Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration
Abstract
:1. Introduction
2. AMD
3. Structure of the RPE
4. Role of the RPE
4.1. Blood-Retinal-Barrier
4.2. Protection from Oxidative Stress
4.3. Transport of Nutrients, Wastes, and Water
4.3.1. Transport from Blood to Photoreceptors
4.3.2. Transport from Subretinal Area to Blood
4.4. Phagocytosis of POS
4.5. Production and Secretion of Growth Factors
4.6. Visual Cycle
4.7. Immune Privilege
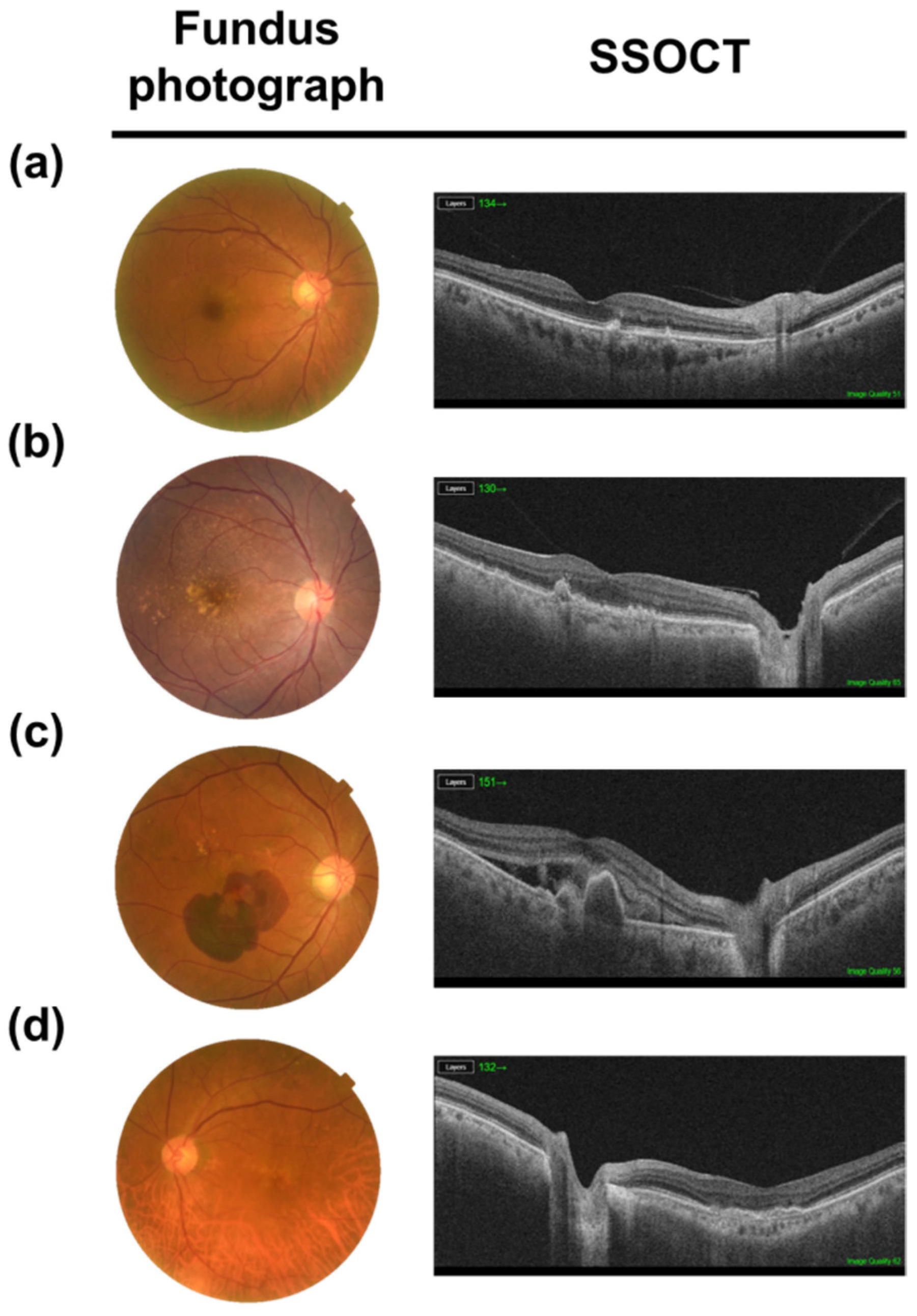
5. AMD Pathogenesis
5.1. Complement Dysregulation in AMD
5.2. Dysfunctional Mitochondria in RPE
5.3. Pathways of RPE Cell Death in AMD
5.4. Autophagy
5.5. α. B Crystallins and RPE Crystallin in AMD
6. Treatment Targeting RPE in AMD
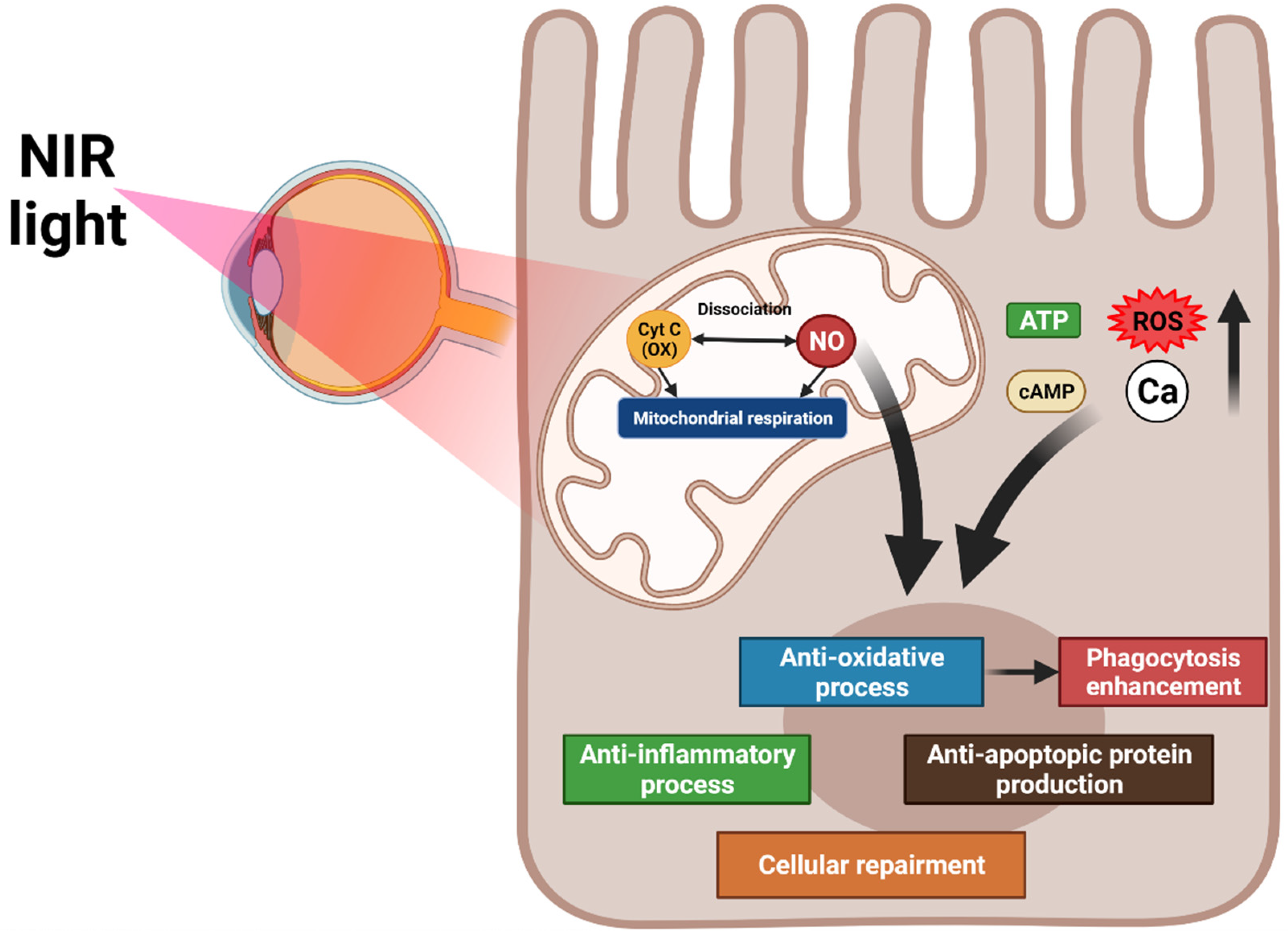
6.1. PBM
6.1.1. Mechanism of PBM
6.1.2. Application of PBM at AMD
6.2. RPE Cell Transplantation
6.2.1. RPE Transplantation for Choroidal Neovascularization in AMD
6.2.2. RPE Transplantation for GA in AMD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Hageman, G.S.; Gehrs, K.; Johnson, L.V.; Anderson, D. Age-related macular degeneration (AMD). N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozing, M.P.; Durhuus, J.A.; Nielsen, M.K.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef] [PubMed]
- Kokotas, H.; Grigoriadou, M.; Petersen, M.B. Age-related macular degeneration: Genetic and clinical findings. Clin. Chem. Lab. Med. 2011, 49, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Bourla, D.H.; Young, T.A. Age-related macular degeneration: A practical approach to a Challenging Disease. J. Am. Geriatr. Soc. 2006, 54, 1130–1135. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: A case-control study in the age-related eye disease study: Age-related eye disease study report number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar] [CrossRef]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report No. 17. Arch. Ophthalmol. 2005, 123, 1484–1498. [Google Scholar]
- Sunness, J.S.; Gonzalez-Baron, J.; Applegate, C.A.; Bressler, N.M.; Tian, Y.; Hawkins, B.; Barron, Y.; Bergman, A. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999, 106, 1768–1779. [Google Scholar] [CrossRef]
- Wong, C.W.; Liao, J.; Cheung, G.C.; Khor, C.C.; Vithana, E.N.; Wang, J.J.; Mitchell, P.; Aung, T.; Wong, T.Y.; Cheng, C.-Y. Lens status influences the association between CFH polymorphisms and age-related macular degeneration: Findings from two population-based studies in Singapore. PloS ONE 2015, 10, e0119570. [Google Scholar] [CrossRef]
- Hogan, M.; Alvarado, J.; Weddell, J. Histology of the human eye. Phila. Saunders 1971. [Google Scholar]
- Marshall, J. The ageing retina: Physiology or pathology. Eye 1987, 1, 282–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streeten, B.W. Development of the human retinal pigment epithelium and the posterior segment. Arch. Ophthalmol. 1969, 81, 383–394. [Google Scholar] [CrossRef]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Baehr, W.; PALCZEWSKI, K.; WU, S.M.; BIRD, A.C. The retinoid cycle and retina disease. Vis. Res. (Oxford) 2003, 43, 2957–2958. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Huisingh, C.; McGwin, G.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.; Tian, J.; Handa, J.T. Similarity of mRNA phenotypes of morphologically normal macular and peripheral retinal pigment epithelial cells in older human eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hollyfield, J. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Del Priore, L.V.; Kuo, Y.-H.; Tezel, T.H. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Feeney-Burns, L.; Hilderbrand, E.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar]
- Caceres, P.S.; Benedicto, I.; Lehmann, G.L.; Rodriguez-Boulan, E.J. Directional fluid transport across organ–blood barriers: Physiology and cell biology. Cold Spring Harb. Perspect. Biol. 2017, 9, a027847. [Google Scholar] [CrossRef] [Green Version]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.S.; Steinberg, R.H. Active transport of ions across frog retinal pigment epithelium. Exp. Eye Res. 1977, 25, 235–248. [Google Scholar] [CrossRef]
- Ved, N.; Hulse, R.P.; Bestall, S.M.; Donaldson, L.F.; Bainbridge, J.W.; Bates, D.O. Vascular endothelial growth factor-A165b ameliorates outer-retinal barrier and vascular dysfunction in the diabetic retina. Clin. Sci. 2017, 131, 1225–1243. [Google Scholar] [CrossRef]
- Desjardins, D.M.; Yates, P.W.; Dahrouj, M.; Liu, Y.; Crosson, C.E.; Ablonczy, Z. Progressive early breakdown of retinal pigment epithelium function in hyperglycemic rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2706–2713. [Google Scholar] [CrossRef] [Green Version]
- Farjood, F.; Vargis, E. Physical disruption of cell–cell contact induces VEGF expression in RPE cells. Mol. Vis. 2017, 23, 431. [Google Scholar] [PubMed]
- Rózanowska, M.; Jarvis-Evans, J.; Korytowski, W.; Boulton, M.E.; Burke, J.M.; Sarna, T.J. Blue light-induced reactivity of retinal age pigment: IN vitro generation OF oxygen-reactive species (∗). J. Biol. Chem. 1995, 270, 18825–18830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Różanowska, M.; Wessels, J.; Boulton, M.; Burke, J.M.; Rodgers, M.A.; Truscott, T.G.; Sarna, T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic. Biol. Med. 1998, 24, 1107–1112. [Google Scholar] [CrossRef]
- Hu, D.N.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef]
- Handa, J.T. How does the macula protect itself from oxidative stress? Mol. Asp. Med. 2012, 33, 418–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, J.; Jones, D.; Sternberg, P.; Wu, M.; Olsen, W. Antioxidant functions of glutathione in human retinal pigment epithelium in relation to age-related macular degeneration. In Retinal Pigment Epithelium and Macular Diseases; Springer: Berlin, Germany, 1998; pp. 47–57. [Google Scholar]
- deS Senanayake, P.; Calabro, A.; Hu, J.G.; Bonilha, V.L.; Darr, A.; Bok, D.; Hollyfield, J.G. Glucose utilization by the retinal pigment epithelium: Evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp. Eye Res. 2006, 83, 235–246. [Google Scholar] [CrossRef]
- Shadrach, K.; Senanayake, P.; Nishiyama, K.; Lee, J.; Hu, J.; Calabro, A.; Bok, D.; Hollyfield, J. Glucose utilization by human RPE cultures. Investig. Ophthalmol. Vis. Sci. 2003, 44, 394. [Google Scholar]
- Khatami, M.; Stramm, L.E.; Rookey, J.H. Ascorbate transport in cultured cat retinal pigment epithelial cells. Exp. Eye Res. 1986, 43, 607–615. [Google Scholar] [CrossRef]
- Bazan, N.G.; Gordon, W.C.; de Turco, E.B.R. Docosahexaenoic acid uptake and metabolism in photoreceptors: Retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Neurobiol. Essent. Fat. Acids 1992, 295–306. [Google Scholar]
- Nagelhus, E.A.; Horio, Y.; Inanobe, A.; Fujita, A.; Haug, F.m.; Nielsen, S.; Kurachi, Y.; Ottersen, O.P. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4. 1 and AQP4 in specific membrane domains. Glia 1999, 26, 47–54. [Google Scholar] [CrossRef]
- Marmor, M.F. Control of subretinal fluid: Experimental and clinical studies. Eye 1990, 4, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.S.; Edelman, J.L. Active ion transport pathways in the bovine retinal pigment epithelium. J. Physiol. 1990, 424, 283–300. [Google Scholar] [CrossRef]
- Verkman, A.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.K.; Sundstrom, J.M.; Antonetti, D.A. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 2007, 10, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.S.; Steinberg, R.H. Passive ionic properties of frog retinal pigment epithelium. J. Membr. Biol. 1977, 36, 337–372. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003, 22, 4143–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomiany, M.G.; Rosenzweig, S.A. Autocrine effects of IGF-I-induced VEGF and IGFBP-3 secretion in retinal pigment epithelial cell line ARPE-19. Am. J. Physiol. Cell Physiol. 2004, 287, C746–C753. [Google Scholar] [CrossRef] [Green Version]
- Walsh, N.; Valter, K.; Stone, J. Cellular and subcellular patterns of expression of bFGF and CNTF in the normal and light stressed adult rat retina. Exp. Eye Res. 2001, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Hackett, S.F.; Vinores, S.A.; Freund, J.; Csaky, C.; LaRochelle, W.; Henderer, J.; Johnson, M.; Rodriguez, I.R.; Friedman, Z. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J. Cell Sci. 1994, 107, 2459–2469. [Google Scholar] [CrossRef]
- Ahuja, P.; Caffe, A.; Holmqvist, I.; Söderpalm, A.; Singh, D.; Shinohara, T.; Van Veen, T. Lens epithelium-derived growth factor (LEDGF) delays photoreceptor degeneration in explants of rd/rd mouse retina. Neuroreport 2001, 12, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Adamis, A.; Shima, D.; Yeo, K.-T.; Yeo, T.; Brown, L.; Berse, B.; Damore, P.; Folkman, J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 1993, 193, 631–638. [Google Scholar] [CrossRef]
- Tombran-Tink, J.; Chader, G.G.; Johnson, L.V. PEDF: A pigment epithelium-derived factor with potent neuronal differentiative activity. Exp. Eye Res. 1991, 53, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Tanihara, H.; Yoshida, M.; Matsumoto, M.; Yoshimura, N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1993, 34, 413–419. [Google Scholar]
- Witmer, A.; Vrensen, G.; Van Noorden, C.; Schlingemann, R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Hargrave, P.A. Rhodopsin structure, function, and topography the Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3–9. [Google Scholar]
- Detrick, B.; Hooks, J.J. Immune regulation in the retina. Immunol. Res. 2010, 47, 153–161. [Google Scholar] [CrossRef]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Zavazava, N.; Halene, M.; Westphal, E.; Nölle, B.; Duncker, G.; Eckstein, E.; Harpprecht, J.; MÜLLER-RUCHHOLTZ, W. Expression of MHC class I and II molecules by cadaver retinal pigment epithelium cells: Optimization of post-mortem HLA typing. Clin. Exp. Immunol. 1991, 84, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, S.J.; Bishop, P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2018, 40, 65–74. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Agodi, A. Complement system and age-related macular degeneration: Implications of gene-environment interaction for preventive and personalized medicine. BioMed Res. Int. 2018, 2018, 7532507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-G.; Park, Y.-S.; Kim, I.-B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Nat. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, J.; Chen, M.; Hogg, R.E.; Toth, L.; Silvestri, G.; Chakravarthy, U.; Xu, H. Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age-related macular degeneration. Immun. Ageing 2016, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipo, E.; Cashman, S.M.; Kumar-Singh, R. Aurintricarboxylic acid inhibits complement activation, membrane attack complex, and choroidal neovascularization in a model of macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7107–7114. [Google Scholar] [CrossRef]
- Schramm, E.C.; Clark, S.J.; Triebwasser, M.P.; Raychaudhuri, S.; Seddon, J.M.; Atkinson, J.P. Genetic variants in the complement system predisposing to age-related macular degeneration: A review. Mol. Immunol. 2014, 61, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433–439. [Google Scholar]
- Lin, M.K.; Yang, J.; Hsu, C.W.; Gore, A.; Bassuk, A.G.; Brown, L.M.; Colligan, R.; Sengillo, J.D.; Mahajan, V.B.; Tsang, S.H. HTRA 1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP 1 and TSP 1. Aging Cell 2018, 17, e12710. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Strainic, M.; Medof, M. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin. Exp. Immunol. 2003, 131, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Baciu, P.; Kerrigan, B.C.P.; Etheridge, M.; Sung, E.; Toimil, B.A.; Berchuck, J.E.; Jaffe, G.J. Retinal pigment epithelial cell death by the alternative complement cascade: Role of membrane regulatory proteins, calcium, PKC, and oxidative stress. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Joseph, K.; Kulik, L.; Coughlin, B.; Kunchithapautham, K.; Bandyopadhyay, M.; Thiel, S.; Thielens, N.M.; Holers, V.M.; Rohrer, B.J. Oxidative stress sensitizes retinal pigmented epithelial (RPE) cells to complement-mediated injury in a natural antibody-, lectin pathway-, and phospholipid epitope-dependent manner. J. Biol. Chem. 2013, 288, 12753–12765. [Google Scholar] [CrossRef] [Green Version]
- Lau, L.-I.; Chiou, S.-H.; Liu, C.J.-L.; Yen, M.-Y.; Wei, Y.-H. The effect of photo-oxidative stress and inflammatory cytokine on complement factor H expression in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6832–6841. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B.J. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef] [Green Version]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD pathobiology: A bioenergetic crisis in the RPE. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef] [Green Version]
- Adijanto, J.; Du, J.; Moffat, C.; Seifert, E.L.; Hurley, J.B.; Philp, N.J. The retinal pigment epithelium utilizes fatty acids for ketogenesis: Implications for metabolic coupling with the outer retina. J. Biol. Chem. 2014, 289, 20570–20582. [Google Scholar] [CrossRef] [Green Version]
- Toms, M.; Burgoyne, T.; Tracey-White, D.; Richardson, R.; Dubis, A.M.; Webster, A.R.; Futter, C.; Moosajee, M. Phagosomal and mitochondrial alterations in RPE may contribute to KCNJ13 retinopathy. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Gottlieb, E.; Brooks, D.G.; Murphy, M.P.; Dunaief, J.L. Mitochondria-derived Reactive Oxygen Species Mediate Blue Light-induced Death of Retinal Pigment Epithelial Cells. Photochem. Photobiol. 2004, 79, 470–475. [Google Scholar] [CrossRef]
- Liang, F.-Q.; Godley, B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [Green Version]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N.Y. Acad. Sci. 2008, 1147, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasahara, E.; Lin, L.-R.; Ho, Y.-S.; Reddy, V.N. SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: Implications for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3426–3434. [Google Scholar] [CrossRef]
- Lascaratos, G.; Ji, D.; Wood, J.P.; Osborne, N.N. Visible light affects mitochondrial function and induces neuronal death in retinal cell cultures. Vis. Res. 2007, 47, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrett, S.G.; Lewin, A.S.; Boulton, M.E. The importance of mitochondria in age-related and inherited eye disorders. Ophthalmic Res. 2010, 44, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candas, D.; Li, J.J. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal. 2014, 20, 1599–1617. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M.J. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Haber, E.; Hirabara, S.; Rebelato, E.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.; Carpinelli, A.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, J.; Zhang, H.; Wang, Z.; Liu, Q.; Zhou, Q.; Wang, S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013, 4, e965. [Google Scholar] [CrossRef] [Green Version]
- Guang-Yu, L.; Bin, F.; Zheng, Y.-C. Calcium overload is a critical step in programmed necrosis of ARPE-19 cells induced by high-concentration H2O2. Biomed. Environ. Sci. 2010, 23, 371–377. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Reed, J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430. [Google Scholar] [CrossRef]
- Kaneko, H.; Dridi, S.; Tarallo, V.; Gelfand, B.D.; Fowler, B.J.; Cho, W.G.; Kleinman, M.E.; Ponicsan, S.L.; Hauswirth, W.W.; Chiodo, V.A. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011, 471, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.T.; Bardwell, A.J.; Grewal, S.; Iverson, C.; Bardwell, L. Interacting JNK-docking sites in MKK7 promote binding and activation of JNK mitogen-activated protein kinases. J. Biol. Chem. 2006, 281, 13169–13179. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Cui, J.Z.; To, E.; Cao, S.; Matsubara, J.A. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J. Neuroinflamm. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.A.; Thein, T.; Kinnunen, K.; Lashkari, K.; Gregory, M.S.; D'Amore, P.A.; Ksander, B.R. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: Implications for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 110–120. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Brandstetter, C.; Patt, J.; Holz, F.G.; Krohne, T.U. Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis. J. Photochem. Photobiol. B Biol. 2016, 161, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kivinen, N. The Role of Autophagy in Age-Related Macular Degeneration (AMD)–Studies into the Pathogenesis of AMD. Ph.D. dissertation, University of Michigan, Ann Arbor, MI, USA, 2018. [Google Scholar]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.K.; Rao, H.V.; Qi, X.; Cai, J.; Sugrue, A.; Dunn, W.A.; Grant, M.B.; Boulton, M.E. Autophagy in the retina: A potential role in age-related macular degeneration. Retin. Degener. Dis. 2012, 83–90. [Google Scholar]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Eye Res. 2010, 90, 465–471. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PloS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [Green Version]
- Alge, C.S.; Priglinger, S.G.; Neubauer, A.S.; Kampik, A.; Zillig, M.; Bloemendal, H.; Welge-Lussen, U. Retinal pigment epithelium is protected against apoptosis by αB-crystallin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3575–3582. [Google Scholar]
- Gangalum, R.K.; Schibler, M.J.; Bhat, S.P. Small heat shock protein αB-crystallin is part of cell cycle-dependent Golgi reorganization. J. Biol. Chem. 2004, 279, 43374–43377. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Rabin, D.M.; Salero, E.; Lederman, P.L.; Temple, S.; Stern, J.H. Human retinal pigment epithelium cell changes and expression of αB-crystallin: A biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch. Ophthalmol. 2007, 125, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimberg, A.; Rylova, S.; Dieterich, L.C.; Olsson, A.-K.; Schiller, P.; Wikner, C.; Bohman, S.; Botling, J.; Lukinius, A.; Wawrousek, E.F. αB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. J. Am. Soc. Hematol. 2008, 111, 2015–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kase, S.; He, S.; Sonoda, S.; Kitamura, M.; Spee, C.; Wawrousek, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. αB-crystallin regulation of angiogenesis by modulation of VEGF. J. Am. Soc. Hematol. 2010, 115, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Yaung, J.; Kannan, R.; Wawrousek, E.F.; Spee, C.; Sreekumar, P.G.; Hinton, D.R. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp. Eye Res. 2008, 86, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, G.; Kato, S.; Nakata, H.; Ishida, T.; Ohuchi, N.; Ishioka, C. αB-crystallin: A novel p53-target gene required for p53-dependent apoptosis. Cancer Sci. 2009, 100, 2368–2375. [Google Scholar] [CrossRef]
- Li, D.W.-C.; Liu, J.-P.; Mao, Y.-W.; Xiang, H.; Wang, J.; Ma, W.-Y.; Dong, Z.; Pike, H.M.; Brown, R.E.; Reed, J.C. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by αB-crystallin through inhibition of RAS activation. Mol. Biol. Cell 2005, 16, 4437–4453. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.C.; Gonzalez-Lima, F. Low-level light therapy of the eye and brain. Eye Brain 2011, 3, 49. [Google Scholar]
- Tata, D.B.; Waynant, R.W. Laser therapy: A review of its mechanism of action and potential medical applications. Laser Photonics Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Ivandic, B.T.; Ivandic, T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef]
- Merry, G.; Dotson, R.; Devenyi, R.; Markowitz, S.; Reyes, S. Photobiomodulation as a new treatment for dry age related macular degeneration. results from the toronto and Oak ridge photobimodulation study in AMD (TORPA). Investig. Ophthalmol. Vis. Sci. 2012, 53, 2049. [Google Scholar]
- Tang, J.; Herda, A.A.; Kern, T.S. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br. J. Ophthalmol. 2014, 98, 1013–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuma, S.; Murase, H.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. Photobiomodulation with 670 nm light increased phagocytosis in human retinal pigment epithelial cells. Mol. Vis. 2015, 21, 883. [Google Scholar]
- Lavey, B.J.; Estlack, L.E.; Schuster, K.J.; Rockwell, B.A.; Wigle, J.C. The response of human retinal pigmented epithelial cells in vitro to changes in nitric oxide concentration stimulated by low levels of red light. Proceedings of Mechanisms for Low-Light Therapy VIII, San Francisco, CA, USA, 2–3 February 2013; p. 85690. [Google Scholar]
- Kokkinopoulos, I.; Colman, A.; Hogg, C.; Heckenlively, J.; Jeffery, G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017, 95, e270–e277. [Google Scholar] [CrossRef] [Green Version]
- Tezel, T.H.; Del Priore, L.V.; Berger, A.S.; Kaplan, H.J. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 584–595. [Google Scholar] [CrossRef]
- Binder, S.; Krebs, I.; Hilgers, R.-D.; Abri, A.; Stolba, U.; Assadoulina, A.; Kellner, L.; Stanzel, B.V.; Jahn, C.; Feichtinger, H.; et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: A prospective trial. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4151–4160. [Google Scholar] [CrossRef]
- Lu, Y.; Han, L.; Wang, C.; Dou, H.; Feng, X.; Hu, Y.; Feng, K.; Wang, X.; Ma, Z. A comparison of autologous transplantation of retinal pigment epithelium (RPE) monolayer sheet graft with RPE–Bruch's membrane complex graft in neovascular age-related macular degeneration. Acta Ophthalmol. 2017, 95, e443–e452. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surendran, H.; Nandakumar, S.; Stoddard, J.; Mohan, V.; Upadhyay, P.K.; McGill, T.J.; Pal, R. Therapy. Transplantation of retinal pigment epithelium and photoreceptors generated concomitantly via small molecule-mediated differentiation rescues visual function in rodent models of retinal degeneration. Stem Cell Rep. 2021, 12, 1–17. [Google Scholar]
- Shrestha, R.; Wen, Y.-T.; Tsai, R.-K. Effective differentiation and biological characterization of retinal pigment epithelium derived from human induced pluripotent stem cells. Curr. Eye Res. 2020, 45, 1155–1167. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q. Clinical-grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Trans. Med. No. 475 (eaat550). 2019, 11. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal transplantation of embryonic stem cell–derived retinal pigment epithelium for the treatment of macular degeneration: An assessment at 4 years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Trans. Med. No. 435 (eaao4097). 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, Y.J.; Won, J.Y. Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12298. https://doi.org/10.3390/ijms222212298
Kim J, Lee YJ, Won JY. Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2021; 22(22):12298. https://doi.org/10.3390/ijms222212298
Chicago/Turabian StyleKim, Jongmin, Yeo Jin Lee, and Jae Yon Won. 2021. "Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration" International Journal of Molecular Sciences 22, no. 22: 12298. https://doi.org/10.3390/ijms222212298
APA StyleKim, J., Lee, Y. J., & Won, J. Y. (2021). Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. International Journal of Molecular Sciences, 22(22), 12298. https://doi.org/10.3390/ijms222212298







