Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience
Abstract
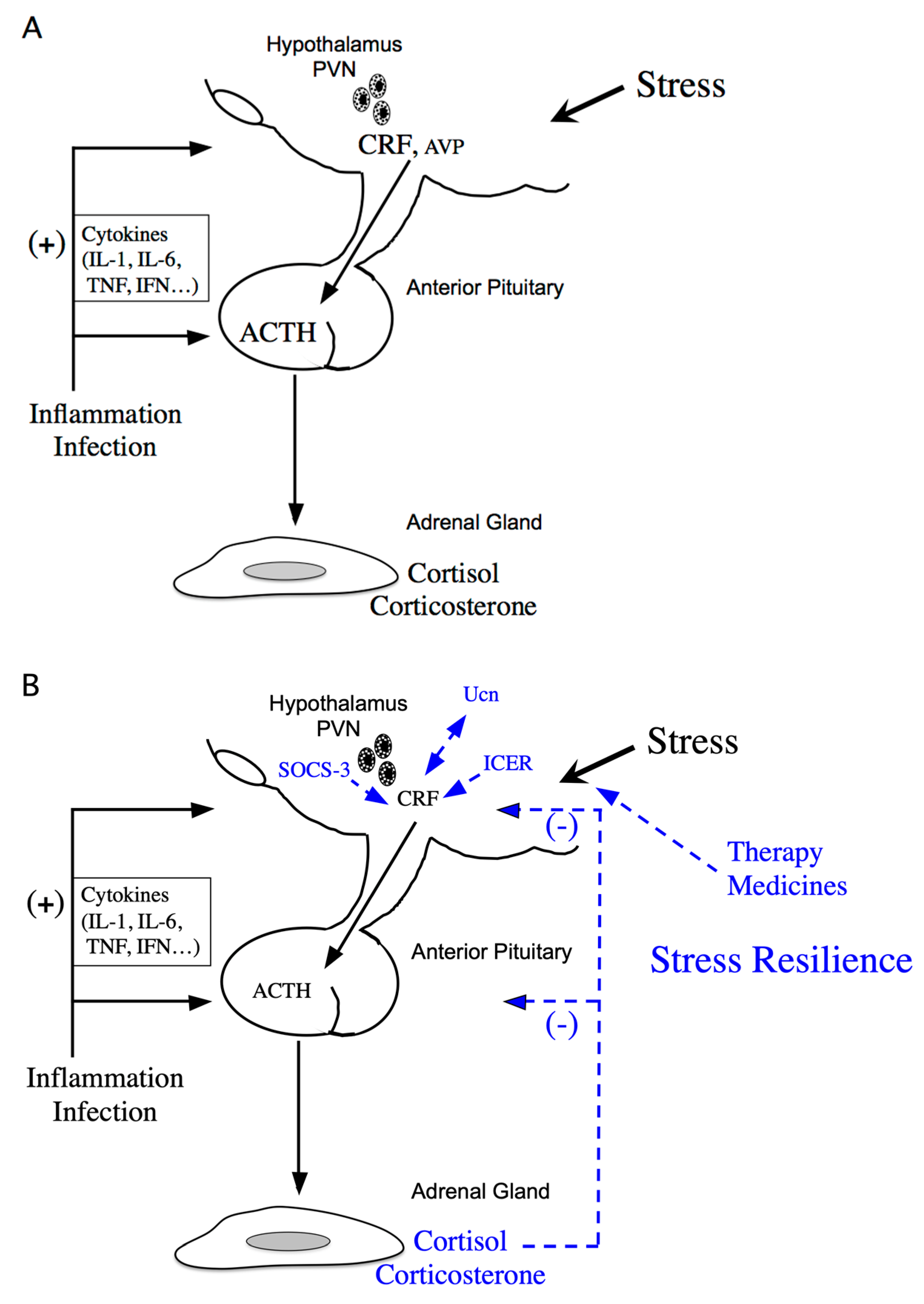
:1. Introduction
2. Molecular Mechanisms of CRF Regulation in the Hypothalamus
3. Roles of the CRF Peptide Family and Stress-Related Peptides and Their Receptors under Stress
4. CRF Dysregulation and Therapy
5. Negative CRF Feedback Mechanisms in the Hypothalamus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef]
- Leon, M.A.G.; Pérez-Mármol, J.M.; Gonzalez-Pérez, R.; García-Ríos, M.D.C.; Peralta-Ramírez, M.I. Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol. Behav. 2019, 202, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef]
- Mukai, Y.; Nagayama, A.; Itoi, K.; Yamanaka, A. Identification of substances which regulate activity of corticotropin-releasing factor-producing neurons in the paraventricular nucleus of the hypothalamus. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Herman, J.P.; Tasker, J.; Ziegler, D.R.; Cullinan, W.E. Local circuit regulation of paraventricular nucleus stress integration: Glutamate–GABA connections. Pharmacol. Biochem. Behav. 2002, 71, 457–468. [Google Scholar] [CrossRef]
- Tasker, J.G.; Boudaba, C.; Schrader, L.A. Local glutamatergic and GABAergic synaptic circuits and metabotropic glutamate receptors in the hypothalamic paraventricular and supraoptic nuclei. Adv. Exp. Med. Biol. 1998, 449, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Linton, E.A.; Lowry, P.J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nat. Cell Biol. 1982, 299, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Mouri, T.; Itoi, K.; Takahashi, K.; Suda, T.; Murakami, O.; Yoshinaga, K.; Andoh, N.; Ohtani, H.; Masuda, T.; Sasano, N. Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology 1993, 57, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Whitnall, M.H. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 1993, 40, 573–629. [Google Scholar] [CrossRef]
- Muglia, L.J.; Jenkins, N.A.; Gilbert, D.J.; Copeland, N.G.; Majzoub, A.J. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J. Clin. Investig. 1994, 93, 2066–2072. [Google Scholar] [CrossRef]
- Muglia, L.J.; Jacobson, L.; Dikkes, P.; Majzoub, J.A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nat. Cell Biol. 1995, 373, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Muglia, L.J.; Jacobson, L.; Weninger, S.C.; Luedke, C.E.; Bae, D.S.; Jeong, K.H.; Majzoub, J.A. Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. J. Clin. Investig. 1997, 99, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Kageyama, K.; Nigawara, T.; Kasagi, Y.; Suda, T. Effects of corticotropin-releasing hormone (CRH) on the synthesis and secretion of proopiomelanocortin-related peptides in the anterior pituitary: A study using CRH-deficient mice. Neurosci. Lett. 2004, 367, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Muglia, L.J.; Jacobson, L.; Luedke, C.; Vogt, S.K.; Schaefer, M.L.; Dikkes, P.; Fukuda, S.; Sakai, Y.; Suda, T.; Majzoub, J.A. Corticotropin-releasing hormone links pituitary adrenocorticotropin gene expression and release during adrenal insufficiency. J. Clin. Investig. 2000, 105, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Kagaya, S.; Takayasu, S.; Hanada, K.; Iwasaki, Y.; Suda, T. Cytokines induce NF-ĸB, Nurr1 and corticotropin-releasing factor gene transcription in hypothalamic 4B cells. Neuroimmunomodulation 2010, 17, 305–313. [Google Scholar] [CrossRef]
- Takaki, A.; Huang, Q.H.; Somogyvári-Vigh, A.; Arimura, A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation 1994, 1, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Kusnecov, A.W.; Shurin, M.R.; DePaoli, M.; Rabin, B.S. Exposure to physical and psychological stressors elevates plasma interleukin 6: Relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 1993, 133, 2523–2530. [Google Scholar] [CrossRef]
- Navarra, P.; Tsagarakis, S.; Faria, M.S.; Rees, L.H.; Besser, G.M.; Grossman, A.B. Interleukins-1 and -6 stimulate the release of corticotropin-releasing Hormone-41 from rat hypothalamus in vitro via the eicosanoid cyclooxygenase pathway. Endocrinology 1991, 128, 37–44. [Google Scholar] [CrossRef]
- Vallieres, L.; Rivest, S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia1. Endocrinology 1999, 140, 3890–3903. [Google Scholar] [CrossRef]
- Karalis, K.; Muglia, L.J.; Bae, D.; Hilderbrand, H.; Majzoub, J.A. CRH and the immune system. J. Neuroimmunol. 1997, 72, 131–136. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef] [Green Version]
- Ono, D.; Mukai, Y.; Hung, C.J.; Chowdhury, S.; Sugiyama, T.; Yamanaka, A. The mammalian circadian pacemaker regulates wakefulness via CRFneurons in the paraventricular nucleus of the hypothalamus. Sci. Adv. 2020, 6, eabd0384. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian mechanisms in medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yin, Y.; Zhang, W. Ghrelin restores the disruption of the circadian clock in steatotic liver. Int. J. Mol. Sci. 2018, 19, 3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, K.; Hanada, K.; Iwasaki, Y.; Sakihara, S.; Nigawara, T.; Kasckow, J.; Suda, T. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. J. Endocrinol. 2007, 195, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Yamagata, S.; Akimoto, K.; Sugiyama, A.; Murasawa, S.; Suda, T. Action of glucagon-like peptide 1 and glucose levels on corticotropin-releasing factor and vasopressin gene expression in rat hypothalamic 4B cells. Mol. Cell. Endocrinol. 2012, 362, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Ueta, Y.; Serino, R.; Kabashima, N.; Shibuya, I.; Yamashita, H. PACAP type I receptor gene expression in the paraventricular and supraoptic nuclei of rats. NeuroReport 1996, 8, 67–70. [Google Scholar] [CrossRef]
- Shioda, S.; Shuto, Y.; Somogyvári-Vigh, A.; Legradi, G.; Onda, H.; Coy, D.H.; Nakajo, S.; Arimura, A. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci. Res. 1997, 28, 345–354. [Google Scholar] [CrossRef]
- Grinevich, V.; Fournier, A.; Pelletier, G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997, 773, 190–196. [Google Scholar] [CrossRef]
- Mozid, A.M.; Tringali, G.; Forsling, M.L.; Hendricks, M.S.; Ajodha, S.; Edwards, R.; Navarra, P.; Grossman, A.B.; Korbonits, M. Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm. Metab. Res. 2003, 35, 455–459. [Google Scholar] [CrossRef]
- Kageyama, K.; Kumata, Y.; Akimoto, K.; Takayasu, S.; Tamasawa, N.; Suda, T. Ghrelin stimulates corticotropin-releasing factor and vasopressin gene expression in rat hypothalamic 4B cells. Stress 2011, 14, 520–529. [Google Scholar] [CrossRef]
- Kageyama, K.; Akimoto, K.; Yamagata, S.; Sugiyama, A.; Murasawa, S.; Watanuki, Y.; Tamasawa, N.; Suda, T. Dexamethasone stimulates the expression of ghrelin and its receptor in rat hypothalamic 4B cells. Regul. Pept. 2012, 174, 12–17. [Google Scholar] [CrossRef]
- Ishigame, N.; Kageyama, K.; Takayasu, S.; Furumai, K.; Nakada, Y.; Daimon, M. Regulation of the expression of corticotropin-releasing factor gene by pyroglutamylated RFamide peptide in rat hypothalamic 4B cells. Endocr. J. 2016, 63, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchimura, T.; Hara, S.; Yazawa, T.; Kamei, Y.; Kitano, T. Involvement of heat shock proteins on the transcriptional regulation of corticotropin-releasing hormone in medaka. Front. Endocrinol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Denver, R.J. Regulation of vertebrate corticotropin-releasing factor genes. Gen. Comp. Endocrinol. 2007, 153, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Tamasawa, N.; Suda, T. Signal transduction in the hypothalamic corticotropin-releasing factor system and its clinical implications. Stress 2011, 14, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Seasholtz, A.F.; Thompson, R.C.; Douglass, J.O. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol. Endocrinol. 1988, 2, 1311–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spengler, D.; Rupprecht, R.; Van, L.P.; Holsboer, F. Identification and characterization of a 3’,5’-cyclic adenosine monophosphate-responsive element in the human corticotropin-releasing hormone gene promoter. Mol. Endocrinol. 1992, 6, 1931–1941. [Google Scholar] [CrossRef] [Green Version]
- Yamamori, E.; Asai, M.; Yoshida, M.; Takano, K.; Itoi, K.; Oiso, Y.; Iwasaki, Y. Calcium/calmodulin kinase IV pathway is involved in the transcriptional regulation of the corticotropin-releasing hormone gene promoter in neuronal cells. J. Mol. Endocrinol. 2004, 33, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Hanada, K.; Takayasu, S.; Iwasaki, Y.; Sakihara, S.; Nigawara, T.; Suda, T. Involvement of regulatory elements on corticotropin-releasing factor gene promoter in hypothalamic 4B cells. J. Endocrinol. Investig. 2008, 31, 1079–1085. [Google Scholar] [CrossRef]
- Kageyama, K.; Itoi, K.; Iwasaki, Y.; Niioka, K.; Watanuki, Y.; Yamagata, S.; Nakada, Y.; Das, G.; Suda, T.; Daimon, M. Stimulation of corticotropin-releasing factor gene expression by FosB in rat hypothalamic 4B cells. Peptides 2013, 51, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Uchida, K.; Kageyama, K.; Iwasaki, Y.; Suda, T.; Itoi, K. Glucocorticoid dependency of surgical stress-induced FosB/DeltaFosB expression in the paraventricular and supraoptic nuclei of the rathypothalamus. J. Neuroendocrinol. 2009, 21, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Yajima, F.; Tomori, N.; Demura, H.; Shizume, K. In vitro study of immunoreactive corticotropin-releasing factor release from the rat hypothalamus. Life Sci. 1985, 37, 1499–1505. [Google Scholar] [CrossRef]
- Agarwal, A.; Halvorson, L.M.; Legradi, G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Mol. Brain Res. 2005, 138, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.H.; Nicholson, R.C.; King, B.; Chan, E.C.; Fitter, J.T.; Smith, R. glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J. Clin. Endocrinol. Metab. 2000, 85, 1937–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochedalski, T.; Subburaju, S.; Wynn, P.C.; Aguilera, G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J. Neuroendocr. 2007, 19, 189–197. [Google Scholar] [CrossRef]
- Ogura, E.; Kageyama, K.; Hanada, K.; Kasckow, J.; Suda, T. Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type beta in hypothalamic 4B cells. Peptides 2008, 29, 456–464. [Google Scholar] [CrossRef]
- Ma, X.M.; Aguilera, G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology 1999, 140, 5642–5650. [Google Scholar] [CrossRef]
- Foulkes, N.S.; Borrelli, E.; Sassone-Corsi, P. CREM gene: Use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell 1991, 64, 739–749. [Google Scholar] [CrossRef]
- Molina, C.A.; Foulkes, N.S.; Lalli, E.; Sassone-Corsi, P. Inducibility and negative autoregulation of CREM: An alternative promoter directs the expression of ICER, an early response repressor. Cell 1993, 75, 875–886. [Google Scholar] [CrossRef]
- Lalli, E.; Sassone-Corsi, P. Signal transduction and gene regulation: The nuclear response to cAMP. J. Biol. Chem. 1994, 269, 17359–17362. [Google Scholar] [CrossRef]
- Krebs, D.; Hilton, D. SOCS: Physiological suppressors of cytokine signaling. J. Cell Sci. 2000, 113, 2813–2819. [Google Scholar] [CrossRef]
- Auernhammer, C.J.; Bousquet, C.; Melmed, S. Autoregulation of pituitary corticotroph SOCS-3 expression: Characterization of the murine SOCS-3 promoter. Proc. Natl. Acad. Sci. USA 1999, 96, 6964–6969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, P.A.; Waxman, D. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 1999, 274, 35553–35561. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Weissenbach, M.; Haan, S.; Heinrich, P.C.; Schaper, F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 2000, 275, 12848–12856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, K.; Hanada, K.; Iwasaki, Y.; Suda, T. Regulation and role of suppressor of cytokine signaling-3 in hypothalamic 4B cells. J. Endocrinol. 2009, 201, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, J.; Donaldson, C.J.; Bittencourt, J.; Perrin, M.H.; Lewis, A.K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nat. Cell Biol. 1995, 378, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Merlo-Pich, E.; Chan, R.K.W.; Basso, A.M.; Rivier, J.; Vale, W.; Koob, G.F. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 1996, 273, 1561–1564. [Google Scholar] [CrossRef]
- Parkes, D.G.; Vaughan, J.; Rivier, J.; Vale, W.; May, C.N. Cardiac inotropic actions of urocortin in conscious sheep. Am. J. Physiol. Content 1997, 272, H2115–H2122. [Google Scholar] [CrossRef]
- Sashinami, H.; Kageyama, K.; Suda, T.; Nakane, A. Urocortin 2 suppresses host resistance to Listeria monocytogenes infection via up-regulation of interleukin-10. Endocrinology 2005, 146, 5003–5011. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Kimura, R.; Suga, S.; Ogawa, Y.; Suda, T.; Wakui, M. Modulation of Ca2+ influx by corticotropin-releasing factor (CRF) family of peptides via CRF receptors in rat pancreatic beta-cells. Peptides 2006, 27, 1814–1819. [Google Scholar] [CrossRef]
- Kavalakatt, S.; Khadir, A.; Madhu, D.; Koistinen, H.A.; Al-Mulla, F.; Tuomilehto, J.; Abubaker, J.; Tiss, A. Urocortin 3 overexpression reduces ER stress and heat shock response in 3T3-L1 adipocytes. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Chang, C.P.; Pearse, R.; O’Connell, S.; Rosenfeld, M.G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 1993, 11, 1187–1195. [Google Scholar] [CrossRef]
- Chen, R.; Lewis, K.A.; Perrin, M.H.; Vale, W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 8967–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vita, N.; Laurent, P.; Lefort, S.; Chalon, P.; Lelias, J.-M.; Kaghad, M.; Le Fur, G.; Caput, D.; Ferrara, P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993, 335, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lovenberg, T.W.; Liaw, C.W.; Grigoriadis, D.E.; Clevenger, W.; Chalmers, D.T.; De Souza, E.B.; Oltersdorf, T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA 1995, 92, 836–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, M.; Donaldson, C.; Chen, R.; Blount, A.; Berggren, T.; Bilezikjian, L.; Sawchenko, P.; Vale, W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. USA 1995, 92, 2969–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenzel, P.; Kesterson, R.; Yeung, W.; Cone, R.D.; Stenzel-Poore, M.P.; Rittenberg, M.B. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 1995, 9, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, T.; Kageyama, K.; Sakihara, S.; Nigawara, T. Physiological roles of urocortins, human homologues of fish urotensin I, and their receptors. Peptides 2004, 25, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Hsueh, A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001, 7, 605–611. [Google Scholar] [CrossRef]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A.; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, K.; Li, C.; Vale, W.W. Corticotropin-releasing factor receptor type 2 messenger ribonucleic acid in rat pituitary: Localization and regulation by immune challenge, restraint stress, and glucocorticoids. Endocrinology 2003, 144, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Lovenberg, T.W.; Chalmers, D.T.; Liu, C.; De Souza, E.B. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 1995, 136, 4139–4142. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.; Stein, D.J.; Gallas-Lopes, M.; Landau, L.; De Almeida, R.M.M. Corticotropin-releasing factor receptor signaling and modulation: Implications for stress response and resilience. Trends Psychiatry Psychother. 2020, 42, 195–206. [Google Scholar] [CrossRef]
- Bale, T.L.; Contarino, A.; Smith, G.W.; Chan, R.; Gold, L.H.; Sawchenko, P.E.; Koob, G.F.; Vale, W.W.; Lee, K.-F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000, 24, 410–414. [Google Scholar] [CrossRef]
- Anthony, T.E.; Dee, N.; Bernard, A.; Lerchner, W.; Heintz, N.; Anderson, D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 2014, 156, 522–536. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Kageyama, K.; Kasagi, Y.; Iwasaki, Y.; Nigawara, T.; Sakihara, S.; Suda, T. Differential regulation of corticotropin-releasing factor receptor type 1 (CRF1 receptor) mRNA via protein kinase A and mitogen-activated protein kinase pathways in rat anterior pituitary cells. Mol. Cell. Endocrinol. 2005, 243, 74–79. [Google Scholar] [CrossRef]
- Kageyama, K.; Hanada, K.; Moriyama, T.; Nigawara, T.; Sakihara, S.; Suda, T. G protein-coupled receptor kinase 2 involvement in desensitization of corticotropin-releasing factor (CRF) receptor type 1 by CRF in murine corticotrophs. Endocrinology 2006, 147, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, F.; Feelders, R.; Van Der Pas, R.; Kros, J.M.; Dogan, F.; Van Koetsveld, P.M.; Van Der Lelij, A.-J.; Neggers, S.J.C.M.M.; Minuto, F.; De Herder, W.; et al. β-Arrestin 1 and 2 and G protein-coupled receptor kinase 2 expression in pituitary adenomas: Role in the regulation of response to somatostatin analogue treatment in patients with acromegaly. Endocrinology 2013, 154, 4715–4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, N.J.; Seasholtz, A.F. CRH-BP: The regulation and function of a phylogenetically conservedbinding protein. Front. Biosci. 2006, 11, 1878–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehuda, R.; Golier, J.A.; Halligan, S.; Meaney, M.; Bierer, L.M. The ACTH response to dexamethasone in PTSD. Am. J. Psychiatry 2004, 161, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.K.; Walker, H.E.; Valentino, R.J.; Bhatnagar, S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: Role of corticotropin-releasing factor. Endocrinology 2010, 151, 1795–1805. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, D.; Sokolowski, M.; Wasserman, J. Genetics of HPA-axis, depression and suicidality. Eur. Psychiatry 2010, 25, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological treatment of anxiety disorders: The role of the HPA Axis. Front. Psychiatry 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cui, S.Y.; Cui, X.Y.; Liu, Y.T.; Hu, X.; Zhao, H.L.; Qin, Y.; Kurban, N.; Zhang, Y.H. Anti-stress effects of combined block of glucocorticoid and mineralocorticoid receptors in the paraventricular nucleus of the hypothalamus. Br. J. Pharmacol. 2021, 178, 3696–3707. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Takayanagi, Y.; Yoshida, M.; Onaka, T. Gentle stroking stimuli induce affiliative responsiveness to humans in male rats. Sci. Rep. 2020, 10, 9135. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Diaz, H.M.; Kolinske, J.S.; Gates, L.H.; Seasholtz, A.F. Negative glucorticoid regulation of cyclic adenosine 3’, 5’-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol. Endocrinol. 1996, 10, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkoski, S.P.; Dorin, R.I. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol. Endocrinol. 1999, 13, 1629–1644. [Google Scholar] [CrossRef]
- King, B.R.; Smith, R.; Nicholson, R.C. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol. Cell. Endocrinol. 2002, 194, 19–28. [Google Scholar] [CrossRef]
- Jacobson, L.; Sharp, F.R.; Dallman, M.F. Induction of fos-like immunoreactivity in hypothalamic corticotropin-releasing factor neurons after adrenalectomy in the rat. Endocrinology 1990, 126, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Légrádi, G.; Holzer, D.; Kapcala, L.P.; Lechan, R.M. Glucocorticoids inhibit stress-induced phosphorylation of CREB in corticotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology 1997, 66, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, N.; Tsawdaroglou, N. The c-fos serum response element (SRE) confers negative response to glucocorticoids. Oncogene 1994, 9, 2327–2334. [Google Scholar]
- Ruiz-Conca, M.; Gardela, J.; Martinez, A.C.; Wright, D.; López-Bejar, M.; Rodríguez-Martínez, H.; Álvarez-Rodríguez, M.; Ruiz-Conca, M. Natural mating differentially triggers expression of glucocorticoid receptor (NR3C1)-related genes in the preovulatory porcine female reproductive tract. Int. J. Mol. Sci. 2020, 21, 4437. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Ridout, K.K.; Parade, S.H. Childhood adversity and epigenetic regulation of glucocorticoidsignaling genes: Associations in children and adults. Dev. Psychopathol. 2016, 28, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Vitellius, G.; Lombes, M. Genetics in endocrinology: Glucocorticoid resistance syndrome. Eur. J. Endocrinol. 2020, 182, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.D.; Ozsan, I.; Ospina, S.R.; Gulick, D.; Blair, L.J. Hsp90 Heterocomplexes regulate steroid hormone receptors: From stress response to psychiatric disease. Int. J. Mol. Sci. 2018, 20, 79. [Google Scholar] [CrossRef] [Green Version]
- Merkulov, V.M.; Merkulova, T.I.; Bondar, N.P. Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry 2017, 82, 351–365. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, K.; Iwasaki, Y.; Watanuki, Y.; Niioka, K.; Daimon, M. Differential effects of Fkbp4 and Fkbp5 on regulation of the Proopiomelanocortin Gene in murine AtT-20 corticotroph cells. Int. J. Mol. Sci. 2021, 22, 5724. [Google Scholar] [CrossRef] [PubMed]
- Häusl, A.S.; Brix, L.M.; Hartmann, J.; Pöhlmann, M.L.; Lopez, J.-P.; Menegaz, D.; Brivio, E.; Engelhardt, C.; Roeh, S.; Bajaj, T.; et al. The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol. Psychiatry 2021, 26, 3060–3076. [Google Scholar] [CrossRef] [PubMed]
- Itoi, K.; Motoike, I.; Liu, Y.; Clokie, S.; Iwasaki, Y.; Uchida, K.; Sato, T.; Aguilera, G. Genome-wide analysis of glucocorticoid-responsive transcripts in the hypothalamic paraventricular region of male rats. Endocrinology 2019, 160, 38–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. https://doi.org/10.3390/ijms222212242
Kageyama K, Iwasaki Y, Daimon M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. International Journal of Molecular Sciences. 2021; 22(22):12242. https://doi.org/10.3390/ijms222212242
Chicago/Turabian StyleKageyama, Kazunori, Yasumasa Iwasaki, and Makoto Daimon. 2021. "Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience" International Journal of Molecular Sciences 22, no. 22: 12242. https://doi.org/10.3390/ijms222212242
APA StyleKageyama, K., Iwasaki, Y., & Daimon, M. (2021). Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. International Journal of Molecular Sciences, 22(22), 12242. https://doi.org/10.3390/ijms222212242





