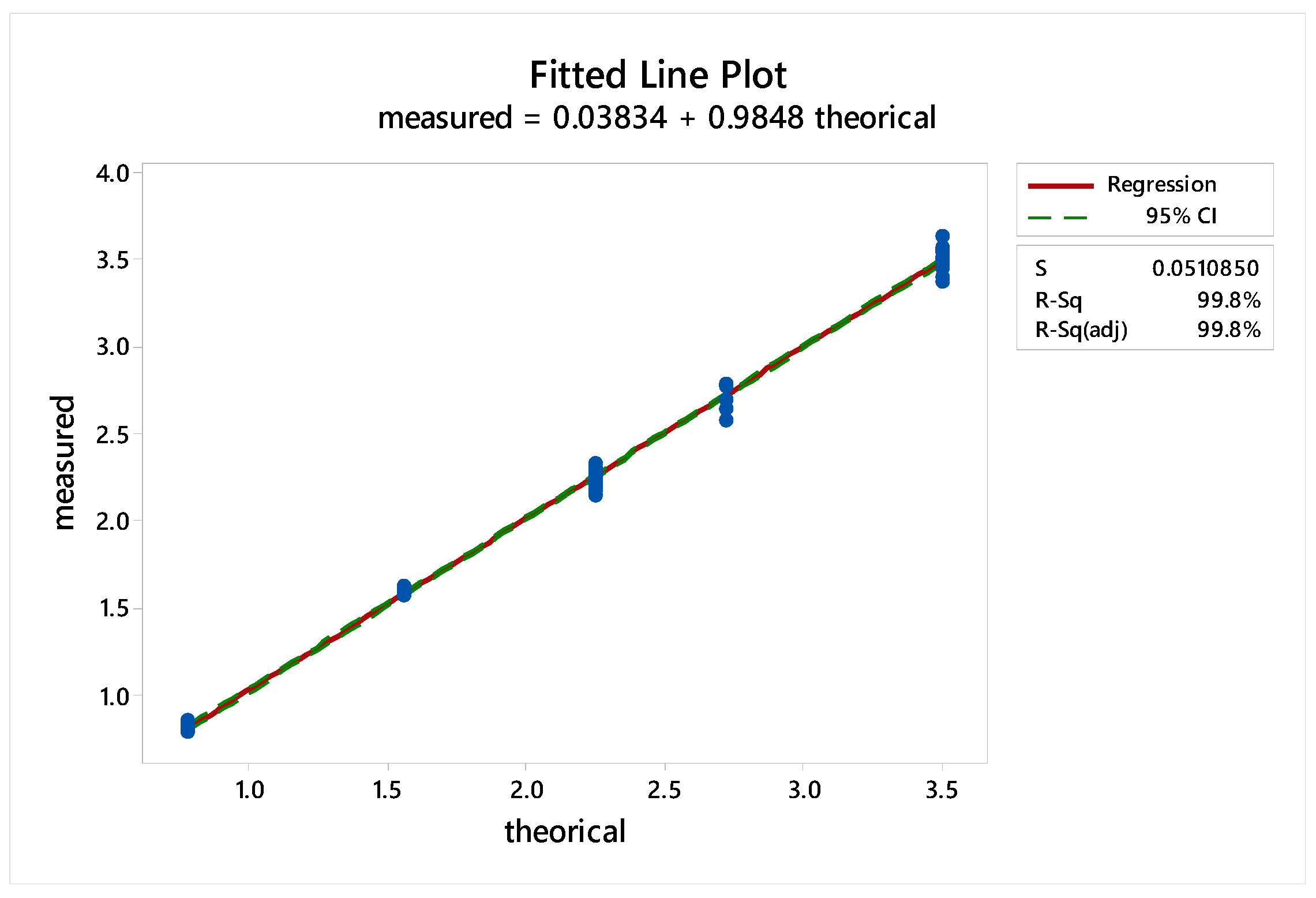
2.2.1. Standard Linearity
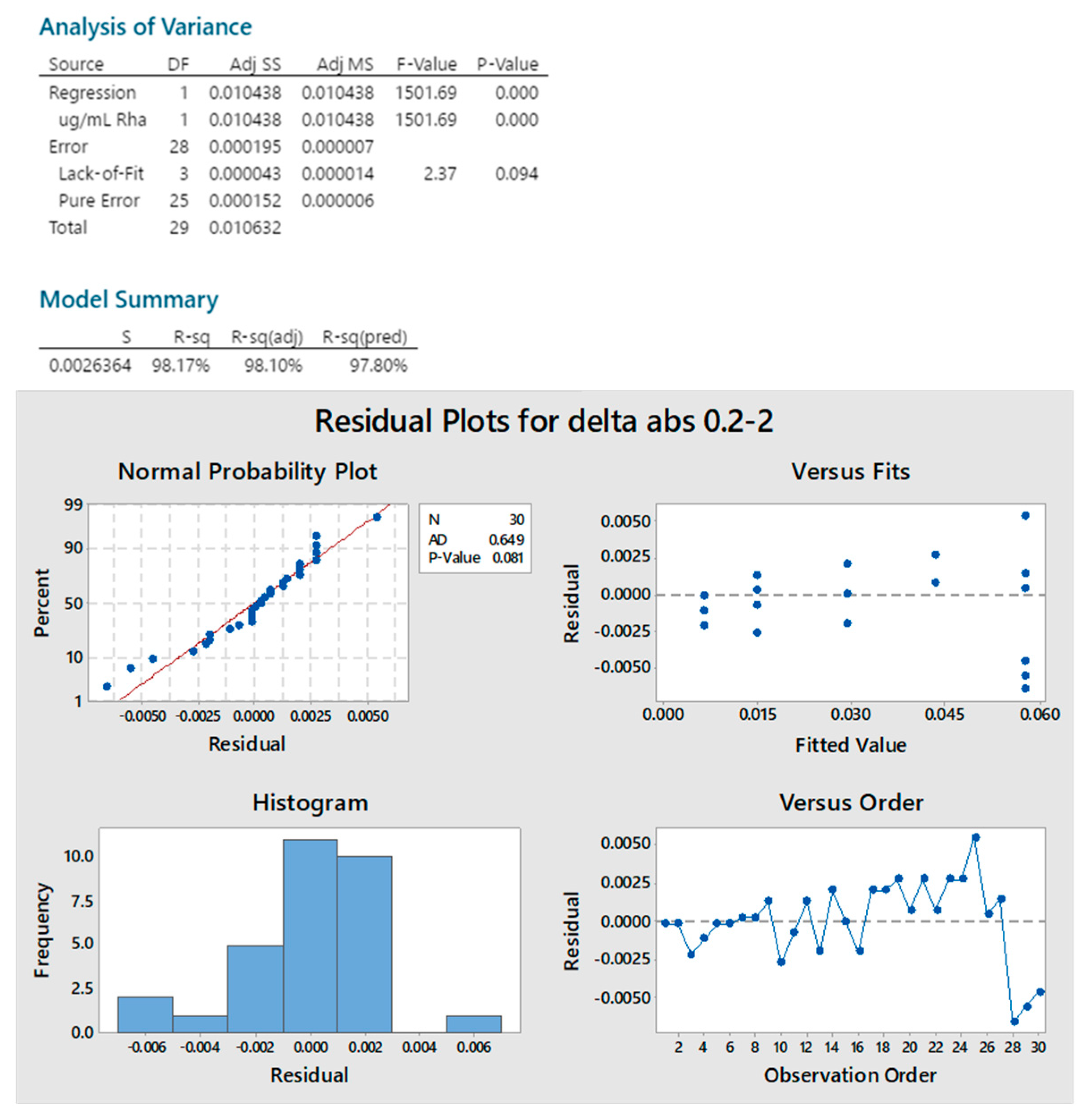
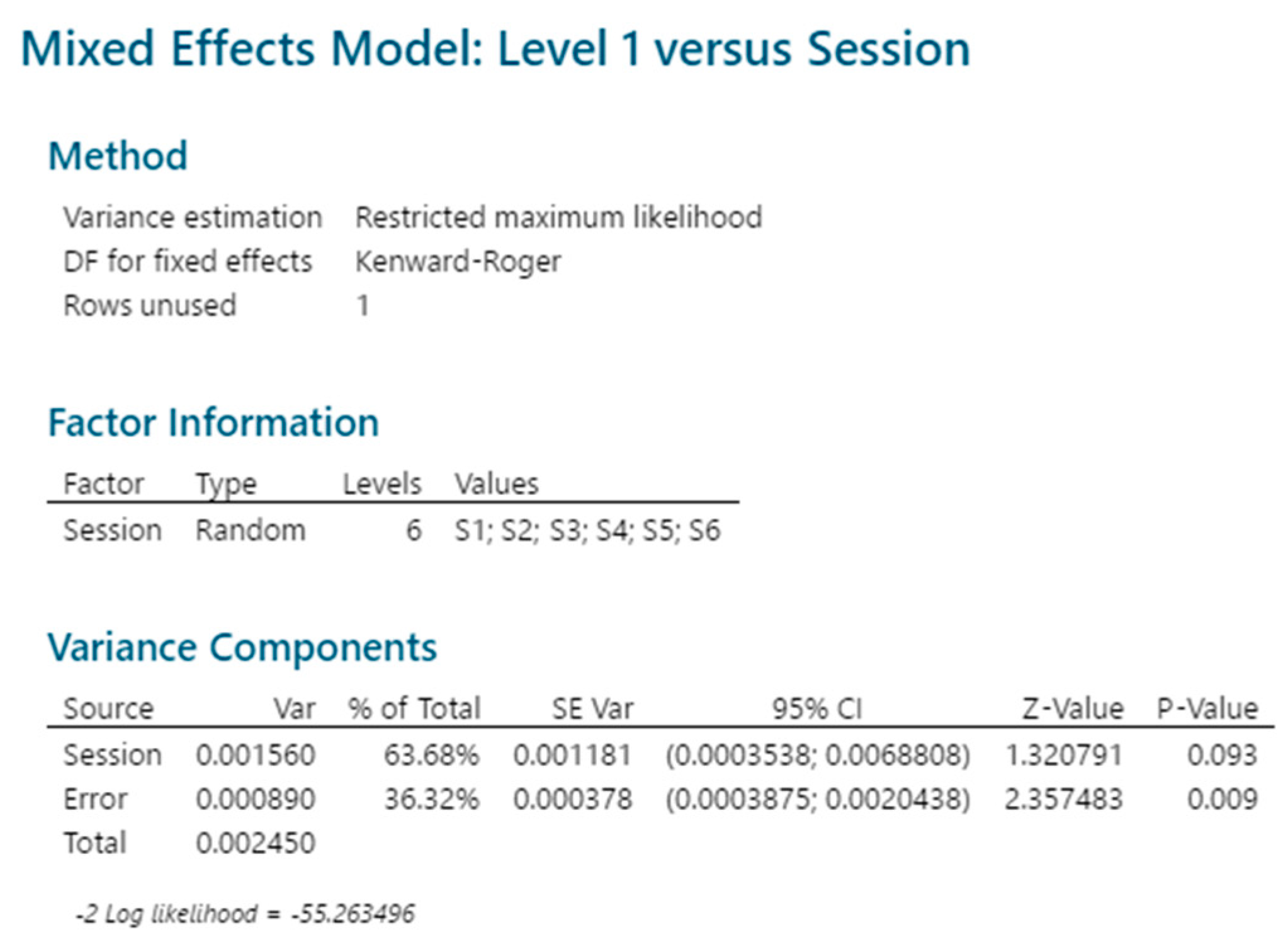
2.2.2. Reproducibility
2.2.3. Sample Linearity
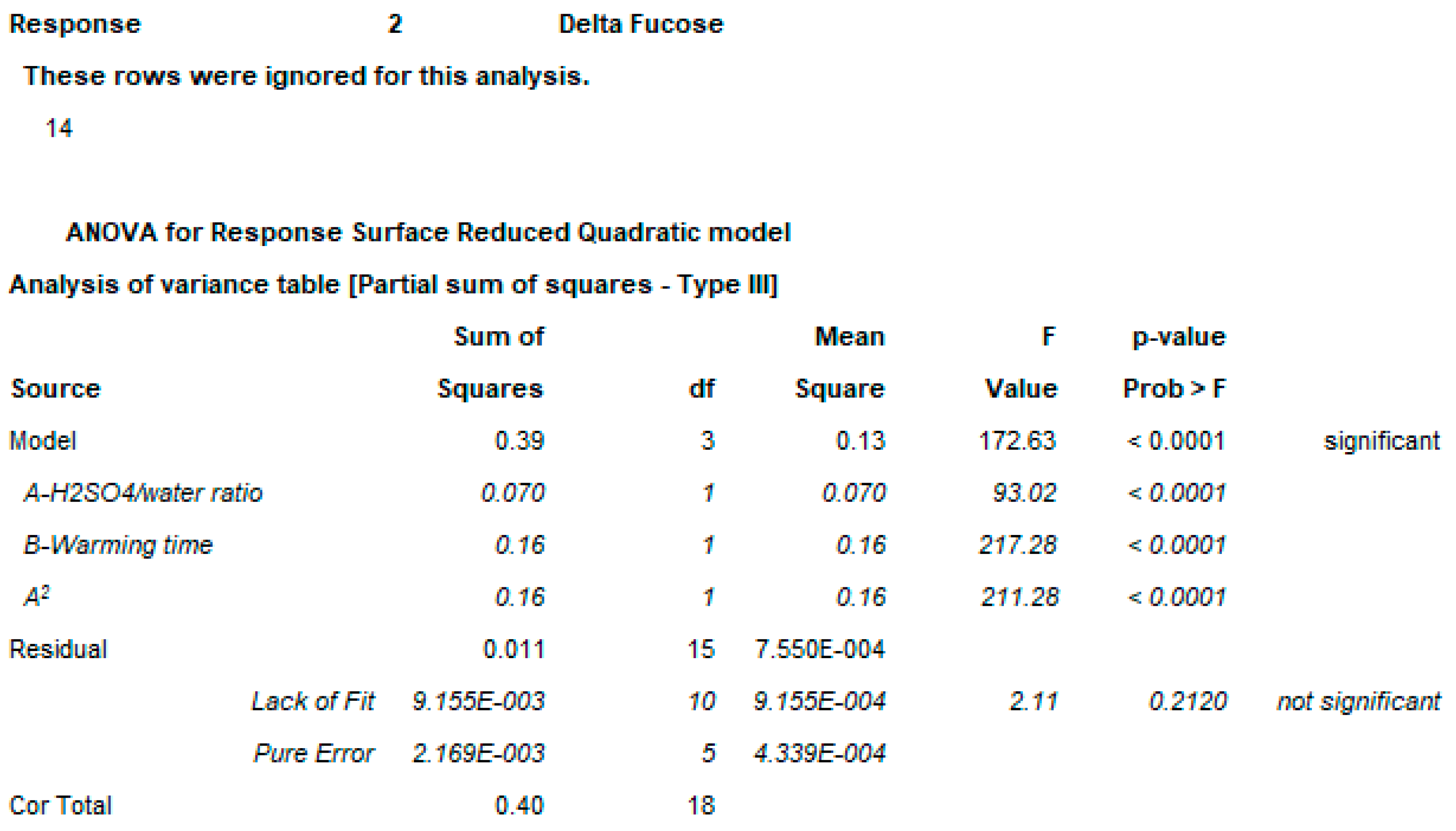
2.3. OAg Hydrolysis Followed by HPAEC-PAD
2.3.1. Polysaccharide Hydrolysis Conditions
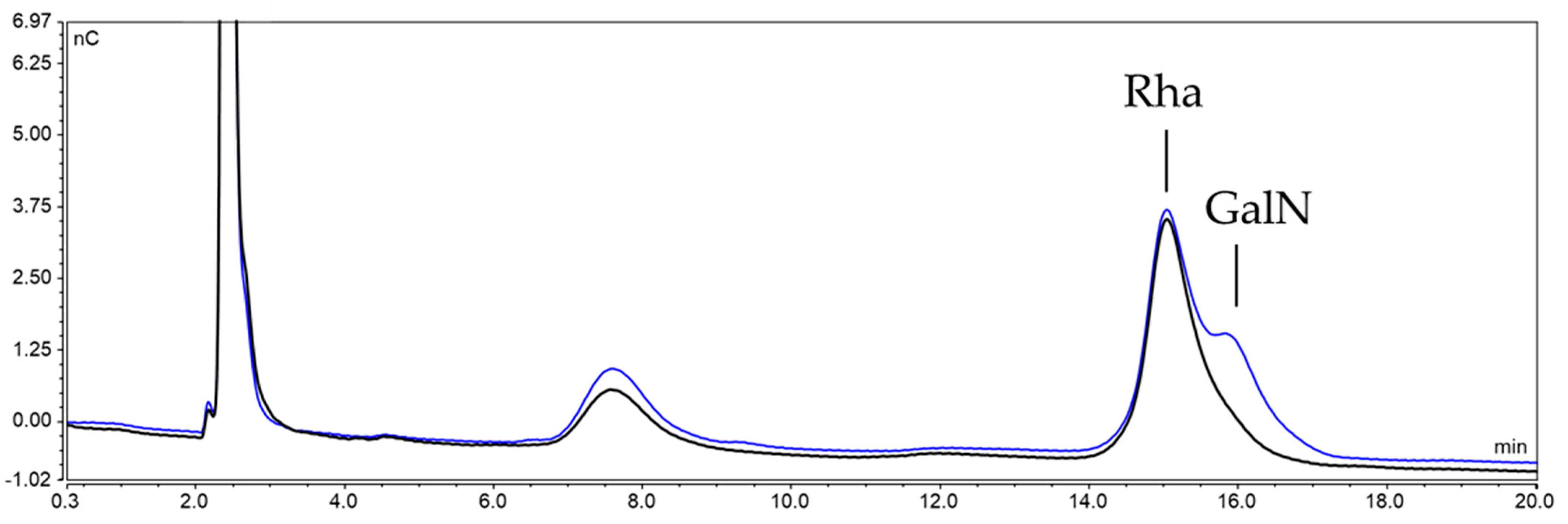
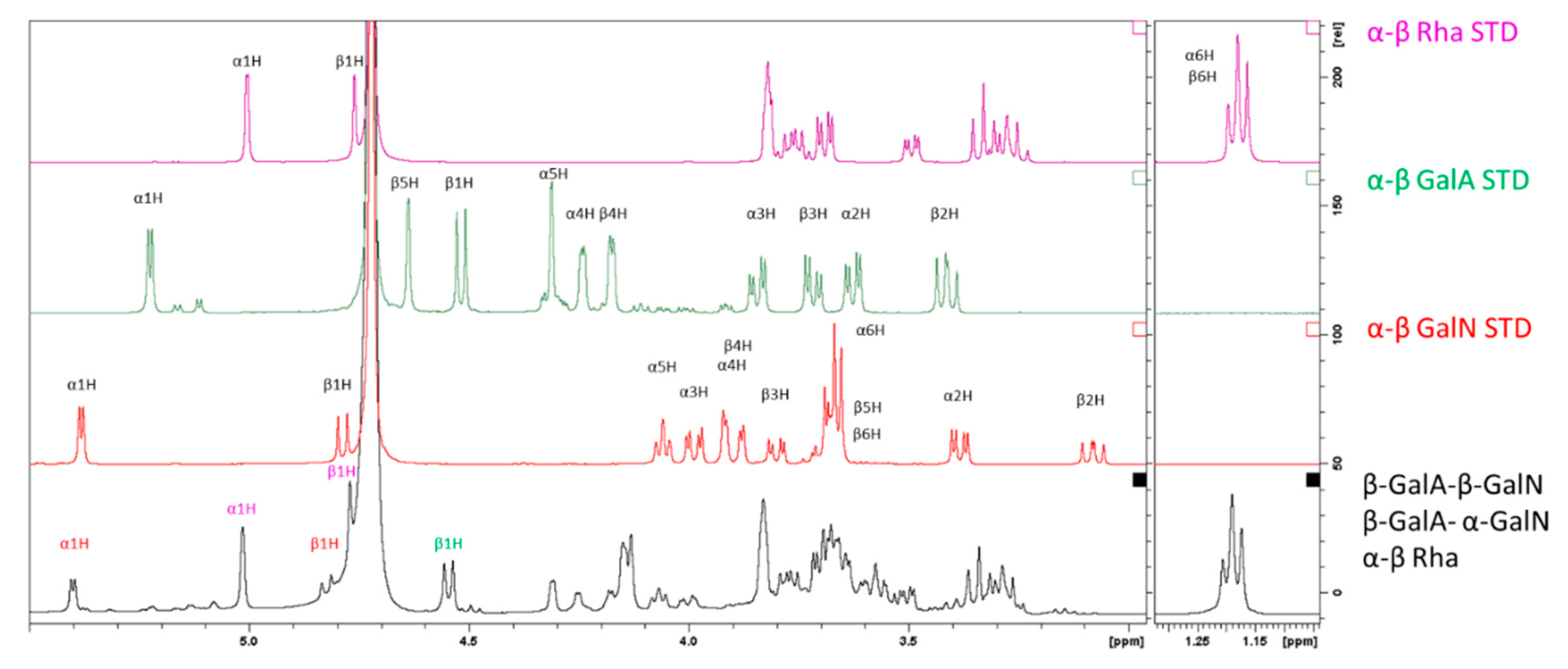
2.3.2. Characterization of the Unknown Peak Coming from Acid Hydrolysis (Dimer GalA-GalN)
2.3.3. Hydrolysis Yield Determination by qNMR
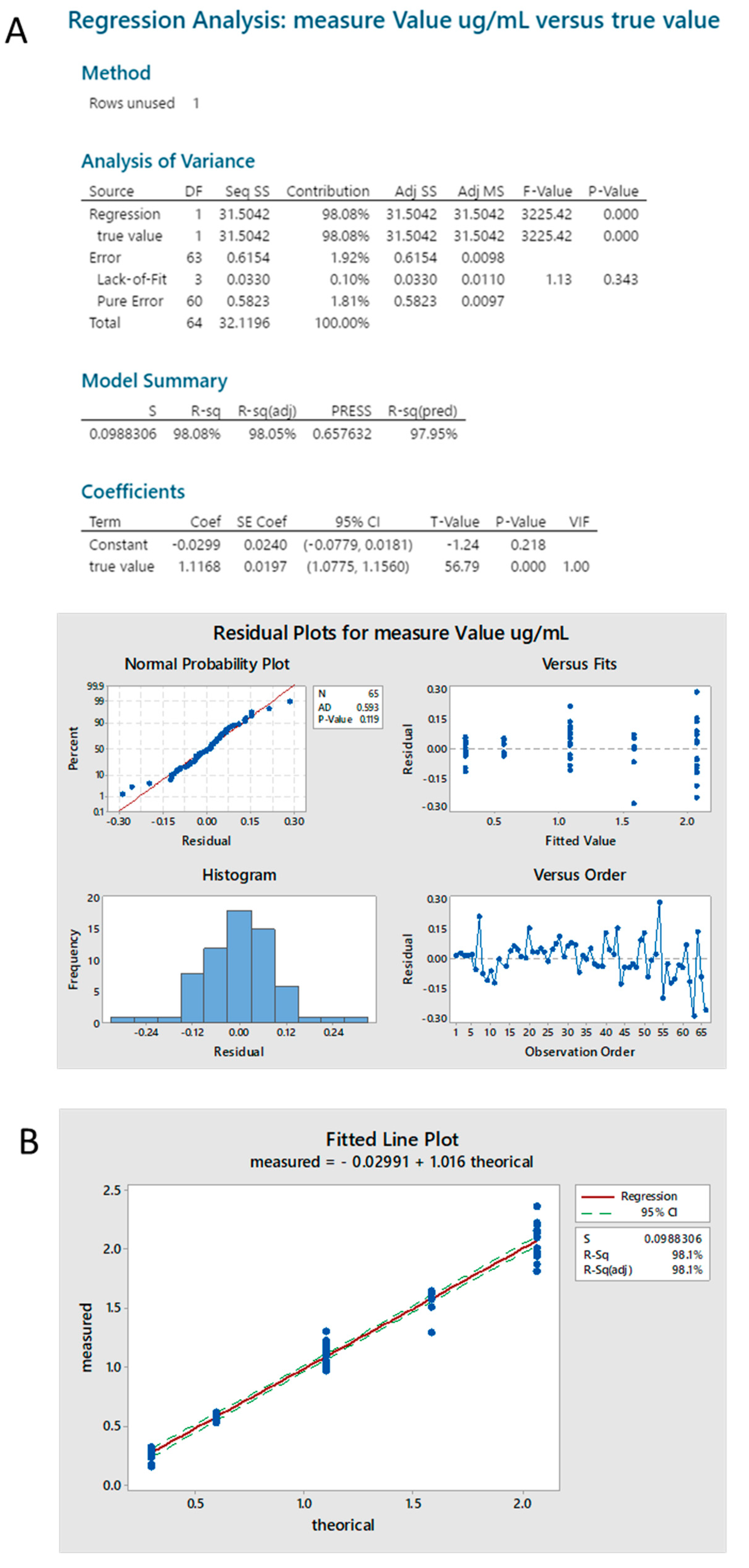
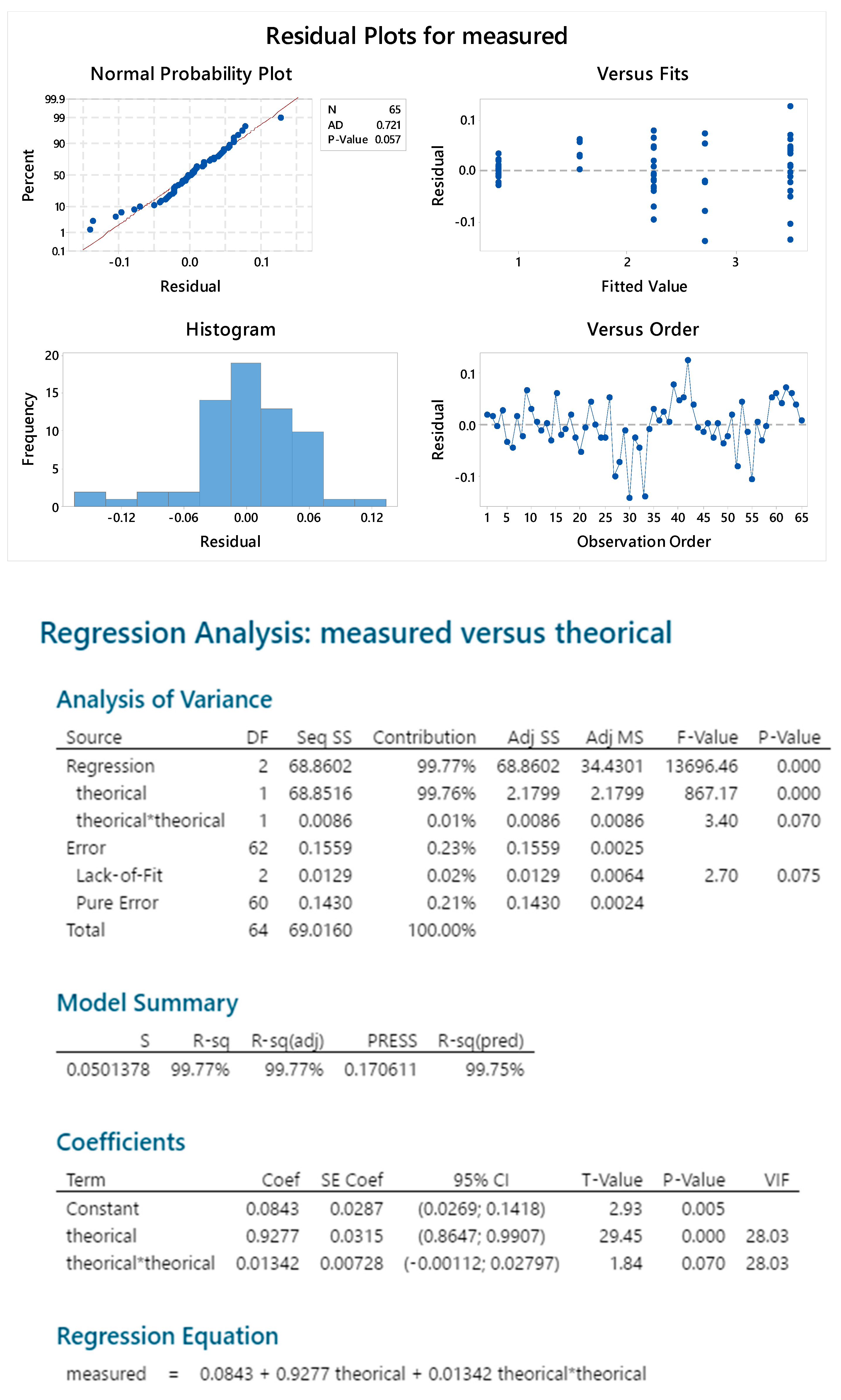
2.3.4. Standard Linearity
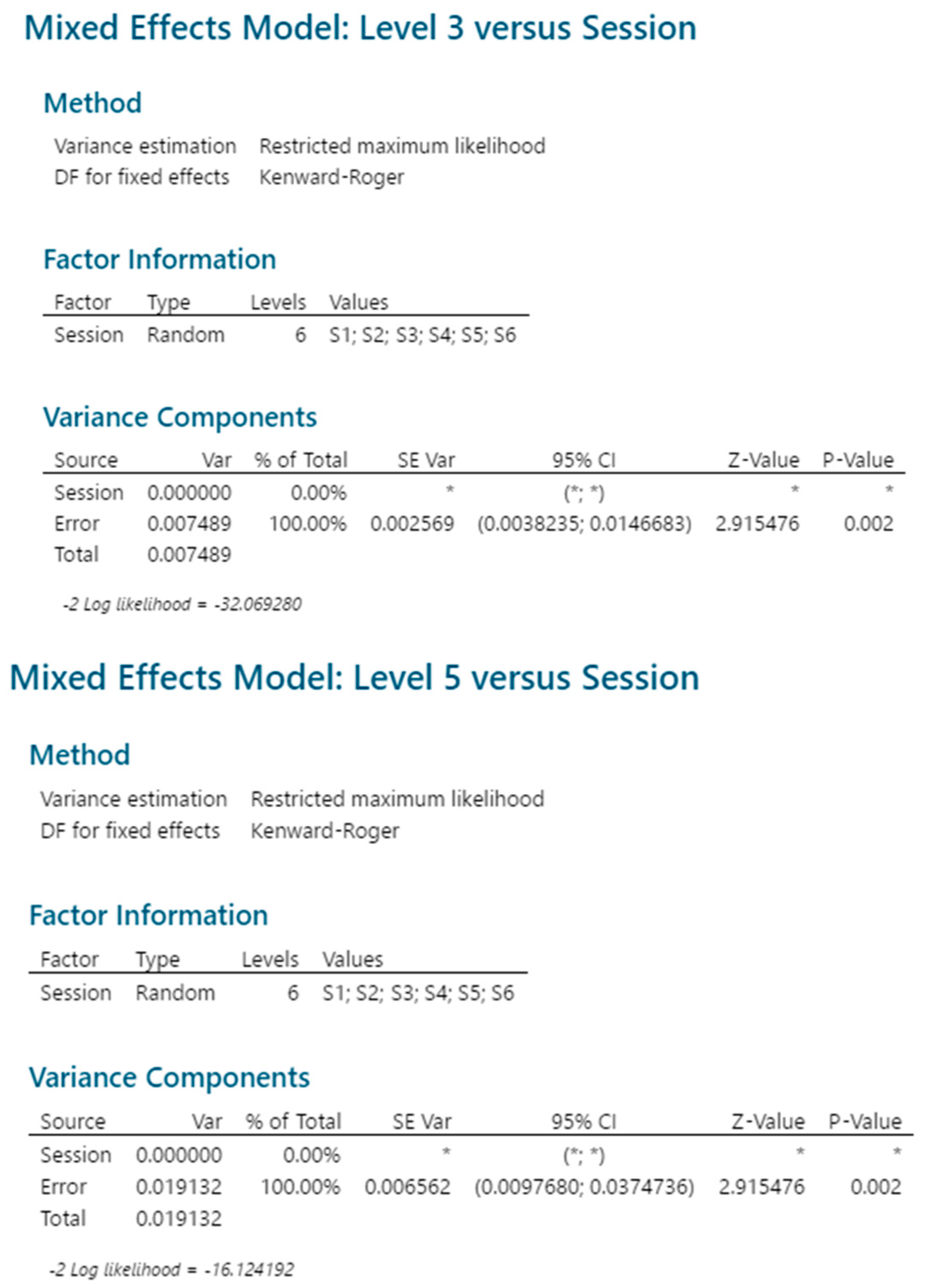
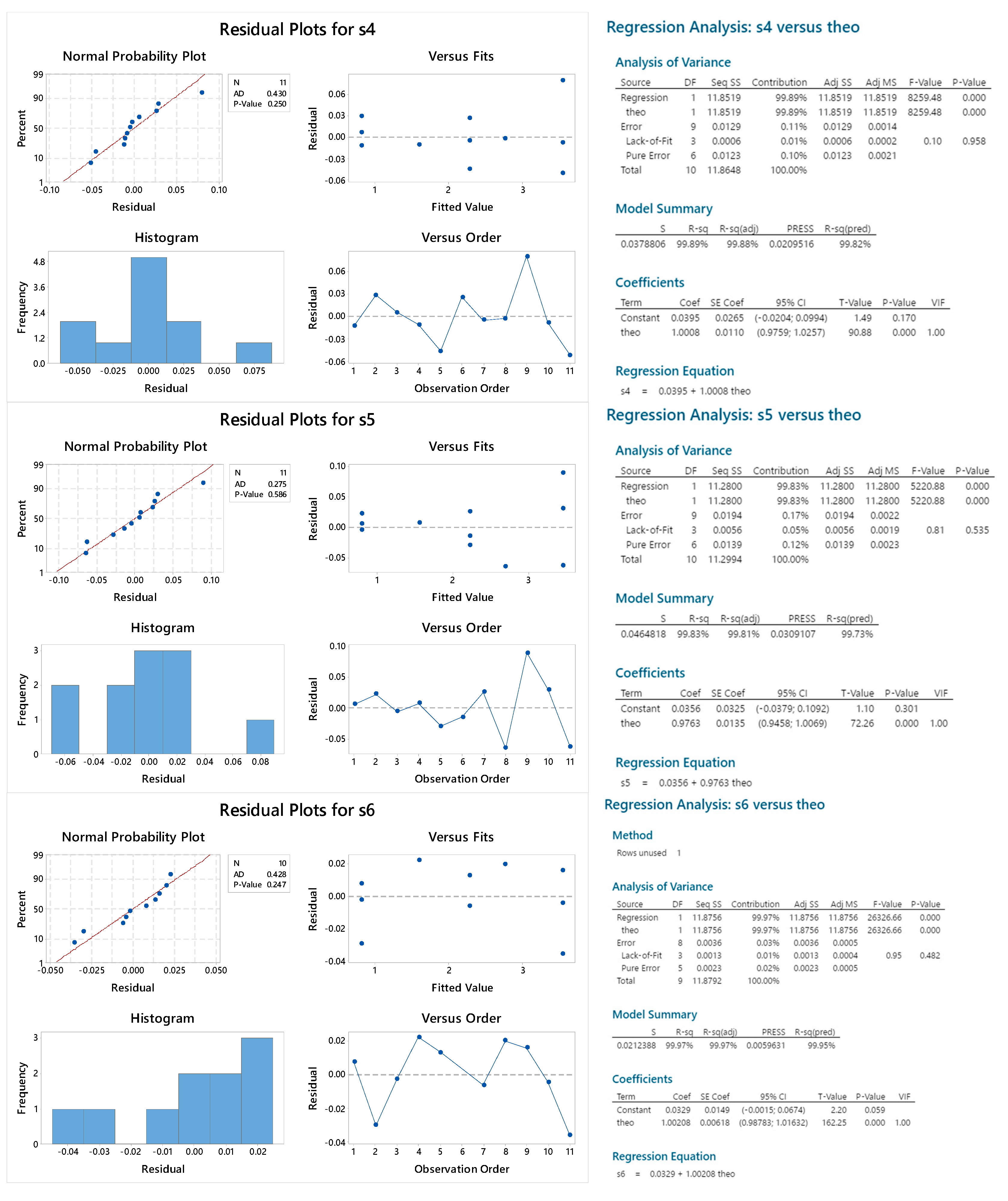
2.3.5. Reproducibility
2.3.6. Sample Linearity
2.3.7. Accuracy (Spike Recovery)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Dische Colorimetric Method
4.2.1. DoE
4.2.2. Optimized Quantification Method
4.2.3. Standard Linearity
4.2.4. Reproducibility and Sample Linearity
4.3. OAg Hydrolysis Followed by HPAEC-PAD
4.3.1. Optimized Analysis Conditions (GalA-GalN Dimer Quantification)
4.3.2. Chromatographic Conditions:
- Neutral sugar determination: 5 μL injection volume, column and detector temperature 25 °C, CarboPac PA10 4 × 50 mm guard column connected in series with PA10 4 × 250 mm column (Thermo). Eluent program: NaOH 18 mM × 30 min (flow rate 0.6 mL/min), NaOH 500 mM × 10 min (flow rate 1.6 mL/min), NaOH 18 mM × 38 min (flow rate 1.6 mL/min).
- Uronic acid determination (analysis conditions reported in Giannelli et al. [20] for 2-aminouronic acids): 25 μL injection volume, column and detector temperature 25 °C, CarboPac PA1 4 × 50 mm guard column connected in series with PA1 4 × 250 mm column (Thermo). Eluent program: NaOH 400 mM × 15 min (flow rate 1.5 mL/min).
4.3.3. DoE
4.3.4. Mass Spectrometry (MS)
4.3.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.3.6. Standard Linearity
4.3.7. Reproducibility and Sample Linearity
4.3.8. Accuracy (Spike Recovery)
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Run | Type | Sample | Water | Cold H2SO4 | Final H2SO4/Water | Cysteine 1 M | Warming Time |
|---|---|---|---|---|---|---|---|
| μL | μL | μL | Ratio | μL | min | ||
| 1 | Factorial | 200 | 205 | 1095 | 2.70 | 32.0 | 5.0 |
| 2 | Factorial | 200 | 205 | 1095 | 2.70 | 32.0 | 15.0 |
| 3 | Axial | 200 | 300 | 1000 | 2.00 | 36.8 | 10.0 |
| 4 | Axial | 200 | 300 | 1000 | 2.00 | 13.2 | 10.0 |
| 5 | Factorial | 200 | 452 | 848 | 1.30 | 18.0 | 5.0 |
| 6 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| 7 | Factorial | 200 | 452 | 848 | 1.30 | 32.0 | 5.0 |
| 8 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| 9 | Axial | 200 | 159 | 1141 | 3.18 | 25.0 | 10.0 |
| 10 | Factorial | 200 | 205 | 1095 | 2.70 | 18.0 | 15.0 |
| 11 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| 12 | Axial | 200 | 300 | 1000 | 2.00 | 25.0 | 18.4 |
| 13 | Axial | 200 | 300 | 1000 | 2.00 | 25.0 | 1.6 |
| 14 1 | Factorial | 200 | 205 | 1095 | 2.70 | 18.0 | 5.0 |
| 15 | Factorial | 200 | 452 | 848 | 1.30 | 18.0 | 15.0 |
| 16 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| 17 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| 18 | Factorial | 200 | 452 | 848 | 1.30 | 32.0 | 15.0 |
| 19 | Axial | 200 | 623 | 677 | 0.82 | 25.0 | 10.0 |
| 20 | Center | 200 | 300 | 1000 | 2.00 | 25.0 | 10.0 |
| Group | Run | Temperature °C | TFA % | HCl M | Time Min |
|---|---|---|---|---|---|
| 1 | 1 | 50 | 10 | 6 | 150 |
| 1 | 2 | 50 | 10 | 6 | 150 |
| 1 | 3 | 50 | 10 | 6 | 150 |
| 2 | 4 | 50 | 10 | 6 | 150 |
| 2 | 5 | 50 | 10 | 6 | 150 |
| 2 | 6 | 50 | 10 | 6 | 150 |
| 3 | 7 | 50 | 10 | 6 | 150 |
| 3 | 8 | 50 | 10 | 6 | 150 |
| 3 | 9 | 50 | 10 | 6 | 150 |
| 4 | 10 | 60 | 11.5 | 4 | 240 |
| 4 | 11 | 60 | 20 | 8 | 240 |
| 4 | 12 | 60 | 0 | 8 | 160.8 |
| 4 | 13 | 60 | 0 | 4 | 60 |
| 4 | 14 | 60 | 20 | 6.2 | 60 |
| 5 | 15 | 50 | 10 | 6 | 150 |
| 5 | 16 | 50 | 10 | 6 | 150 |
| 5 | 17 | 50 | 10 | 6 | 150 |
| 6 | 18 | 50 | 10 | 6 | 150 |
| 6 | 19 | 50 | 10 | 6 | 150 |
| 6 | 20 | 50 | 10 | 6 | 150 |
| 7 | 21 | 40 | 20 | 8 | 60 |
| 7 | 22 | 40 | 10 | 6 | 150 |
| 7 | 23 | 40 | 0 | 8 | 240 |
| 7 | 24 | 40 | 20 | 4 | 240 |
| 7 | 25 | 40 | 0 | 4 | 60 |
| 8 | 26 | 60 | 8.5 | 8 | 240 |
| 8 | 27 | 60 | 0 | 4 | 240 |
| 8 | 28 | 60 | 20 | 4 | 159 |
| 8 | 29 | 60 | 12.9 | 8 | 60 |
| 8 | 30 | 60 | 0 | 5.92 | 60 |
| 9 | 31 | 40 | 0 | 8 | 60 |
| 9 | 32 | 40 | 0 | 4 | 240 |
| 9 | 33 | 40 | 20 | 4 | 60 |
| 9 | 34 | 40 | 20 | 8 | 240 |
| 9 | 35 | 40 | 10 | 6 | 150 |
| 10 | 36 | 60 | 9 | 4 | 60 |
| 10 | 37 | 60 | 20 | 4 | 60 |
| 10 | 38 | 60 | 0 | 6.3 | 240 |
| 10 | 39 | 60 | 0 | 8 | 60 |
| 10 | 40 | 60 | 20 | 5.68 | 240 |
| 10 | 41 | 60 | 20 | 8 | 141.9 |
| 11 | 42 | 50 | 10 | 6 | 150 |
| 11 | 43 | 50 | 10 | 6 | 150 |
| 11 | 44 | 50 | 10 | 6 | 150 |
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | |
|---|---|---|---|---|---|---|
| lack of fit | 0.042 | 0.290 | 0.122 | 0.958 | 0.535 | 0.482 |
| residual normality (p, AD) | 0.297 | 0.229 | 0.853 | 0.250 | 0.586 | 0.247 |
| linear coefficient (95% CI) | 0.96–1.02 | 0.96–1.01 | 0.92–0.99 | 0.98–1.03 | 0.95–1.01 | 0.99–1.02 |
| Intercept (95% CI) | −0.03–0.10 | −0.02–0.09 | −0.04–0.14 | −0.02–0.10 | −0.04–0.11 | −0.002–1.02 |
| Spike | |||
|---|---|---|---|
| μg/mL | 1 μg/mL | 2 μg/mL | 3 μg/mL |
| Session 1 | 91% | 92% | 101% |
| Session 2 | 91% | 97% | 102% |
| Session 3 | 98% | 95% | 103% |
| Session 4 | 96% | 98% | 103% |
| Concentration | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 |
|---|---|---|---|---|---|---|
| Level 1 | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates |
| Level 2 | 1 replicate | 1 replicate | 1 replicate | 1 replicate | 1 replicate | 1 replicate |
| Level 3 | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates |
| Level 4 | 1 replicate | 1 replicate | 1 replicate | 1 replicate | 1 replicate | 1 replicate |
| Level 5 | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates | 3 replicates |

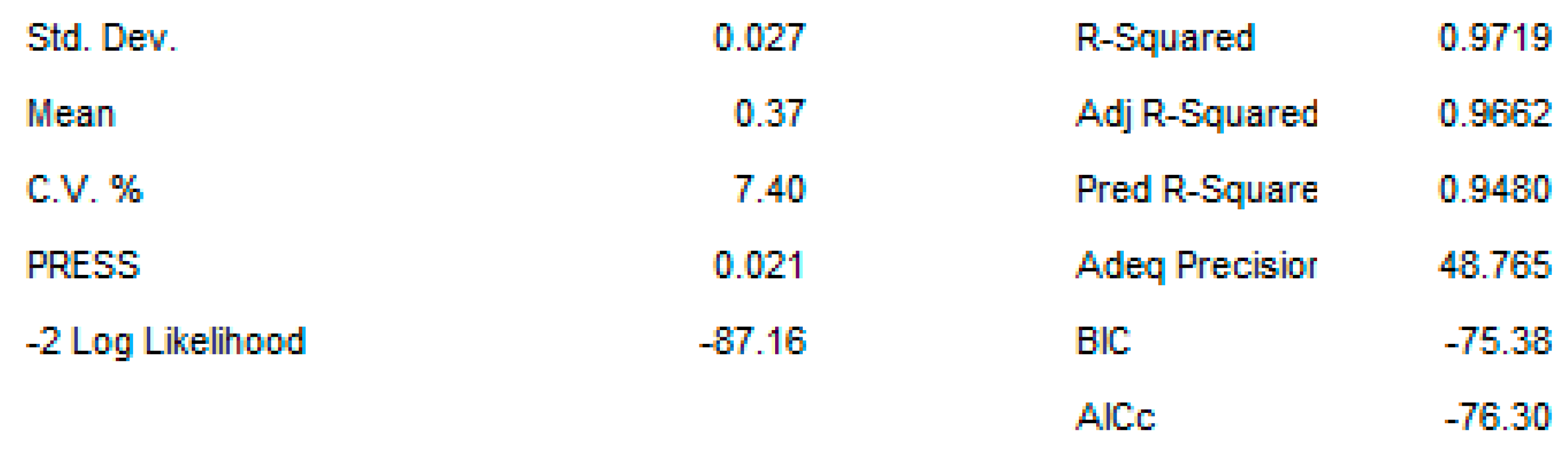









References
- Schroeder, G.N.; Hilbi, H. Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 2008, 21, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Klontz, K.C.; Singh, N. Treatment of drug-resistant Shigella infections. Expert Rev. Anti-Infect. Ther. 2015, 13, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Kaminski, R.W.; Porter, C.; Choy, R.K.M.; White, J.A.; Fleckenstein, J.M.; Cassels, F.; Bourgeois, L. Vaccines for Protecting Infants from Bacterial Causes of Diarrheal Disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Barel, L.A.; Mulard, L.A. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: From concept to efficacy in human. Hum. Vaccines Immunother. 2019, 15, 1338–1356. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.I.; Ravenscroft, N.; Arato, V.; Oldrini, D.; Micoli, F.; Kuttel, M.M. Conformational and Immunogenicity Studies of the Shigella flexneri Serogroup 6 O-Antigen: The Effect of O-Acetylation. Vaccines 2021, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Perepelov, A.V.; Shekht, M.E.; Liu, B.; Shevelev, S.D.; Ledov, V.A.; Senchenkova, S.N.; L’Vov, L.; Shashkov, A.S.; Feng, L.; Aparin, P.G.; et al. Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 2012, 66, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Dische, Z.; Shettles, L.B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 1948, 175, 595–603. [Google Scholar] [CrossRef]
- Dische, Z.; Shettles, L.B. A new spectrophotometric test for the detection of methylpentose. J. Biol. Chem. 1951, 192, 579–582. [Google Scholar] [CrossRef]
- Bailey, R.W. The Reaction of Pentoses with Anthrone. Biochem. J. 1958, 68, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F.; et al. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines 2020, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Coxon, B.; Robbins, J.B.; Schneerson, R. Immunochemical studies of Shigella flexneri 2a and 6, and Shigella dysenteriae type 1 O-specific polysaccharide-core fragments and their protein conjugates as vaccine candidates. Carbohydr. Res. 2010, 345, 1600–1608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cataldi, T.R.I.; Campa, C.; De Benedetto, G.E. Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, C.; Raso, M.M.; Palmieri, E.; De Felice, A.; Pippi, F.; Micoli, F. Development of a Specific and Sensitive HPAEC-PAD Method for Quantification of Vi Polysaccharide Applicable to other Polysaccharides Containing Amino Uronic Acids. Anal. Chem. 2020, 92, 6304–6311. [Google Scholar] [CrossRef] [PubMed]
- 2.5.21 Methylpentoses in polysaccharide vaccine. In European Pharmacopoeia (Ph. Eur.), 10th ed.; European Directorate for the Quality of Medicines and Healthcare, Council of Europe: Strasbourg, France; p. 173.
- Micoli, F.; Rondini, S.; Gavini, M.; Lanzilao, L.; Medaglini, D.; Saul, A.; Martin, L.B. O:2-CRM197 conjugates against Salmonella Paratyphi A. PLoS ONE 2012, 7, e47039. [Google Scholar] [CrossRef] [PubMed]
- Park, B.T.; Kim, B.S.; Park, H.; Jeong, J.; Hyun, H.; Hwang, H.S.; Kim, H.H. Binding Specificity of Philyra pisum Lectin to Pathogen-Associated Molecular Patterns, and Its Secondary Structure. Korean J. Physiol. Pharm. 2013, 17, 547–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
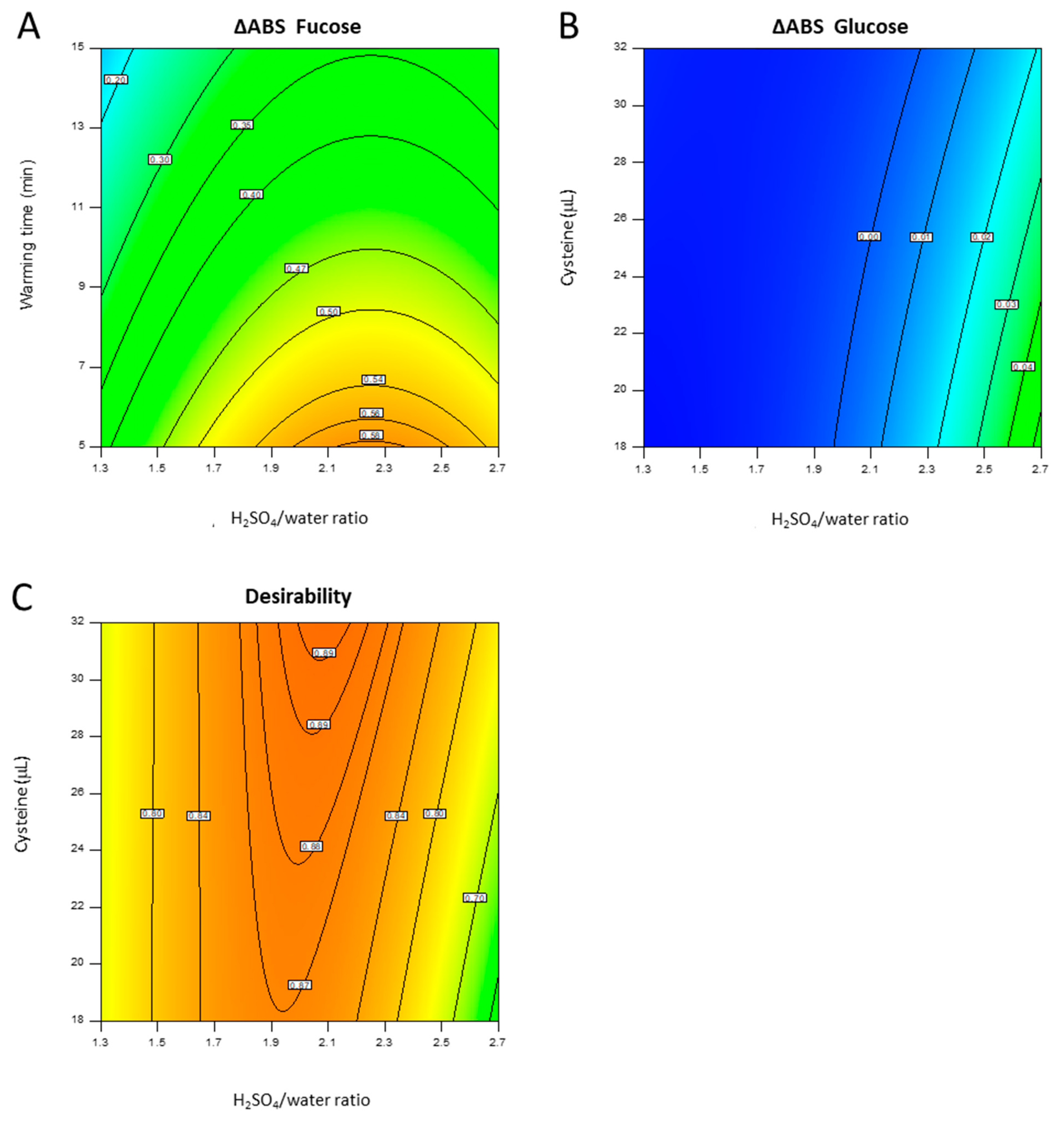

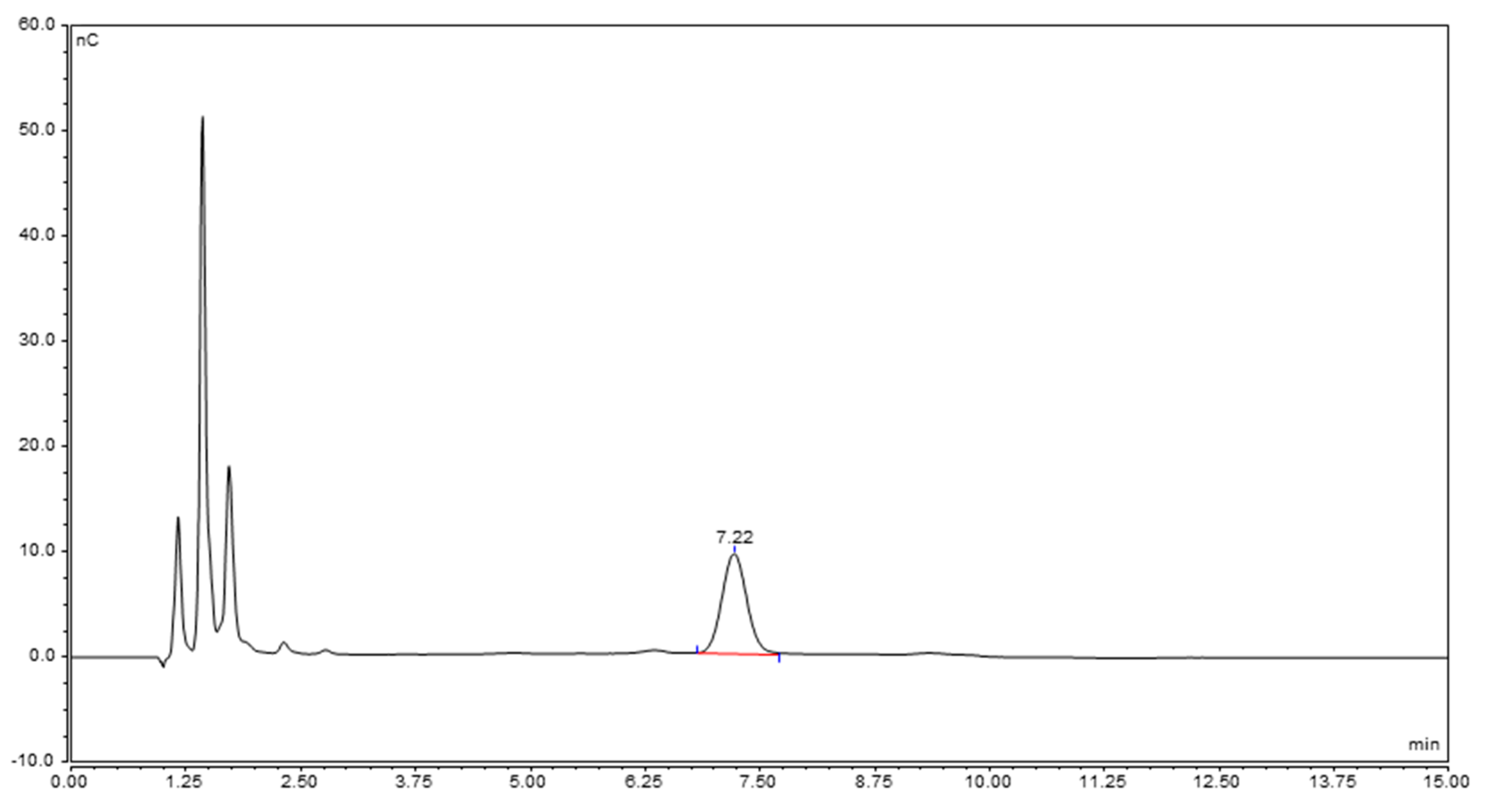
| Source | 0.41 µg/mL | 2.05 µg/mL | 4.10 µg/mL | |||
|---|---|---|---|---|---|---|
| CV | p Value | CV | p Value | CV | p Value | |
| Session | 15.3% | 0.093 | 0 | - | 0% | - |
| Repeatability | 11.6% | 0.009 | 7.7% | 0.002 | 6.7% | 0.002 |
| Intermediate precision | 19.2% | 7.7% | 6.7% | |||
| m/z | Theo. Mass | Delta (ppm) | Composition | Note | |
|---|---|---|---|---|---|
| GalN | 180.0866 | 180.0866 | 0 | C6H14NO5 | [MH]+ |
| 162.0760 | 162.0761 | −0.6 | C6H12NO4 | [MH-H2O]+ | |
| GalA-GalN | 356.1181 | 356.1187 | −1.7 | C12H22NO11 | [MH]+ |
| 338.1077 | 338.1082 | −1.5 | C12H20NO10 | [MH-H2O]+ |
| Source | 1 μg/mL | 2.89 μg/mL | 4.5 μg/mL | |||
|---|---|---|---|---|---|---|
| CV | p Value | CV | p Value | CV | p Value | |
| Session | 0.9% | 0.287 | 1.6% | 0.114 | 1.0% | 0.213 |
| Repeatability | 2.0% | 0.007 | 1.4% | 0.010 | 1.6% | 0.007 |
| Intermediate precision | 2.3% | 2.2% | 1.8% | |||
| Spike | |||
|---|---|---|---|
| 1 μg/mL | 2 μg/mL | 3 μg/mL | |
| Recovery value (95% CI) | 88.1–100% | 91.6–99.8% | 101–104% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raso, M.M.; Vassallo, O.; Micoli, F.; Giannelli, C. Comparison and Optimization of Quantification Methods for Shigella flexneri Serotype 6 O-antigen Containing Galacturonic Acid and Methyl-Pentose. Int. J. Mol. Sci. 2021, 22, 12160. https://doi.org/10.3390/ijms222212160
Raso MM, Vassallo O, Micoli F, Giannelli C. Comparison and Optimization of Quantification Methods for Shigella flexneri Serotype 6 O-antigen Containing Galacturonic Acid and Methyl-Pentose. International Journal of Molecular Sciences. 2021; 22(22):12160. https://doi.org/10.3390/ijms222212160
Chicago/Turabian StyleRaso, Maria Michelina, Oscar Vassallo, Francesca Micoli, and Carlo Giannelli. 2021. "Comparison and Optimization of Quantification Methods for Shigella flexneri Serotype 6 O-antigen Containing Galacturonic Acid and Methyl-Pentose" International Journal of Molecular Sciences 22, no. 22: 12160. https://doi.org/10.3390/ijms222212160
APA StyleRaso, M. M., Vassallo, O., Micoli, F., & Giannelli, C. (2021). Comparison and Optimization of Quantification Methods for Shigella flexneri Serotype 6 O-antigen Containing Galacturonic Acid and Methyl-Pentose. International Journal of Molecular Sciences, 22(22), 12160. https://doi.org/10.3390/ijms222212160








