Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection
Abstract
:1. Introduction
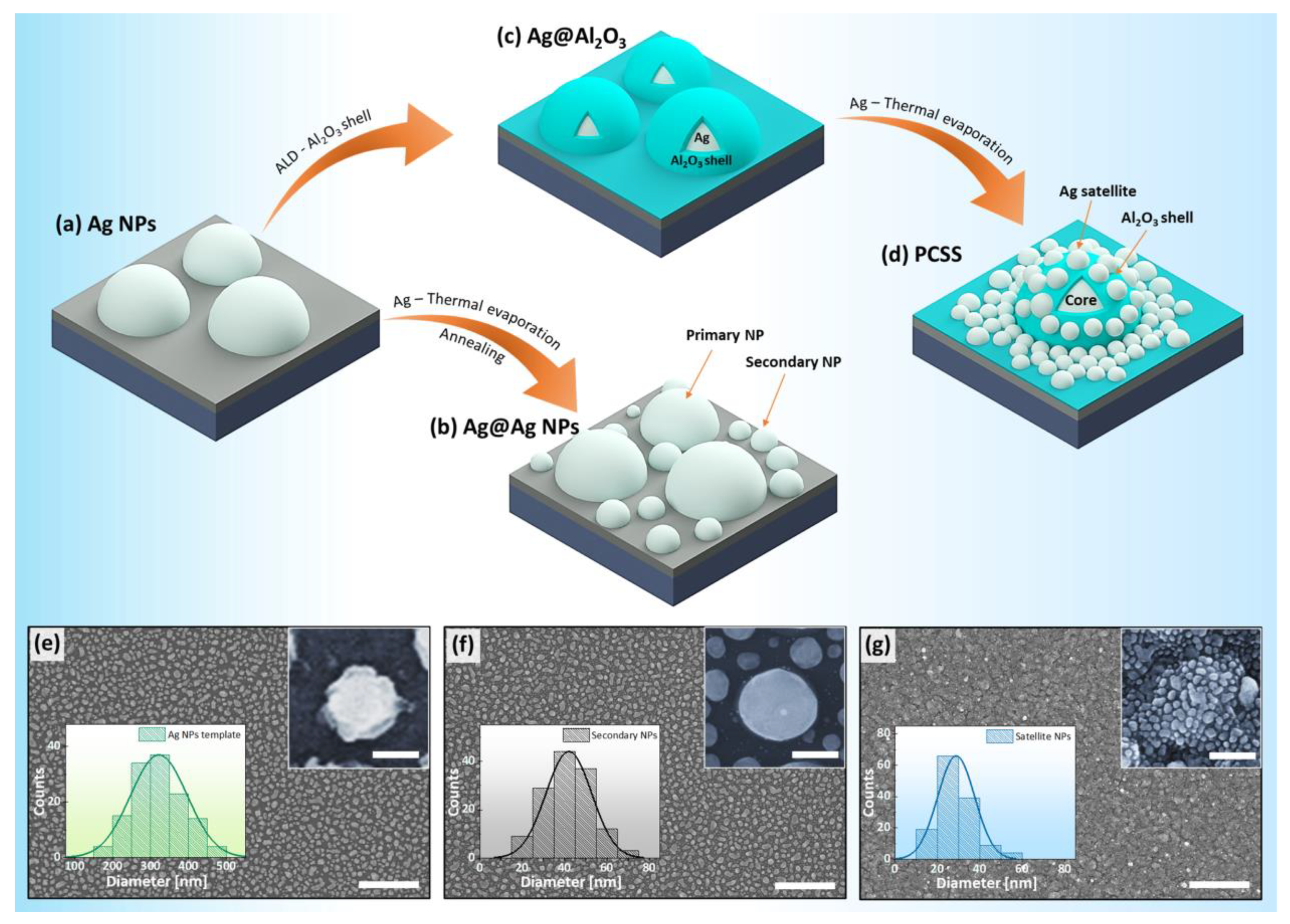
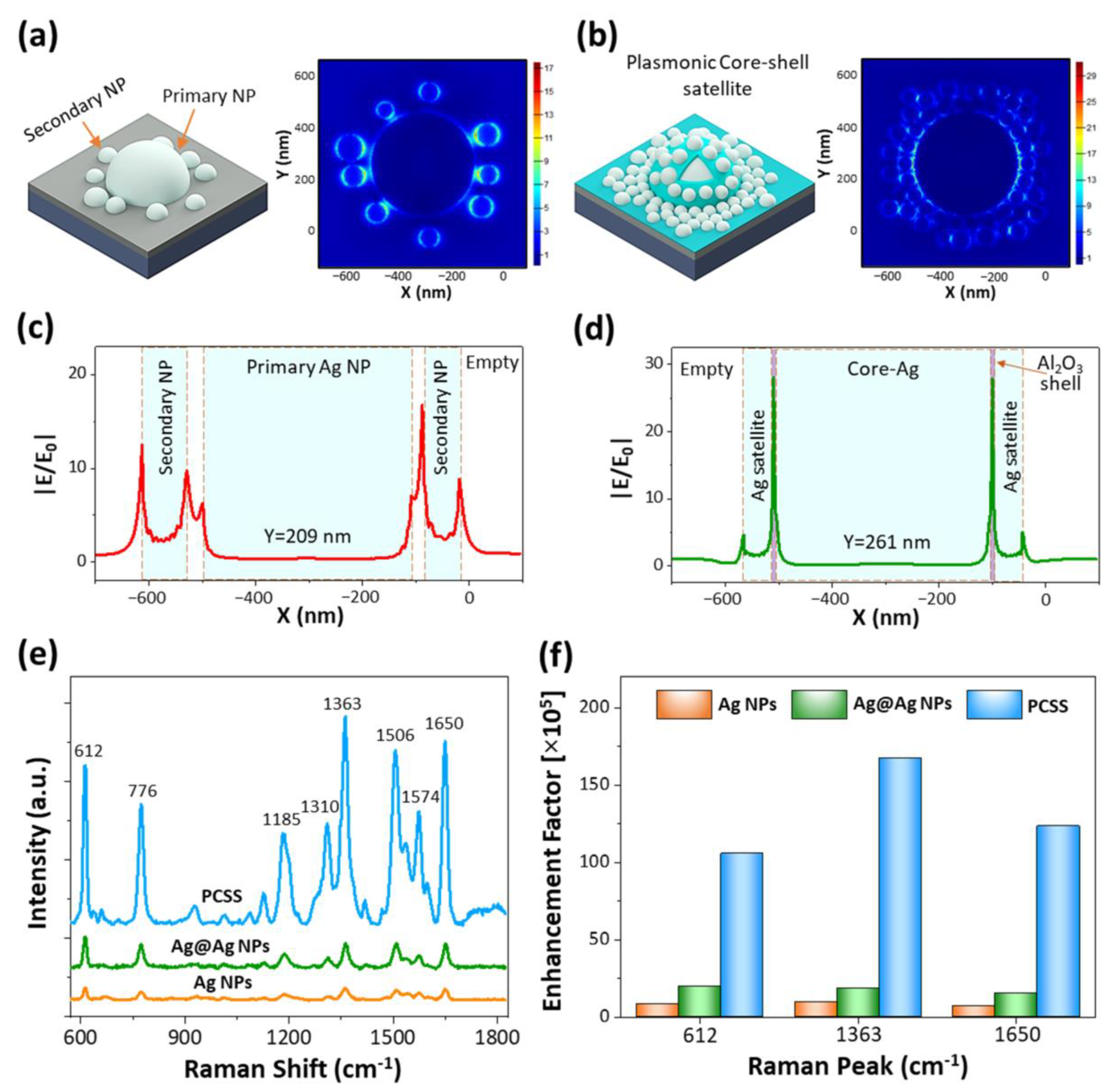
2. Results and Discussion
3. Materials and Methods
3.1. Fabrication of Ag NP Templates and Ag@Ag NPs
3.2. Fabrication of Plasmonic Core–Shell Satellites
3.3. Sample Characterization and SERS Measurement
3.4. FDTD Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, T.; Jiang, R.; Jiang, N.; Ruan, Q.; Wang, J.; Sun, L.-D.; Yan, C.-H.; Lin, H.Q. Thickness Control Produces Gold Nanoplates with Their Plasmon in the Visible and Near-Infrared Regions. Adv. Opt. Mater. 2016, 4, 76–85. [Google Scholar] [CrossRef]
- Lin, Z.-W.; Tsao, Y.-C.; Yang, M.-Y.; Huang, M.H. Seed-Mediated Growth of Silver Nanocubes in Aqueous Solution with Tunable Size and Their Conversion to Au Nanocages with Efficient Photothermal Property. Chem.-A Eur. J. 2016, 22, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, L.; Cheng, Z.-Q.; Yang, D.-J.; Zhou, L.; Hao, Z.-H.; Wang, Q.-Q. Plasmon-assisted site-selective growth of Ag nanotriangles and Ag-Cu2O hybrids. Sci. Rep. 2017, 7, 44806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, H.; Kersey, F.R.; Register, J.K.; Parrott, M.C.; Vo-Dinh, T. Plasmonic Gold Nanostars for Multi-Modality Sensing and Diagnostics. Sensors 2015, 15, 3706–3720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, J.A.; Ferhan, A.R.; Cho, N.-J. Nanoplasmonic sensors for biointerfacial science. Chem. Soc. Rev. 2017, 46, 3615–3660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Dhiman, M. Plasmonic nanocatalysis for solar energy harvesting and sustainable chemistry. J. Mater. Chem. A 2020, 8, 10074–10095. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Yang, J.-K.; Jo, A.; Lee, H.; Heo, E.; Jeong, D.; Jun, B.-H.; Chang, H.; Lee, Y.-S. Template-Assisted Plasmonic Nanogap Shells for Highly Enhanced Detection of Cancer Biomarkers. Int. J. Mol. Sci. 2021, 22, 1752. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Vongphachanh, S.; Yoon, J.; Kim, B.; Choi, C.-J.; Sohn, J.I.; Hong, W.-K. Silver nanowire-network-film-coated soft substrates with wrinkled surfaces for use as stretchable surface enhanced Raman scattering sensors. J. Alloy. Compd. 2021, 859, 157862. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yu, C.C.; Sheu, S.F. Low concentration rhodamine 6G observed by surface-enhanced Raman scattering on op-timally electrochemically roughened silver substrates. J. Mater. Chem. 2006, 16, 3546–3551. [Google Scholar] [CrossRef]
- Kochylas, I.; Gardelis, S.; Likodimos, V.; Giannakopoulos, K.P.; Falaras, P.; Nassiopoulou, A.G. Improved sur-face-enhanced-Raman scattering sensitivity using Si nanowires/silver nanostructures by a single step metal-assisted chemical etching. Nanomaterials 2021, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Visible-light-driven reduction of nitrostyrene utilizing plasmonic silver nanoparticle catalysts immobilized on oxide supports. Catal. Today 2020, 355, 620–626. [Google Scholar] [CrossRef]
- Nong, J.; Tang, L.; Lan, G.; Luo, P.; Li, Z.; Huang, D.; Shen, J.; Wei, W. Combined Visible Plasmons of Ag Nanoparticles and Infrared Plasmons of Graphene Nanoribbons for High-Performance Surface-Enhanced Raman and Infrared Spectroscopies. Small 2021, 17, 2004640. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Jiang, Z.; Shi, Z.; Wang, J.; Du, L. Highly Sensitive and Reproducible SERS Substrates Based on Ordered Micropyramid Array and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 29222–29229. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Quan, J.; Zhang, J.; Qi, X.; Li, J.; Wang, N.; Zhu, Y. A study on the correlation between the dewetting temperature of Ag film and SERS intensity. Sci. Rep. 2017, 7, 14771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochowska, K.; Siuzdak, K.; Macewicz, Ł.; Skiba, F.; Szkoda, M.; Karczewski, J.; Burczyk, Ł.; Śliwiński, G. Nanostructuring of thin Au films deposited on ordered Ti templates for applications in SERS. Appl. Surf. Sci. 2017, 418, 472–480. [Google Scholar] [CrossRef]
- Pandey, P.; Shin, K.; Jang, A.-R.; Seo, M.-K.; Hong, W.-K.; Sohn, J.I. Highly sensitive multiplex-detection of surface-enhanced Raman scattering via self-assembly arrays of porous AuAg nanoparticles with built-in nanogaps. J. Alloy. Compd. 2021, 888, 161504. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Cheng, Z.; Wei, J.; Yang, L.; Zhong, Z.; Hu, H.; Wang, Y.; Zhou, B.; Li, P. Emerging core–shell nanostructures for surface-enhanced Raman scattering (SERS) detection of pesticide residues. Chem. Eng. J. 2021, 424, 130323. [Google Scholar] [CrossRef]
- Pazos-Perez, N.; Fitzgerald, J.M.; Giannini, V.; Guerrini, L.; Alvarez-Puebla, R.A. Modular assembly of plasmonic core–satellite structures as highly brilliant SERS-encoded nanoparticles. Nanoscale Adv. 2019, 1, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Tang, J.; Luo, W.; Zhang, Z.; Zhu, Y.; Wang, R.; Yang, H.; Chen, X. Core-shell-satellite microspheres-modified glass capillary for microsampling and ultrasensitive SERS spectroscopic detection of methotrexate in serum. Sens. Actuators B Chem. 2018, 275, 267–276. [Google Scholar] [CrossRef]
- Preston, A.S.; Hughes, R.A.; Dominique, N.L.; Camden, J.P.; Neretina, S. Stabilization of Plasmonic Silver Nanostructures with Ultrathin Oxide Coatings Formed Using Atomic Layer Deposition. J. Phys. Chem. C 2021, 125, 17212–17220. [Google Scholar] [CrossRef]
- Chugh, D.; Jagadish, C.; Tan, H. Large-Area Hexagonal Boron Nitride for Surface Enhanced Raman Spectroscopy. Adv. Mater. Technol. 2019, 4, 1900220. [Google Scholar] [CrossRef]
- Chen, Y.; Lerch, S.; Say, Z.; Tiburski, C.; Langhammer, C.; Moth-Poulsen, K. Catalytically active and thermally stable core–shell gold–silica nanorods for CO oxidation. RSC Adv. 2021, 11, 11642–11650. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Yan, L.; Chen, S.; Yang, Z.-L.; Jing, C. Multifunctional Fe3O4@SiO2–Au Satellite Structured SERS Probe for Charge Selective Detection of Food Dyes. ACS Appl. Mater. Interfaces 2016, 8, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, J.; Weng, G.J.; Li, J.J.; Zhao, J.W. Gold nanoring core-shell satellites with abundant built-in hotspots and great analyte penetration: An immunoas say platform for the SERS/fluorescence-based detection of carcinoembryonic antigen. Chem. Eng. J. 2021, 409, 128173. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Wang, X.-Y.; Song, G.; You, L.-J.; Li, J.-M. Core-satellite Ag/TiO2/Ag composite nanospheres for multiple SERS applications in solution by a portable Raman spectrometer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124013. [Google Scholar] [CrossRef]
- Kunwar, S.; Sui, M.; Zhang, Q.; Pandey, P.; Li, M.-Y.; Lee, J. Various Silver Nanostructures on Sapphire Using Plasmon Self-Assembly and Dewetting of Thin Films. Nano-Micro Lett. 2016, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffino, F.; Carria, E.; Kimiagar, S.; Crupi, I.; Grimaldi, M.G. Rayleigh-instability-driven dewetting of thin Au and Ag films on indium-tin-oxide surface under nanosecond laser irradiations. Micro. Nano Lett. 2013, 8, 127–130. [Google Scholar] [CrossRef]
- Xu, R.; Li, R.; Jia, L.; Zheng, Z.; Zhou, T. An Efficient Strategy to Prepare Ultra-High Sensitivity SERS-Active Substrate Based on Laser-Induced Selective Metallization of Polymers. ACS Sustain. Chem. Eng. 2021, 9, 5038–5049. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Mistry, K.; Nguyen, V.H.; Ibrahim, K.H.; Muñoz-Rojas, D.; Yavuz, M.; Musselman, K.P. Quantum-Tunneling Metal-Insulator-Metal Diodes Made by Rapid Atmospheric Pressure Chemical Vapor Deposition. Adv. Funct. Mater. 2019, 29, 1805533. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, L.; Xiao, D.; Li, N.; Li, Y.; Cao, D.; Shi, Z.; Cui, Z.; Lu, N. A silver nanoislands on silica spheres platform: Enriching trace amounts of analytes for ultrasensitive and reproducible SERS detection. Nanoscale 2017, 9, 16749–16754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, F.; Liu, M.; Liu, D.; Men, D.; Cai, W.; Duan, G.; Li, Y. Spherical Nanoparticle Arrays with Tunable Nanogaps and Their Hydrophobicity Enhanced Rapid SERS Detection by Localized Concentration of Droplet Evaporation. Adv. Mater. Interfaces 2015, 2, 1500031. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y. Enhancing light reflective properties on ITO glass by plasmonic effect of silver nanoparticles. Results Phys. 2017, 7, 2874–2877. [Google Scholar] [CrossRef]
- Thouti, E.; Chander, N.; Dutta, V.; Komarala, V.K. Optical properties of Ag nanoparticle layers deposited on silicon substrates. J. Opt. 2013, 15, 035005. [Google Scholar] [CrossRef]
- Park, G.; Min, K.S.; Kwon, H.; Yoon, S.; Park, S.; Kwon, J.; Lee, S.; Jo, J.; Kim, M.; Kim, S.K. Strain-Induced Modulation of Localized Surface Plasmon Resonance in Ultrathin Hexagonal Gold Nanoplates. Adv. Mater. 2021, 33, 2100653. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Qin, Y.; Mu, Y.; Fang, X.; Zhai, T.; Zhang, X. Gold-Stabilized Gold–Silver Alloy Nanostructures as High-Performance SERS Substrate. Plasmonics 2020, 15, 2027–2032. [Google Scholar] [CrossRef]
- Zhong, F.; Wu, Z.; Guo, J.; Jia, D. Porous Silicon Photonic Crystals Coated with Ag Nanoparticles as Efficient Substrates for Detecting Trace Explosives Using SERS. Nanomaterial 2018, 8, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Jung, D.; Wi, J.-S.; Lim, H.; Lee, J.-J. Surfactant-free galvanic replacement for synthesis of raspberry-like silver nanostructure pattern with multiple hot-spots as sensitive and reproducible SERS substrates. Appl. Surf. Sci. 2020, 505, 144548. [Google Scholar] [CrossRef]
- Martins, N.C.; Fateixa, S.; Fernandes, T.; Nogueira, H.I.; Trindade, T. Inkjet Printing of Ag and Polystyrene Nanoparticle Emulsions for the One-Step Fabrication of Hydrophobic Paper-Based Surface-Enhanced Raman Scattering Substrates. ACS Appl. Nano Mater. 2021, 4, 4484–4495. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, L.; Li, X.; Li, B.; Huang, J.; Sun, J.; Wang, Z.; Xu, Z.; Qu, L.; Lu, Y.; et al. Hybrid superhydrophilic–superhydrophobic micro/nanostructures fabricated by femtosecond laser-induced forward transfer for sub-femtomolar Raman detection. Microsyst. Nanoeng. 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.; Kunwar, S.; Shin, K.-H.; Seo, M.-K.; Yoon, J.; Hong, W.-K.; Sohn, J.-I. Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection. Int. J. Mol. Sci. 2021, 22, 12191. https://doi.org/10.3390/ijms222212191
Pandey P, Kunwar S, Shin K-H, Seo M-K, Yoon J, Hong W-K, Sohn J-I. Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection. International Journal of Molecular Sciences. 2021; 22(22):12191. https://doi.org/10.3390/ijms222212191
Chicago/Turabian StylePandey, Puran, Sundar Kunwar, Ki-Hoon Shin, Min-Kyu Seo, Jongwon Yoon, Woong-Ki Hong, and Jung-Inn Sohn. 2021. "Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection" International Journal of Molecular Sciences 22, no. 22: 12191. https://doi.org/10.3390/ijms222212191
APA StylePandey, P., Kunwar, S., Shin, K.-H., Seo, M.-K., Yoon, J., Hong, W.-K., & Sohn, J.-I. (2021). Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection. International Journal of Molecular Sciences, 22(22), 12191. https://doi.org/10.3390/ijms222212191





