Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth
Abstract
:1. Introduction
2. Results
2.1. Negative Effect of MMP14Sf−/− Fibroblast Matrix on Melanoma Proliferation and Migration
2.2. Altered Composition of MMP14Sf−/− Fibroblast ECM
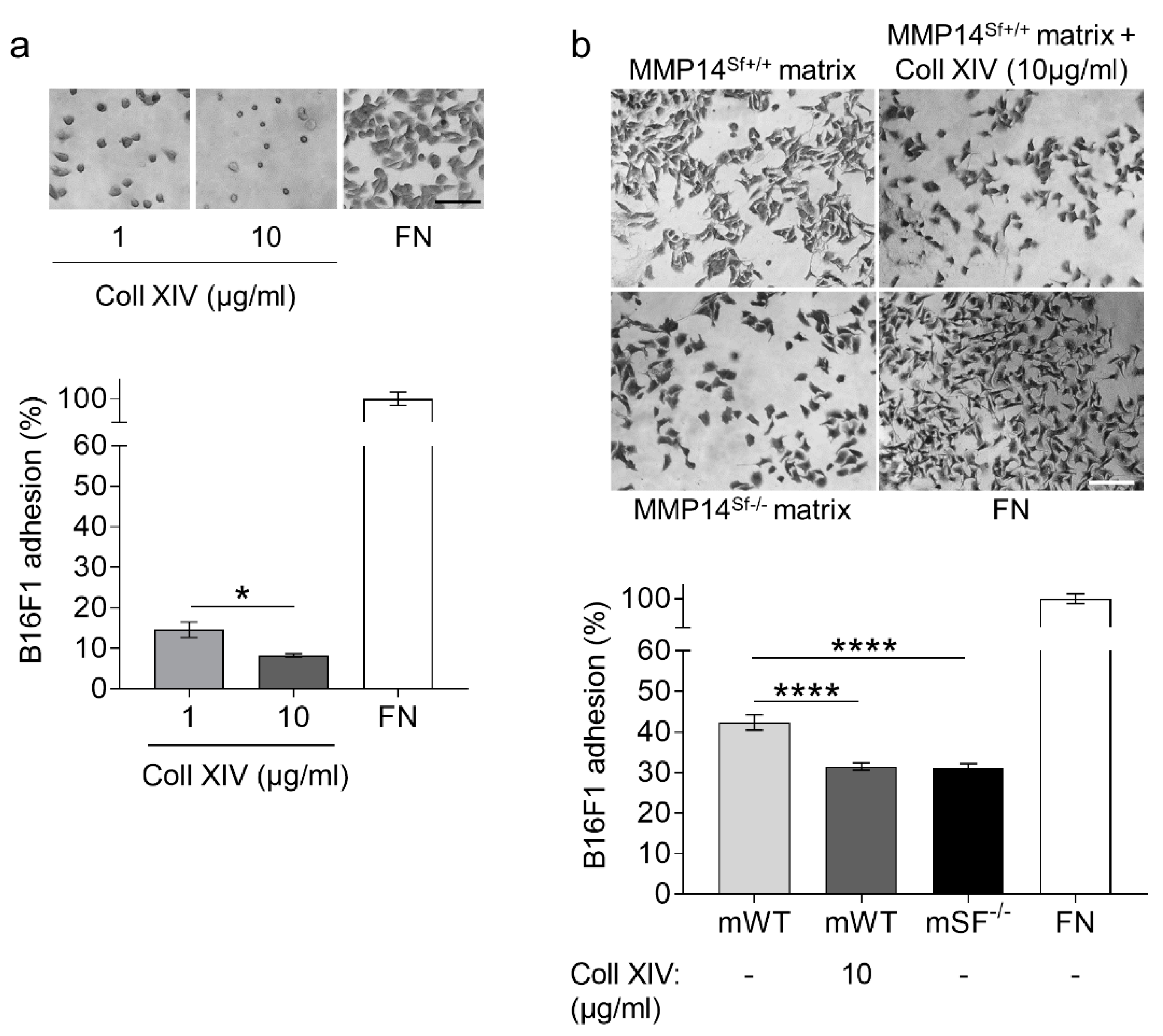
2.3. Collagen XIV Inhibits Pro-Invasive Activities
2.4. Collagen XIV Is Accumulated in Melanoma Grown in Fibrotic Skin
2.5. Collagen XIV Expression in Human Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Migration: Colony Outgrow Assay
4.3. BrdU Incorporation Assay
4.4. In Vitro Cleavage Assay
4.5. Immunoblot
4.6. Preparation of Samples for Proteome Analysis
4.7. Tumor Grafting Experiments
4.8. Immunofluorescence Staining
4.9. Immunohistochemical Staining
4.10. RNA Isolation, RT-PCR, and Real-Time PCR
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stetler-Stevenson, W.G.; Liotta, L.A.; Kleiner, D.E., Jr. Extracellular matrix 6: Role of matrix metalloproteinases in tumor invasion and metastasis. Faseb J. 1993, 7, 1434–1441. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Takino, T.; Kinoshita, T.; Imai, K.; Okada, Y.; Stetler Stevenson, W.G.; Seiki, M. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett. 1996, 385, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Hillebrand, L.E.; Wickberg, S.M.; Gomez-Auli, A.; Follo, M.; Maurer, J.; Busch, H.; Boerries, M.; Reinheckel, T. MMP14 empowers tumor-initiating breast cancer cells under hypoxic nutrient-depleted conditions. FASEB J. 2019, 33, 4124–4140. [Google Scholar] [CrossRef]
- Kasurinen, A.; Gramolelli, S.; Hagström, J.; Laitinen, A.; Kokkola, A.; Miki, Y.; Lehti, K.; Yashiro, M.; Ojala, P.M.; Böckelman, C.; et al. High tissue MMP14 expression predicts worse survival in gastric cancer, particularly with a low PROX1. Cancer Med. 2019, 8, 6995–7005. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.G.; Davies, G.; Martin, T.A.; Parr, C.; Watkins, G.; Mason, M.D.; Mansel, R.E. Expression of membrane type-1 matrix metalloproteinase, MT1-MMP in human breast cancer and its impact on invasiveness of breast cancer cells. Int. J. Mol. Med. 2006, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, A.; Soria-Valles, C.; Osorio, F.G.; Gutierrez-Abril, J.; Garabaya, C.; Aguirre, A.; Fueyo, A.; Fernandez-Garcia, M.S.; Puente, X.S.; Lopez-Otin, C. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 2015, 34, 1875–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmbeck, K.; Bianco, P.; Caterina, J.; Yamada, S.; Kromer, M.; Kuznetsov, S.A.; Mankani, M.; Robey, P.G.; Poole, A.R.; Pidoux, I.; et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigrino, P.; Ayachi, O.; Schild, A.; Kaltenberg, J.; Zamek, J.; Nischt, R.; Koch, M.; Mauch, C. Loss of epidermal MMP-14 expression interferes with angiogenesis but not with re-epithelialization. Eur. J. Cell. Biol. 2012, 91, 748–756. [Google Scholar] [CrossRef]
- Klose, A.; Zigrino, P.; Mauch, C. Monocyte/macrophage MMP-14 modulates cell infiltration and T-cell attraction in contact dermatitis but not in murine wound healing. Am. J. Pathol. 2013, 182, 755–764. [Google Scholar] [CrossRef]
- Taylor, S.H.; Yeung, C.Y.; Kalson, N.S.; Lu, Y.; Zigrino, P.; Starborg, T.; Warwood, S.; Holmes, D.F.; Canty-Laird, E.G.; Mauch, C.; et al. Matrix metalloproteinase 14 is required for fibrous tissue expansion. Elife 2015, 4, e09345. [Google Scholar] [CrossRef] [PubMed]
- Zigrino, P.; Brinckmann, J.; Niehoff, A.; Lu, Y.; Giebeler, N.; Eckes, B.; Kadler, K.E.; Mauch, C. Fibroblast-Derived MMP-14 Regulates Collagen Homeostasis in Adult Skin. J. Invest. Dermatol. 2016, 136, 1575–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pach, E.; Brinckmann, J.; Rübsam, M.; Kümper, M.; Mauch, C.; Zigrino, P. Fibroblast MMP14-Dependent Collagen Processing Is Necessary for Melanoma Growth. Cancers 2021, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Beachley, V.Z.; Wolf, M.T.; Sadtler, K.; Manda, S.S.; Jacobs, H.; Blatchley, M.R.; Bader, J.S.; Pandey, A.; Pardoll, D.; Elisseeff, J.H. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods 2015, 12, 1197–1204. [Google Scholar] [CrossRef]
- Pickering, J.G. Regulation of vascular cell behavior by collagen: Form is function. Circ. Res. 2001, 88, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y.; Zeisberg, M.; Sugimoto, H.; Lively, J.C.; Maeshima, Y.; Yang, C.; Hynes, R.O.; Werb, Z.; Sudhakar, A.; Kalluri, R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 2003, 3, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Ramchandran, R.; Dhanabal, M.; Volk, R.; Waterman, M.J.; Segal, M.; Lu, H.; Knebelmann, B.; Sukhatme, V.P. Antiangiogenic activity of restin, NC10 domain of human collagen XV: Comparison to endostatin. Biochem. Biophys. Res. Commun. 1999, 255, 735–739. [Google Scholar] [CrossRef]
- Hong, J.; Chu, M.; Qian, L.; Wang, J.; Guo, Y.; Xu, D. Fibrillar Type I Collagen Enhances the Differentiation and Proliferation of Myofibroblasts by Lowering α2β1 Integrin Expression in Cardiac Fibrosis. Biomed. Res. Int. 2017, 2017, 1790808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharidis, G.; Connelly, J.T. Minor collagens of the skin with not so minor functions. J. Anat. 2019, 235, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumour. Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Fields, G.B. The Expanding Role of MT1-MMP in Cancer Progression. Pharmaceuticals 2019, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerecke, D.R.; Meng, X.; Liu, B.; Birk, D.E. Complete primary structure and genomic organization of the mouse Col14a1 gene. Matrix Biol 2004, 22, 595–601. [Google Scholar] [CrossRef]
- Ansorge, H.L.; Meng, X.; Zhang, G.; Veit, G.; Sun, M.; Klement, J.F.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Type XIV Collagen Regulates Fibrillogenesis: Premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 2009, 284, 8427–8438. [Google Scholar] [CrossRef] [Green Version]
- Tzortzaki, E.G.; Tischfield, J.A.; Sahota, A.; Siafakas, N.M.; Gordon, M.K.; Gerecke, D.R. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1073–1080. [Google Scholar] [CrossRef]
- Tzortzaki, E.G.; Koutsopoulos, A.V.; Dambaki, K.I.; Lambiri, I.; Plataki, M.; Gordon, M.K.; Gerecke, D.R.; Siafakas, N.M. Active remodeling in idiopathic interstitial pneumonias: Evaluation of collagen types XII and XIV. J. Histochem. Cytochem. 2006, 54, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Liu, H.; Shi, Y.; Man, Q.; Liu, S. COL14A1 promotes self-renewal of human liver cancer stem cells through activation of ERK signaling. J. Bio-X Res. 2021, 4, 10–17. [Google Scholar] [CrossRef]
- Agarwal, P.; Zwolanek, D.; Keene, D.R.; Schulz, J.N.; Blumbach, K.; Heinegård, D.; Zaucke, F.; Paulsson, M.; Krieg, T.; Koch, M.; et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012, 287, 22549–22559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kempen, L.C.; van Muijen, G.N.; Ruiter, D.J. Stromal responses in human primary melanoma of the skin. Front Biosci. 2005, 10, 2922–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, U.B.; Westphal, J.R.; Zendman, A.J.; Becker, J.C.; Ruiter, D.J.; van Muijen, G.N. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J. Pathol. 2000, 191, 245–256. [Google Scholar] [CrossRef]
- Miskolczi, Z.; Smith, M.P.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene 2018, 37, 3166–3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Tan, Y.; Zhang, H.; Zhang, Y.; Xu, P.; Chen, J.; Poh, Y.C.; Tang, K.; Wang, N.; Huang, B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 2012, 11, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fanous, M.J.; Kilian, K.A.; Popescu, G. Quantitative phase imaging reveals matrix stiffness-dependent growth and migration of cancer cells. Sci. Rep. 2019, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Henriet, P.; Zhong, Z.D.; Brooks, P.C.; Weinberg, K.I.; DeClerck, Y.A. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc. Natl. Acad. Sci. USA 2000, 97, 10026–10031. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadeh, H.; Webster, M.R.; Behera, R.; Jimenez Valencia, A.M.; Wirtz, D.; Weeraratna, A.T.; Shenoy, V.B. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc. Natl. Acad. Sci. USA 2017, 114, E1617–E1626. [Google Scholar] [CrossRef] [Green Version]
- Ju, R.J.; Stehbens, S.J.; Haass, N.K. The Role of Melanoma Cell-Stroma Interaction in Cell Motility, Invasion, and Metastasis. Front. Med. 2018, 5, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedl, P.; Wolf, K. Proteolytic interstitial cell migration: A five-step process. Cancer Metastasis Rev. 2009, 28, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventure, J.; Domingues, M.J.; Larue, L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigment Cell Melanoma Res. 2013, 26, 316–325. [Google Scholar] [CrossRef]
- Berthod, F.; Germain, L.; Guignard, R.; Lethias, C.; Garrone, R.; Damour, O.; van der Rest, M.; Auger, F.A. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J. Invest. Dermatol. 1997, 108, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Ruehl, M.; Erben, U.; Schuppan, D.; Wagner, C.; Zeller, A.; Freise, C.; Al-Hasani, H.; Loesekann, M.; Notter, M.; Wittig, B.M.; et al. The elongated first fibronectin type III domain of collagen XIV is an inducer of quiescence and differentiation in fibroblasts and preadipocytes. J. Biol. Chem. 2005, 280, 38537–38543. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Paetow, C. Collagen XIV Reduces Proliferation of Human CD44-Positive Tumour Cell Lines. Ph.D. Thesis, Freie University, Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Freise, C.; Bobb, V.; Querfeld, U. Collagen XIV and a related recombinant fragment protect human vascular smooth muscle cells from calcium-/phosphate-induced osteochondrocytic transdifferentiation. Exp. Cell Res. 2017, 358, 242–252. [Google Scholar] [CrossRef]
- Tao, G.; Levay, A.K.; Peacock, J.D.; Huk, D.J.; Both, S.N.; Purcell, N.H.; Pinto, J.R.; Galantowicz, M.L.; Koch, M.; Lucchesi, P.A.; et al. Collagen XIV is important for growth and structural integrity of the myocardium. J. Mol. Cell Cardiol. 2012, 53, 626–638. [Google Scholar] [CrossRef] [Green Version]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Riker, A.I.; Enkemann, S.A.; Fodstad, O.; Liu, S.; Ren, S.; Morris, C.; Xi, Y.; Howell, P.; Metge, B.; Samant, R.S.; et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genomics 2008, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Tobys, D.; Kowalski, L.M.; Cziudaj, E.; Müller, S.; Zentis, P.; Pach, E.; Zigrino, P.; Blaeske, T.; Höning, S. Inhibition of clathrin-mediated endocytosis by knockdown of AP-2 leads to alterations in the plasma membrane proteome. Traffic 2021, 22, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Irmak, D.; Fatima, A.; Gutiérrez-Garcia, R.; Rinschen, M.M.; Wagle, P.; Altmüller, J.; Arrigoni, L.; Hummel, B.; Klein, C.; Frese, C.K.; et al. Mechanism suppressing H3K9 trimethylation in pluripotent stem cells and its demise by polyQ-expanded huntingtin mutations. Hum. Mol. Genet. 2018, 27, 4117–4134. [Google Scholar] [CrossRef]
- Chakraborty, A.; Barajas, S.; Lammoglia, G.M.; Reyna, A.J.; Morley, T.S.; Johnson, J.A.; Scherer, P.E.; Rutkowski, J.M. Vascular Endothelial Growth Factor-D (VEGF-D) Overexpression and Lymphatic Expansion in Murine Adipose Tissue Improves Metabolism in Obesity. Am. J. Pathol. 2019, 189, 924–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pach, E.; Kümper, M.; Fromme, J.E.; Zamek, J.; Metzen, F.; Koch, M.; Mauch, C.; Zigrino, P. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. Int. J. Mol. Sci. 2021, 22, 12276. https://doi.org/10.3390/ijms222212276
Pach E, Kümper M, Fromme JE, Zamek J, Metzen F, Koch M, Mauch C, Zigrino P. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. International Journal of Molecular Sciences. 2021; 22(22):12276. https://doi.org/10.3390/ijms222212276
Chicago/Turabian StylePach, Elke, Maike Kümper, Julia E. Fromme, Jan Zamek, Fabian Metzen, Manuel Koch, Cornelia Mauch, and Paola Zigrino. 2021. "Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth" International Journal of Molecular Sciences 22, no. 22: 12276. https://doi.org/10.3390/ijms222212276
APA StylePach, E., Kümper, M., Fromme, J. E., Zamek, J., Metzen, F., Koch, M., Mauch, C., & Zigrino, P. (2021). Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. International Journal of Molecular Sciences, 22(22), 12276. https://doi.org/10.3390/ijms222212276






