Eco-Friendly Ether and Ester-Urethane Prepolymer: Structure, Processing and Properties
Abstract
:1. Introduction
- designing pipelines, processing equipment, pumps, etc.;
- the quality control of intermediate and end products;
- the analysis of properties of substances during their use.
2. Materials and Methods
2.1. Materials
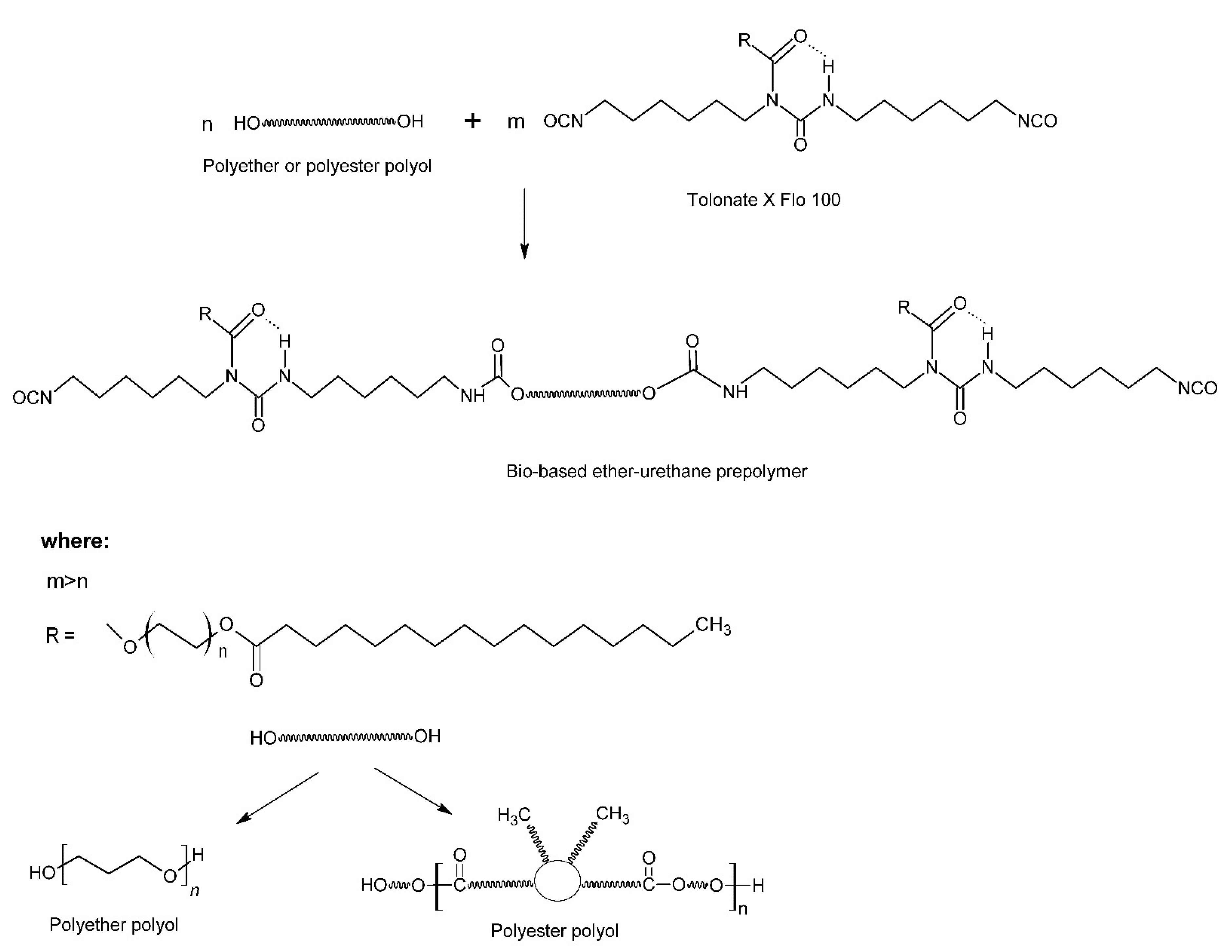
2.2. Prepolymer Preparation
2.3. Characterization
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. 1H NMR
2.3.3. Rheological Measurement
- increasing shear rate from 0 to 300 s−1 in 120 s,
- constant shear rate of 300 s−1 for 120 s,
- decreasing shear rate from 300 to 0 s−1 in 120 s.
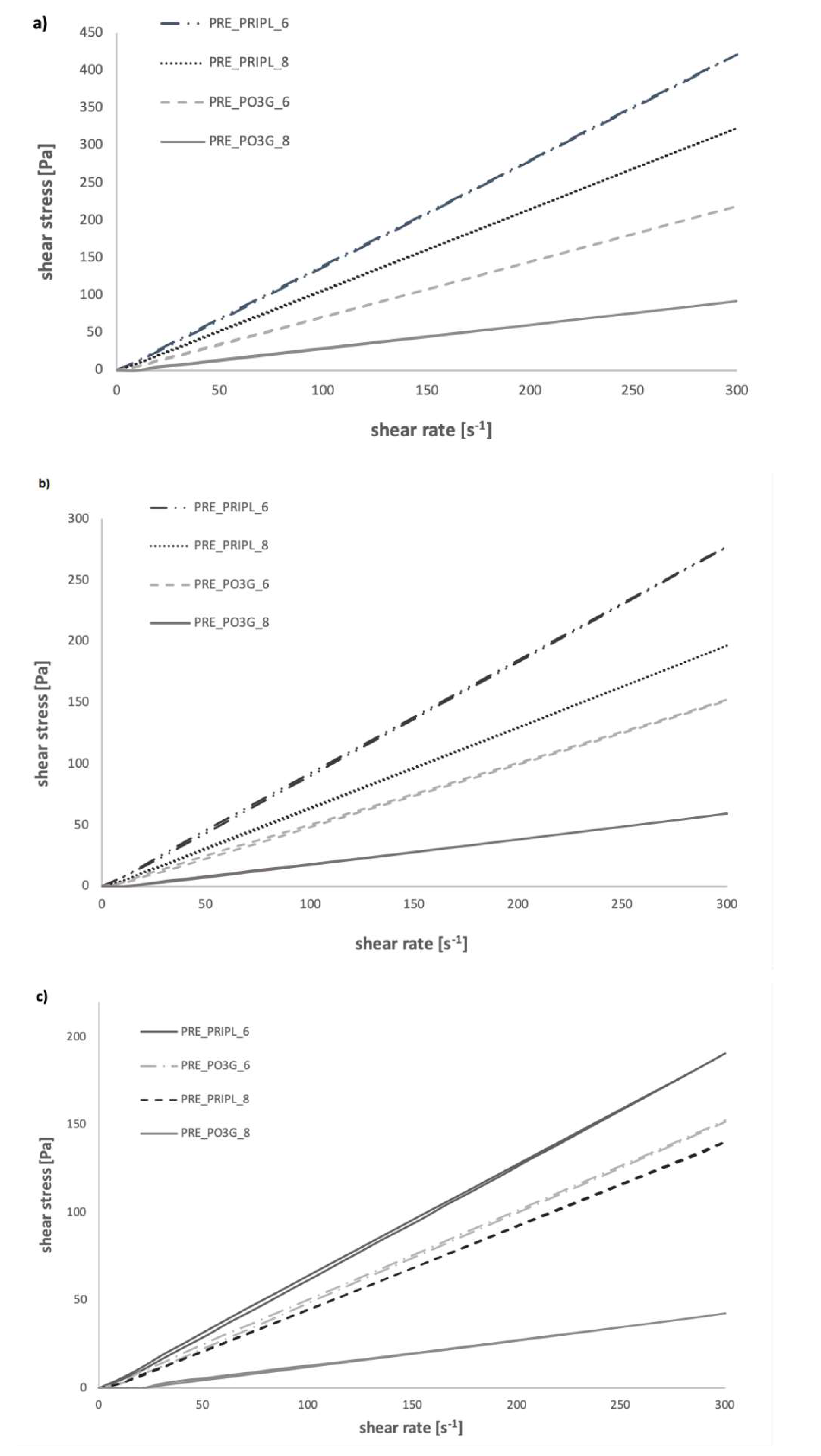
- x—shear rate [s−1]
- y—shear stress [Pa]
- k1—yield stress [Pa]
- k2—consistency index [-]
- k3—flow behavior index [-]
2.3.4. Thermogravimetry (TGA)
3. Results and Discussion
3.1. Chemical Strucutre
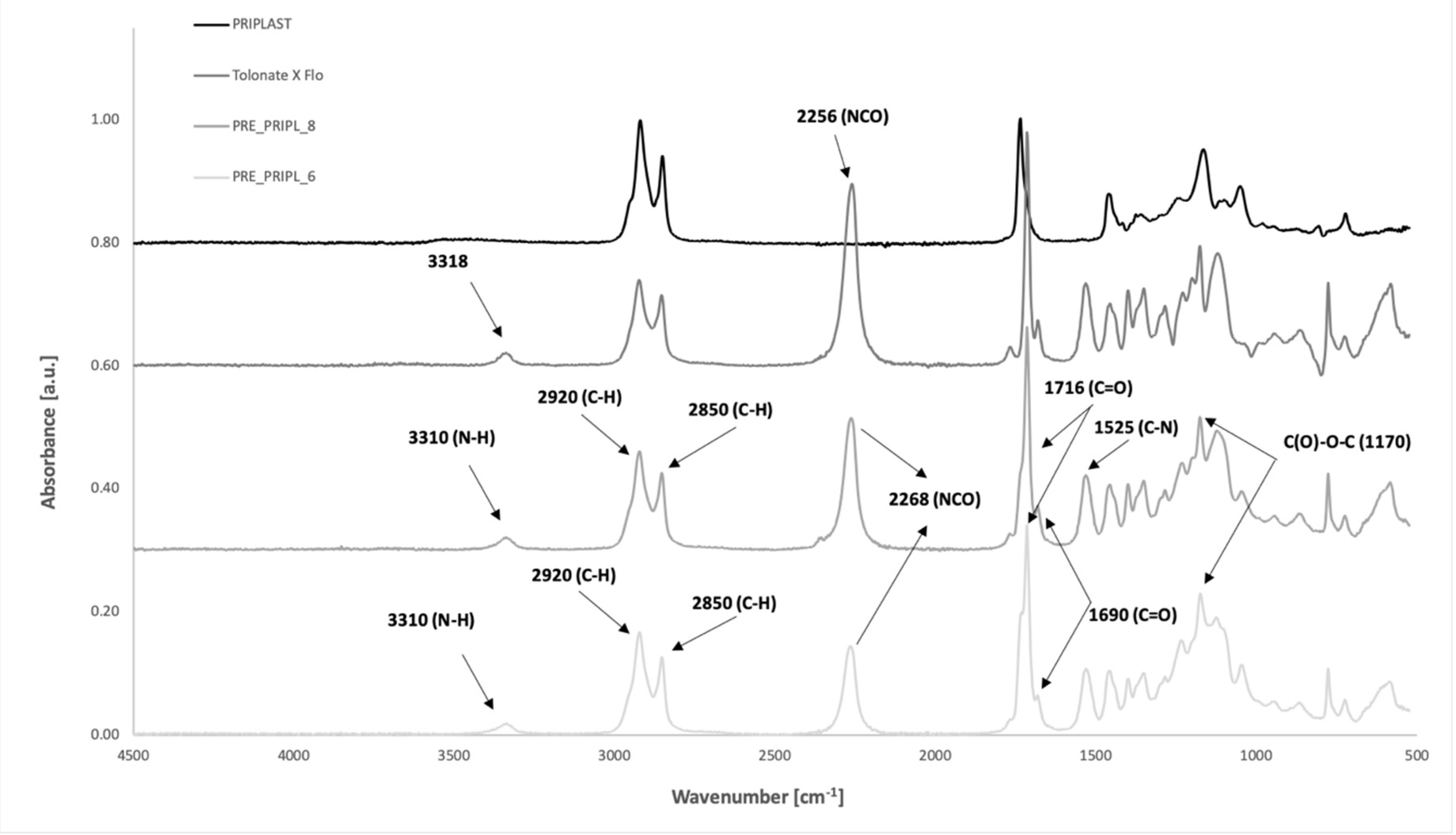
3.1.1. Fourier-Transform Infrared Spectroscopy
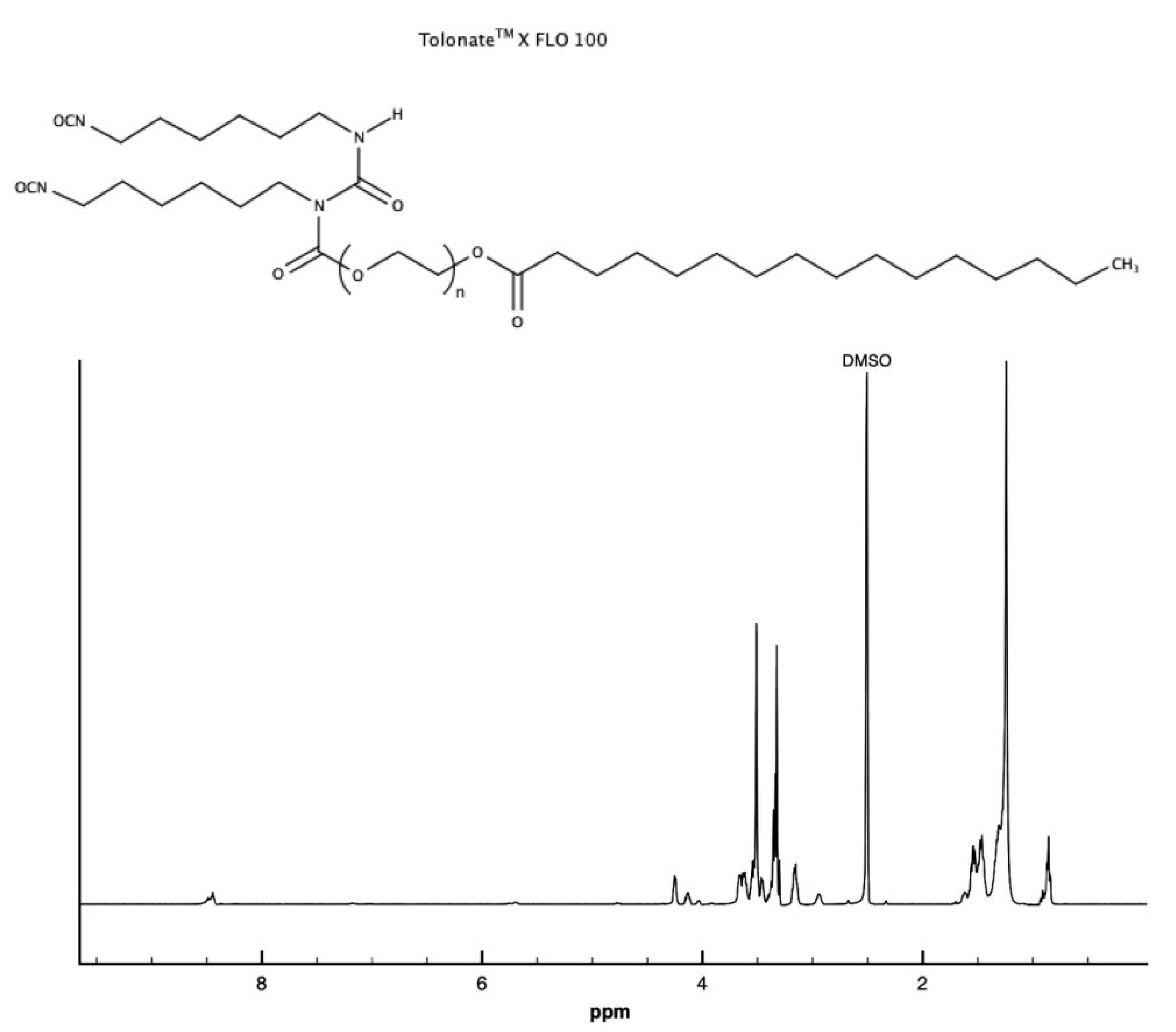
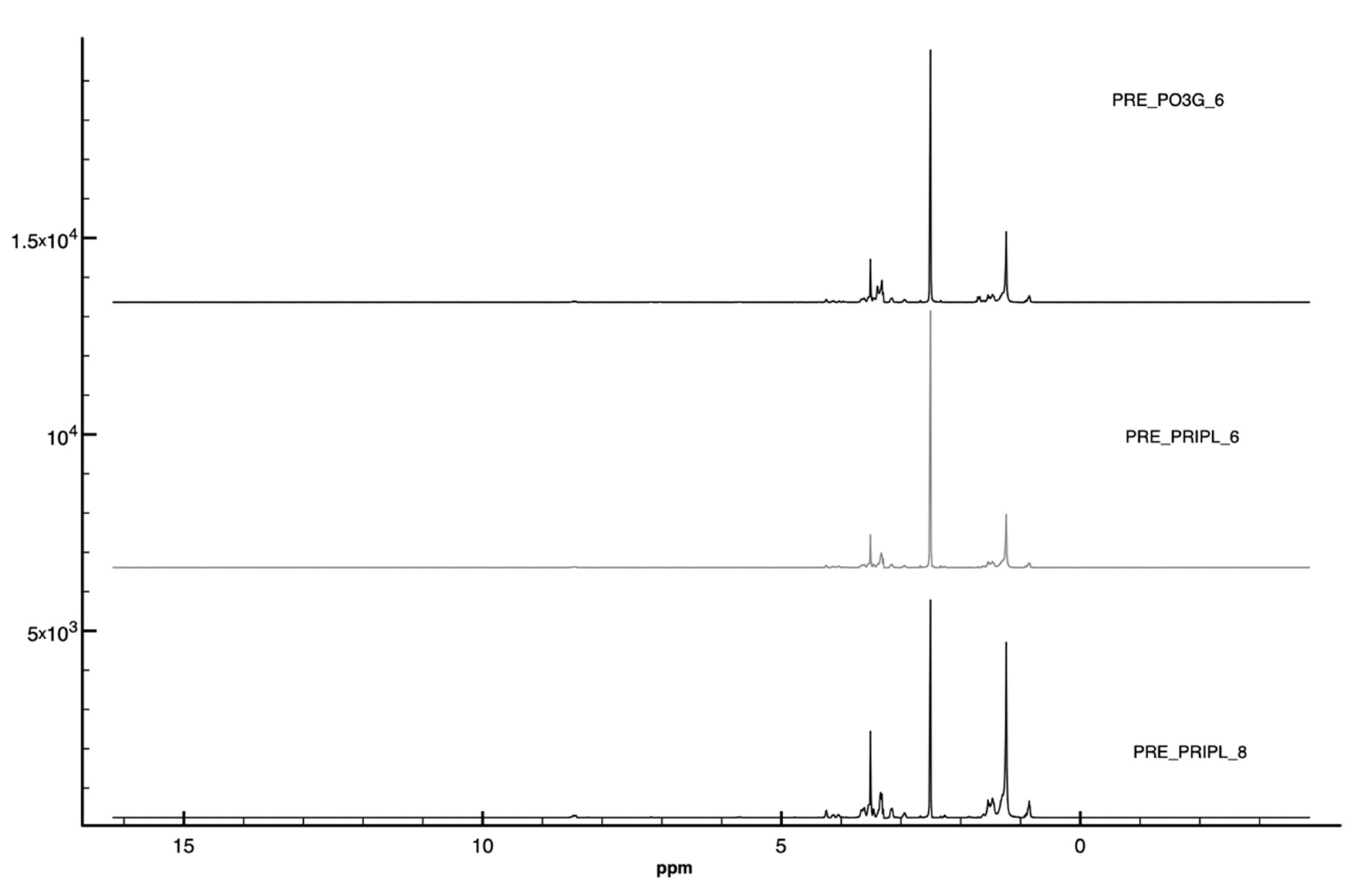
3.1.2. Proton Nuclear Magnetic Resonance
3.2. Processing Properties
3.2.1. Rheological Measurement
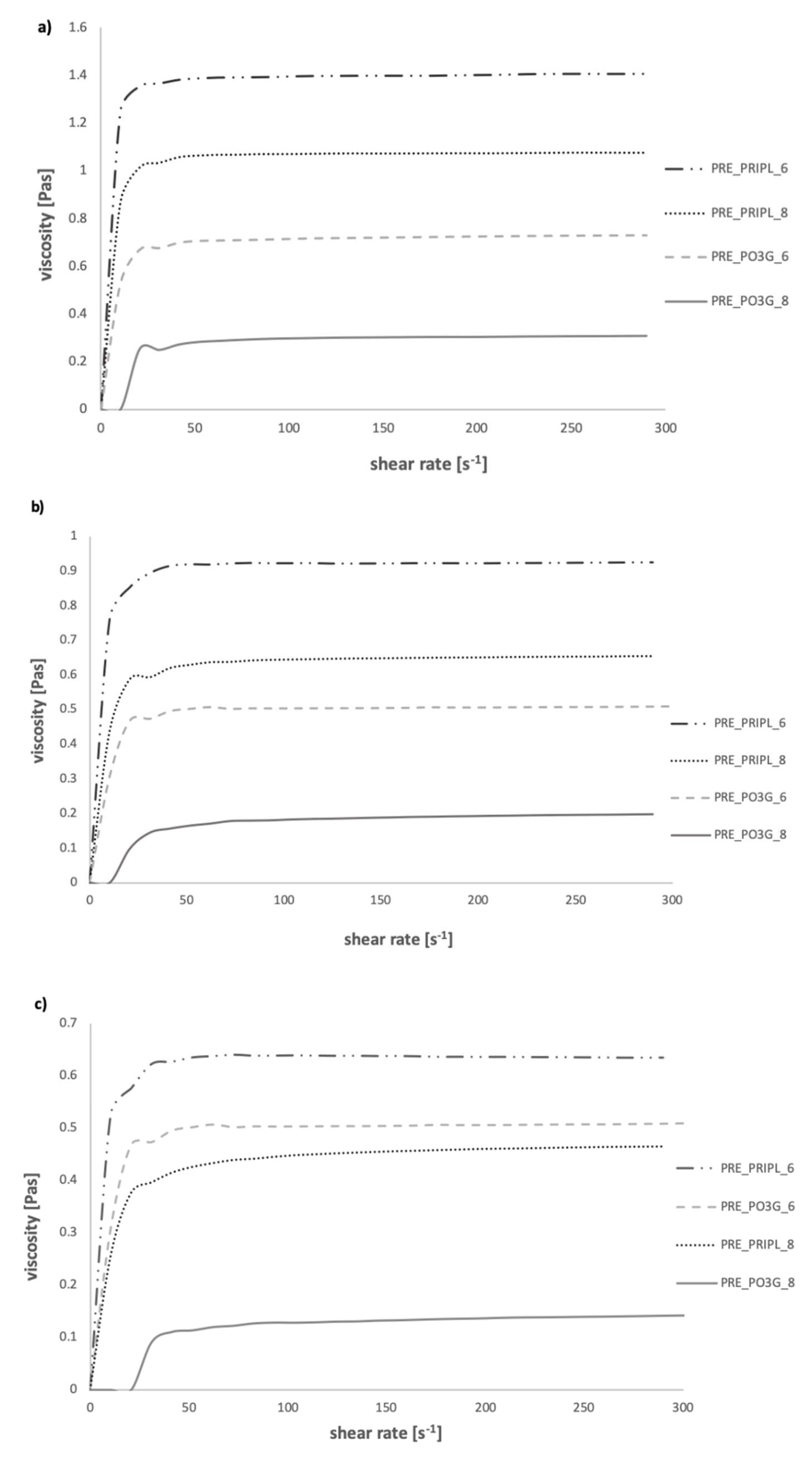
3.2.2. Viscosity Curves of Prepolymers
3.2.3. Flow Curves of Prepolymers
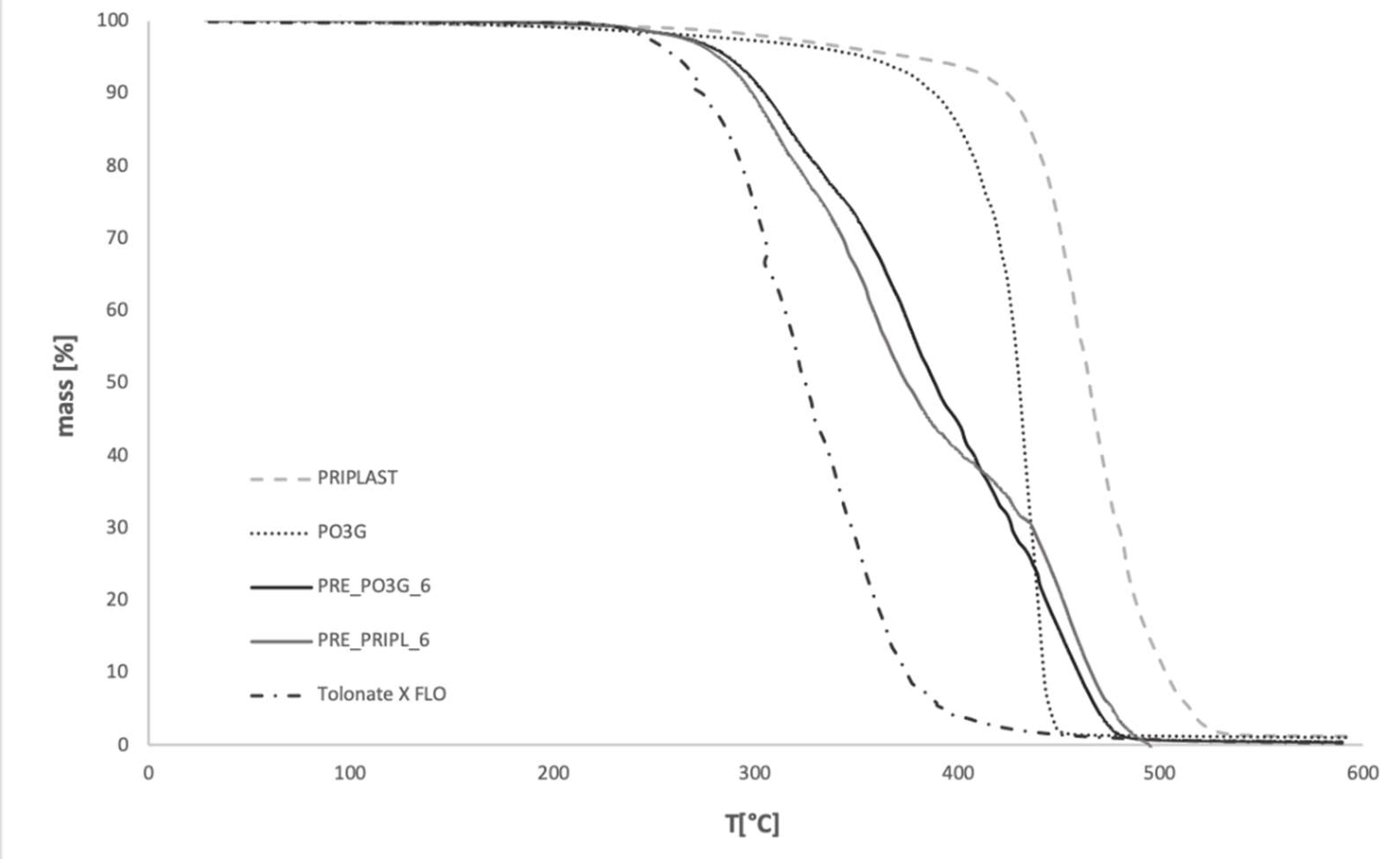
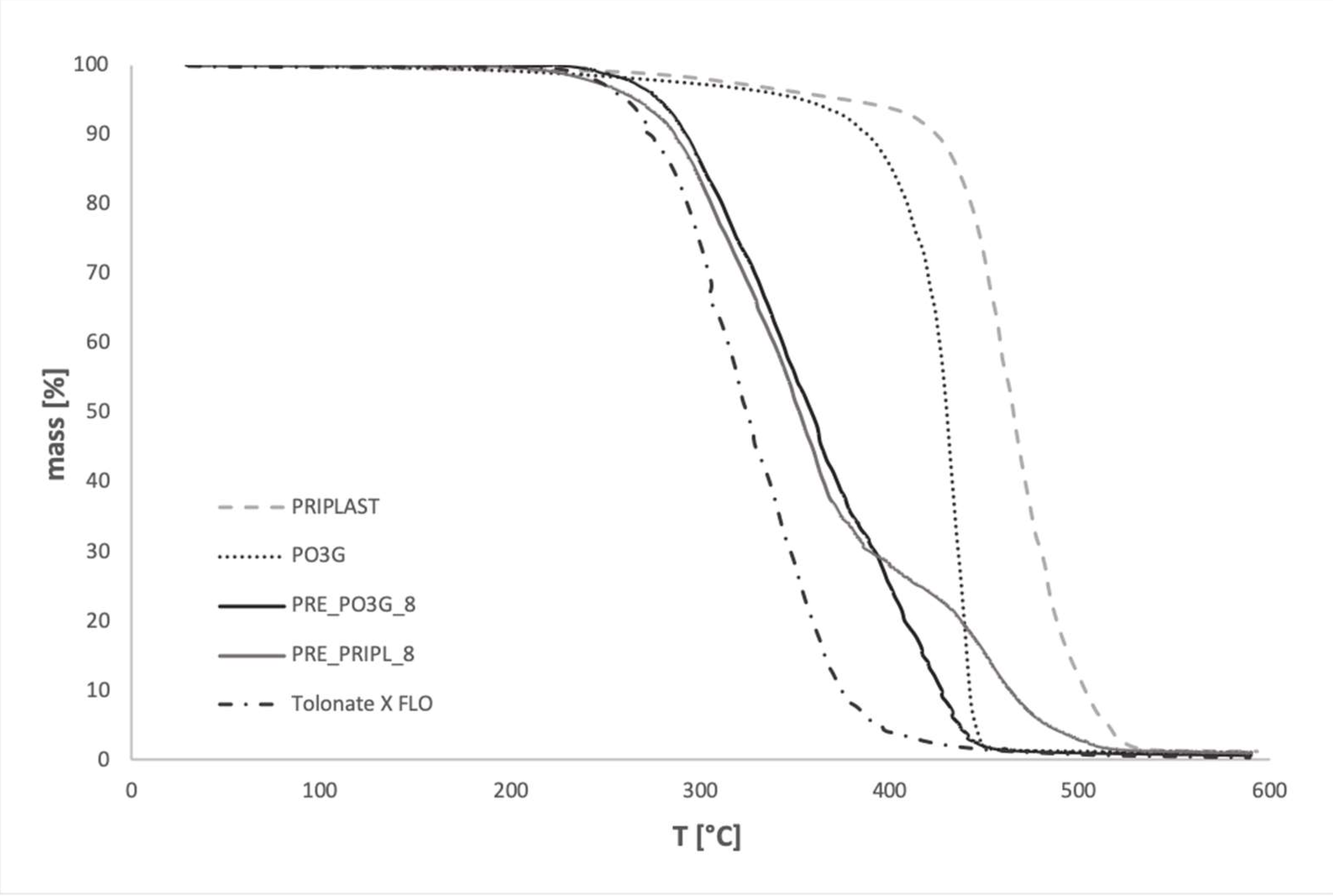
3.3. Thermal Stability
Thermogravimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wirpsza, Z. Polyurethanes: Chemistry, Technology and Applications; Ellis Horwood Limited: Warsaw, Poland, 1991. [Google Scholar]
- Seymour, R.B.; Kauffman, G.B. Polyurethanes: A Class of Modern Versatile Materials. J. Chem. Educ. 1992, 69, 909–910. [Google Scholar] [CrossRef]
- American Chemistry Council GUIDE to the Business of Chemistry. 2019. Available online: https://www.americanchemistry.com/chemistry-in-america/data-industry-statistics/resources/2019-guide-to-the-business-of-chemistry (accessed on 26 March 2021).
- World Economic Forum The Global Risks Report. 2019. Available online: https://www.weforum.org/reports/the-global-risks-report-2019 (accessed on 26 March 2021).
- Crude Oil WTI. Available online: https://oilprice.com (accessed on 26 March 2021).
- Corn Price. Available online: https://markets.businessinsider.com/commodities/corn-price (accessed on 23 March 2021).
- Tersac, G. Chemistry and Technology of Polyols for Polyurethanes. Milhail Ionescu; Rapra Technology: Shrewsbury, UK, 2007; Volume 56, ISBN 9781847350350. [Google Scholar]
- Datta, J.; Kasprzyk, P. Thermoplastic Polyurethanes Derived From Petrochemical or Renewable Resources: A Comprehensive Review. Polym. Eng. Sci. 2017, 58, E14–E35. [Google Scholar] [CrossRef] [Green Version]
- Szycher, M. Structure–Property Relations in Polyurethanes. In Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 37–86. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons.: Hoboken, NJ, USA, 2021; ISBN 9781119669418. [Google Scholar]
- Kasprzyk, P.; Głowińska, E.; Datta, J. Structure and Properties Comparison of Poly(Ether-Urethane)s Based on Nonpetrochemical and Petrochemical Polyols Obtained by Solvent Free Two-Step Method. Eur. Polym. J. 2021, 157. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. A Mathematical Model of Rheological Behavior of Novel Bio-Based Isocyanate-Terminated Polyurethane Prepolymers. Ind. Crop. Prod. 2014, 60, 123–129. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narra, M.; Westkamper, M. Zamówienia Na Bioprodukty i Biousługi.Podręcznik; Agencja ds. Zasobów Odnawialnych: Gulzow-Pruzen, Germany, 2017. [Google Scholar]
- Shastri, V.; Stevens, M. Polymer Foams. Encycl. Biomater. Biomed. Eng. Second Ed.-Four 2008, 2270–2274. [Google Scholar] [CrossRef]
- Hess, D. Allessa VELVETOL—Market Oriented Platforms, Basel, Switzerland. 2019. Available online: https://www.chemspeceurope.com/2021/assets/5._2019_06_26_Allessa_Velvetol_Presentation_Chemspec_Basel.pdf (accessed on 26 March 2021).
- Croda PriplastTM 3238. Available online: https://www.crodasmartmaterials.com/en-gb/product-finder/product/573-Priplast_1_3238 (accessed on 22 March 2021).
- Gesa, B.; Hecking, A.; Sánchez, B.V. High Performance Enabled By Nature: First Bio-Based Crosslinker. pp. 46–50.
- Mitsui Chemicals STABiOTM PDI. Available online: https://jp.mitsuichemicals.com/en/service/packaging/coatings/stabio/index.htm (accessed on 22 March 2021).
- Tolonate X FLO 100. Available online: https://www.palmerholland.com/Assets/User/Documents/Product/44709/3831/MITM09115.PDF (accessed on 23 March 2021).
- Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers 2021, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty Acid-Derived Diisocyanate and Biobased Polyurethane Produced from Vegetable Oil: Synthesis, Polymerization, and Characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zheng, Z.; Yang, Z.; Chen, Y.; Shen, L. A Fully Bio-Based Waterborne Polyurethane Dispersion from Vegetable Oils: From Synthesis of Precursors by Thiol-Ene Reaction to Study of Final Material. Prog. Org. Coat. 2014, 77, 53–60. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Structure-Rheology Relationship of Fully Bio-Based Linear Polyester Polyols for Polyurethanes—Synthesis and Investigation. Polym. Test. 2018, 67, 110–121. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Influence of NCO/OH and Transesterified Castor Oil on the Structure and Properties of Polyurethane: Synthesis and Characterization. Mater. Express 2015, 5, 377–389. [Google Scholar] [CrossRef]
- Głowińska, E.; Wolak, W.; Datta, J. Eco-Friendly Route for Thermoplastic Polyurethane Elastomers with Bio-Based Hard Segments Composed of Bio-Glycol and Mixtures of Aromatic–Aliphatic and Aliphatic–Aliphatic Diisocyanate. J. Polym. Environ. 2021, 29, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, P.; Datta, J. Novel Bio-Based Thermoplastic Poly(Ether-Urethane)s. Correlations between the Structure, Processing and Properties. Polymer 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Lizymol, P.P.; Jayabalan, M. Rheological Behaviour of Polyurethane Prepolymers as Potting Compound for Hollow Fibre Haemodialyzer. Indian J. Chem. Technol. 1997, 4, 89–93. [Google Scholar]
- Kosmela, P.; Gosz, K.; Kazimierski, P.; Hejna, A.; Haponiuk, J.T.; Piszczyk, Ł. Chemical Structures, Rheological and Physical Properties of Biopolyols Prepared via Solvothermal Liquefaction of Enteromorpha and Zostera Marina Biomass. Cellulose 2019, 26, 5893–5912. [Google Scholar] [CrossRef] [Green Version]
- Amado, J.C.Q. Thermal Resistance Properties of Polyurethanes and Its Composites: A Short Review. J. Res. Updates Polym. Sci. 2019, 8, 66–84. [Google Scholar] [CrossRef]

| Composition of Sample | Calculated Percentage of Unreacted-NCO Groups | Sample Code | Determined Percentage of Unreacted Isocyanate Groups [%] |
|---|---|---|---|
| Tolonate X FLO + Velvetol H2400 | 6% | PRE_PO3G_6 | 5.22 ± 0.03 |
| Tolonate X FLO + PRIPLAST 3294TM | 6% | PRE_PRIPL_6 | 5.51 ± 0.14 |
| Tolonate X FLO + Velvetol H2400 | 8% | PRE_PO3G_8 | 7.28 ± 0.03 |
| Tolonate X FLO + PRIPLAST 3294TM | 8% | PRE_PRIPL_8 | 8.42 ± 0.08 |
| Sample Code | Equation | k1 | k2 | k3 | R |
|---|---|---|---|---|---|
| PRE_PO3G_6 (60 °C) | y = 0.65x1.02 | 0 | 0.65 | 1.02 | 0.9999 |
| PRE_PO3G_6 (70 °C) | y = 0.47x1.01 | 0 | 0.47 | 1.01 | 0.9999 |
| PRE_PO3G_6 (80 °C) | y = 0.38x0.99 | 0 | 0.38 | 0.99 | 0.9998 |
| PRE_PO3G_8 (60 °C) | y = 0.25x1.04 | 0 | 0.25 | 1.04 | 0.9998 |
| PRE_PO3G_8 (70 °C) | y = 0.12x1.09 | 0 | 0.12 | 1.09 | 0.9996 |
| PRE_PO3G_8 (80 °C) | y = 0.08x1.10 | 0 | 0.08 | 1.10 | 0.9998 |
| PRE_PRIPL_6 (60 °C) | y = 1.34x1.01 | 0 | 1.34 | 1.01 | 0.9999 |
| PRE_PRIPL_6 (70 °C) | y = 0.90x1.01 | 0 | 0.90 | 1.01 | 0.9999 |
| * PRE_PRIPL_6 (80 °C) | y = 0.65x | a | 0.65 | - | 0.9999 |
| ** PRE_PRIPL_8 (60 °C) | y = x1.01 | 0 | 1 | 1.01 | 0.9999 |
| PRE_PRIPL_8 (70 °C) | y = 0.59x1.02 | 0 | 0.59 | 1.02 | 0.9999 |
| PRE_PRIPL_8 (80 °C) | y = 0.37x1.04 | 0 | 0.37 | 1.04 | 0.9998 |
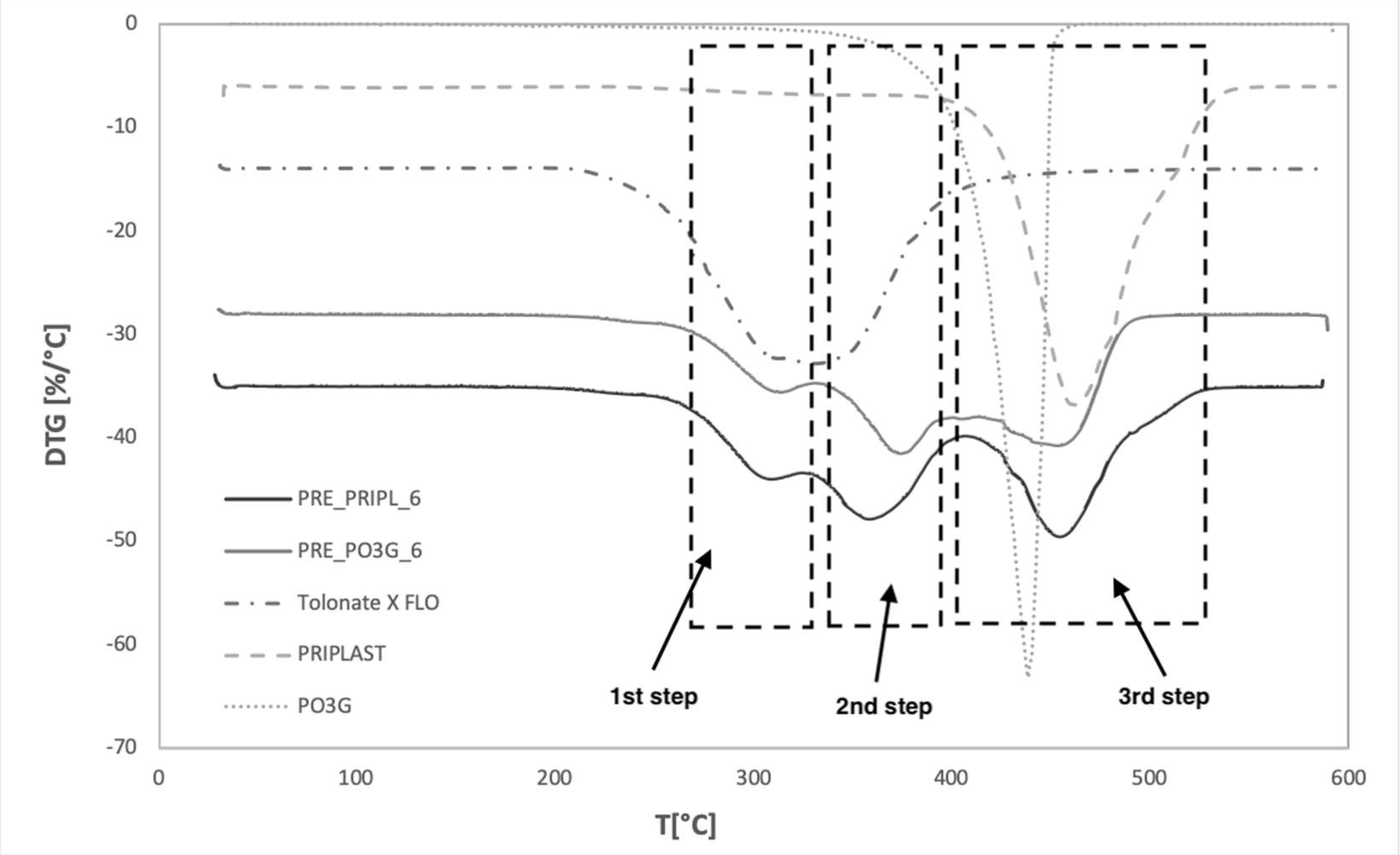
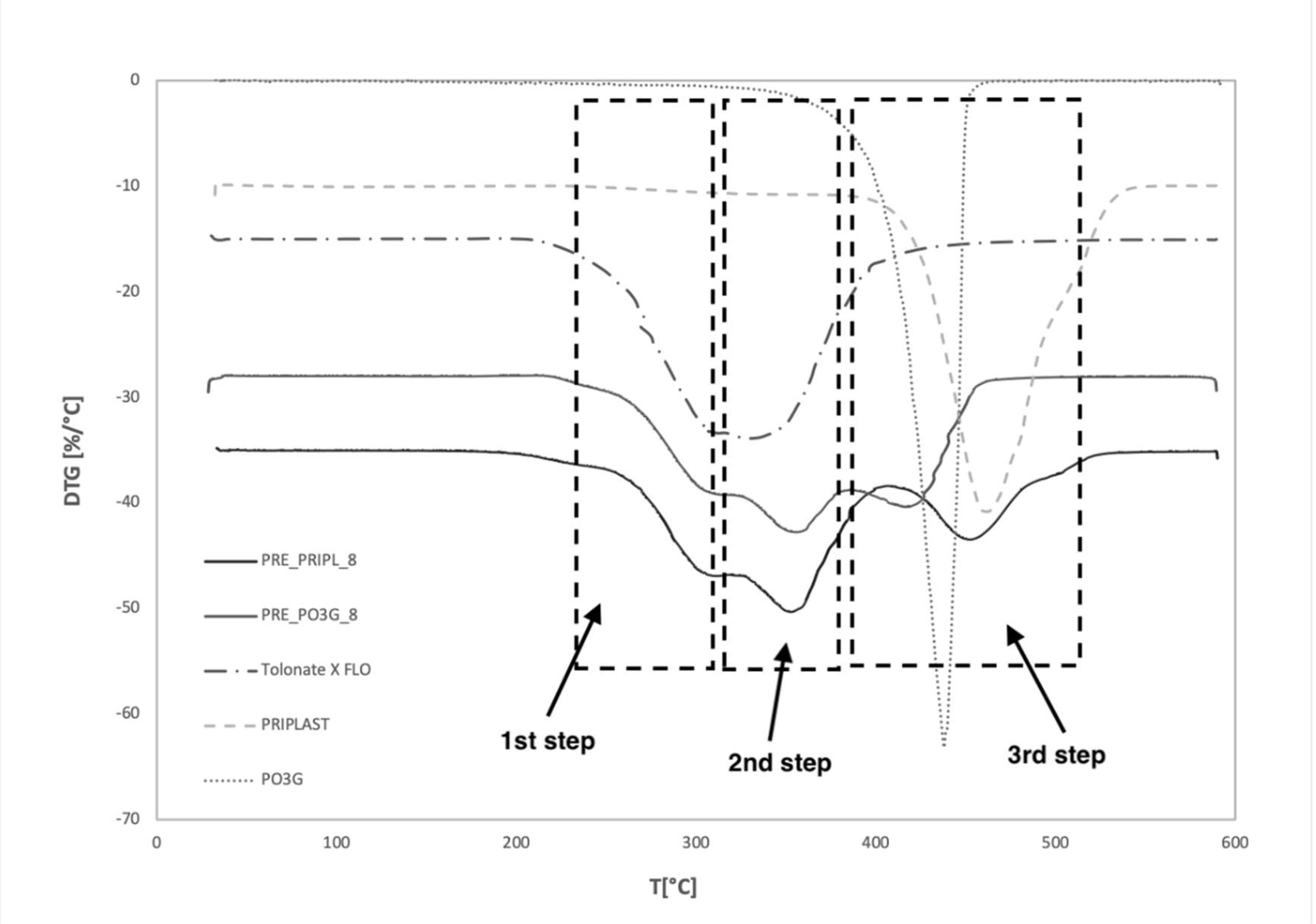
| Sample Code | T5 [°C] | T10 [°C] | T50 [°C] | T90 [°C] | mT550 [°C] [%] |
|---|---|---|---|---|---|
| PO3G | 353.9 | 387.9 | 429.9 | 441.9 | 1.1 |
| PRIPL | 376.7 | 424.1 | 464.8 | 502.7 | 1.3 |
| FLO | 261.5 | 274.2 | 324.7 | 374.5 | 0.45 |
| PRE_PRIPL_6 | 280.0 | 297.8 | 372.3 | 475.1 | 1.1 |
| PRE_PO3G_6 | 286.4 | 303.9 | 388.3 | 459.4 | 0.4 |
| PRE_PRIPL_8 | 266.9 | 286.2 | 352.4 | 463.1 | 1.24 |
| PRE_PO3G_8 | 277.9 | 292.2 | 358.4 | 426.9 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niesiobędzka, J.; Głowińska, E.; Datta, J. Eco-Friendly Ether and Ester-Urethane Prepolymer: Structure, Processing and Properties. Int. J. Mol. Sci. 2021, 22, 12207. https://doi.org/10.3390/ijms222212207
Niesiobędzka J, Głowińska E, Datta J. Eco-Friendly Ether and Ester-Urethane Prepolymer: Structure, Processing and Properties. International Journal of Molecular Sciences. 2021; 22(22):12207. https://doi.org/10.3390/ijms222212207
Chicago/Turabian StyleNiesiobędzka, Joanna, Ewa Głowińska, and Janusz Datta. 2021. "Eco-Friendly Ether and Ester-Urethane Prepolymer: Structure, Processing and Properties" International Journal of Molecular Sciences 22, no. 22: 12207. https://doi.org/10.3390/ijms222212207
APA StyleNiesiobędzka, J., Głowińska, E., & Datta, J. (2021). Eco-Friendly Ether and Ester-Urethane Prepolymer: Structure, Processing and Properties. International Journal of Molecular Sciences, 22(22), 12207. https://doi.org/10.3390/ijms222212207






