Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry
Abstract
:1. Introduction
2. Results
2.1. Identification of XTH and PG Family Genes in Prunus avium
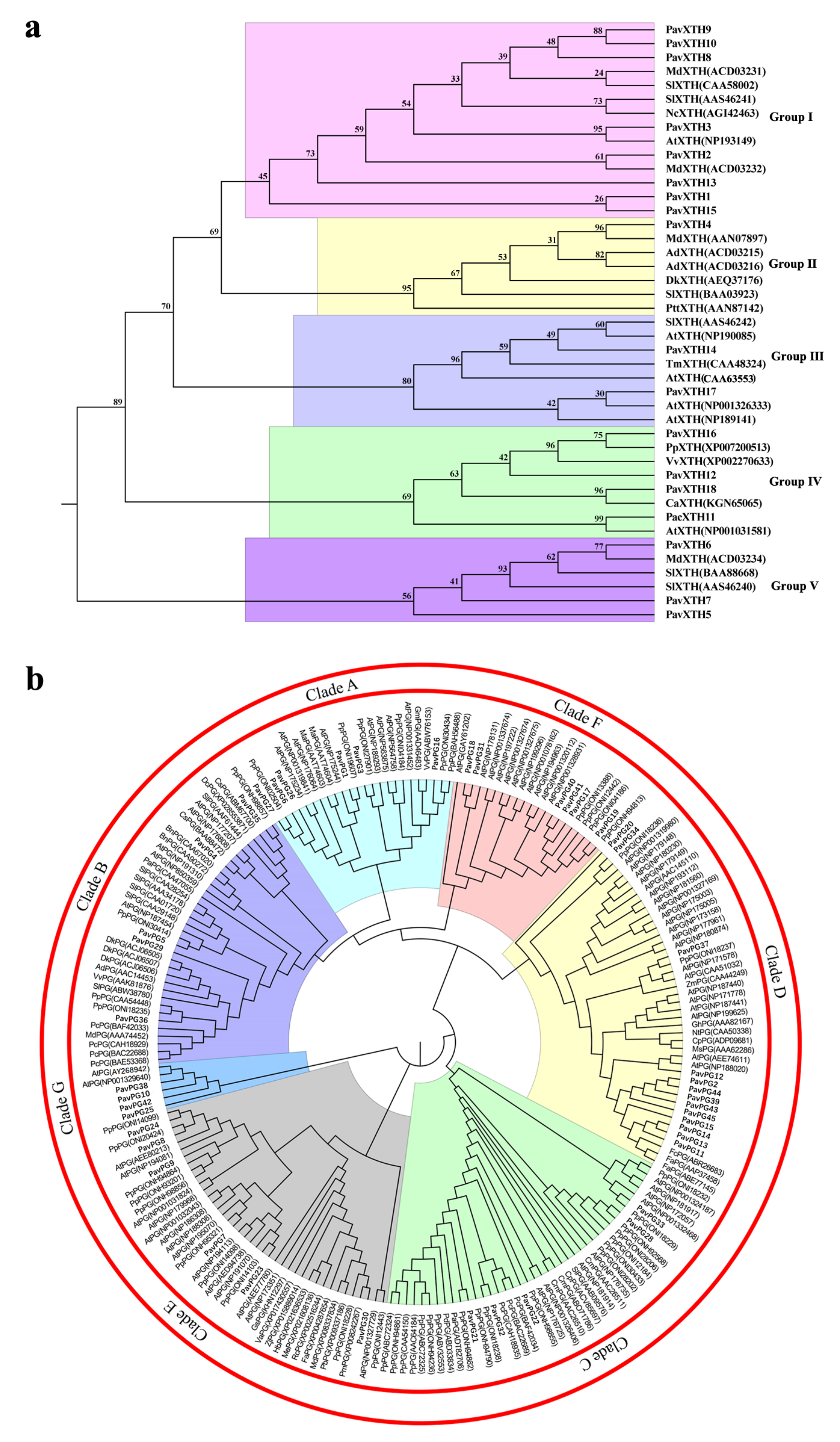
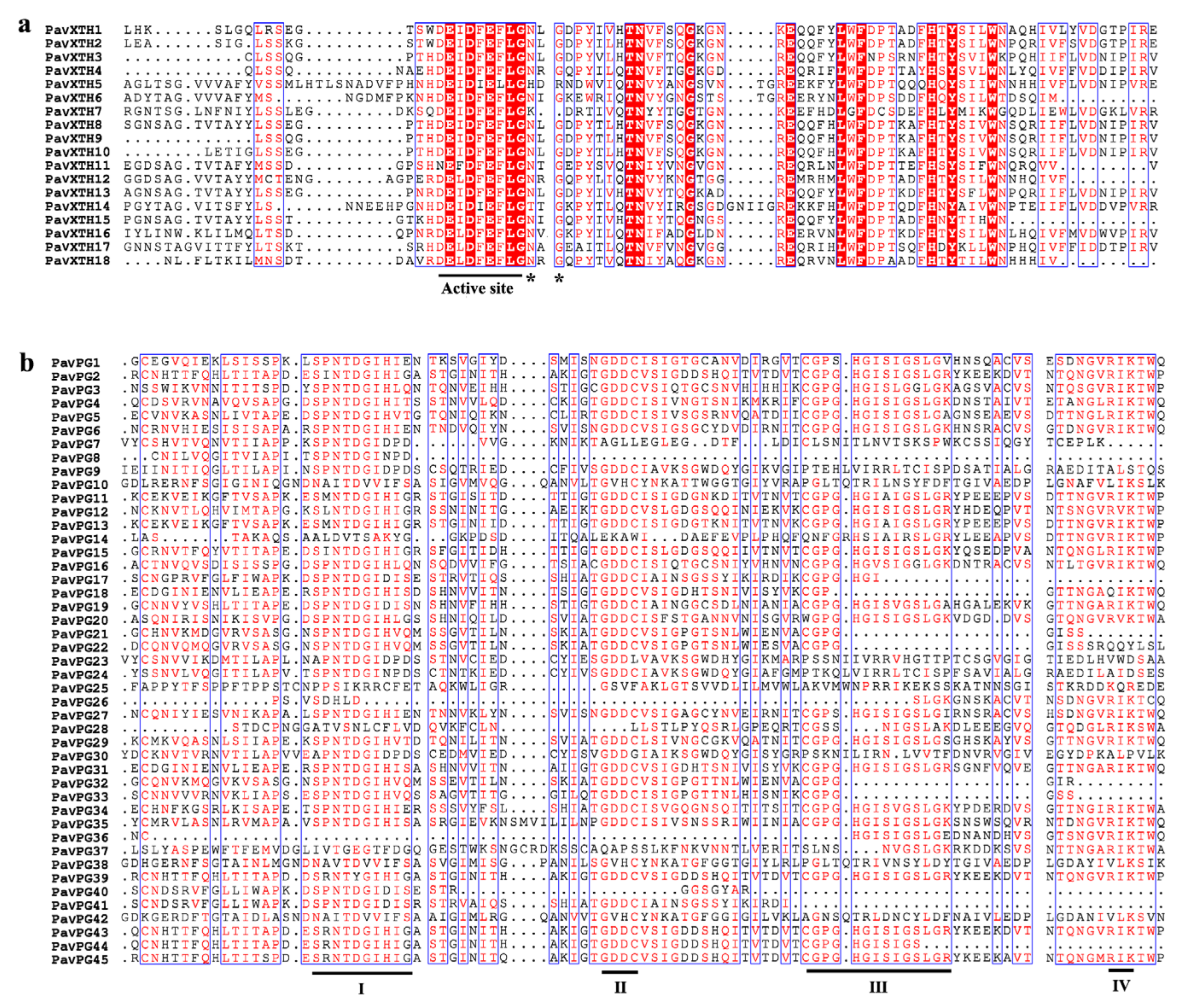
2.2. Phylogenetic Analysis and Multiple Sequence Alignments of the PavXTH and PavPG Gene Family Members
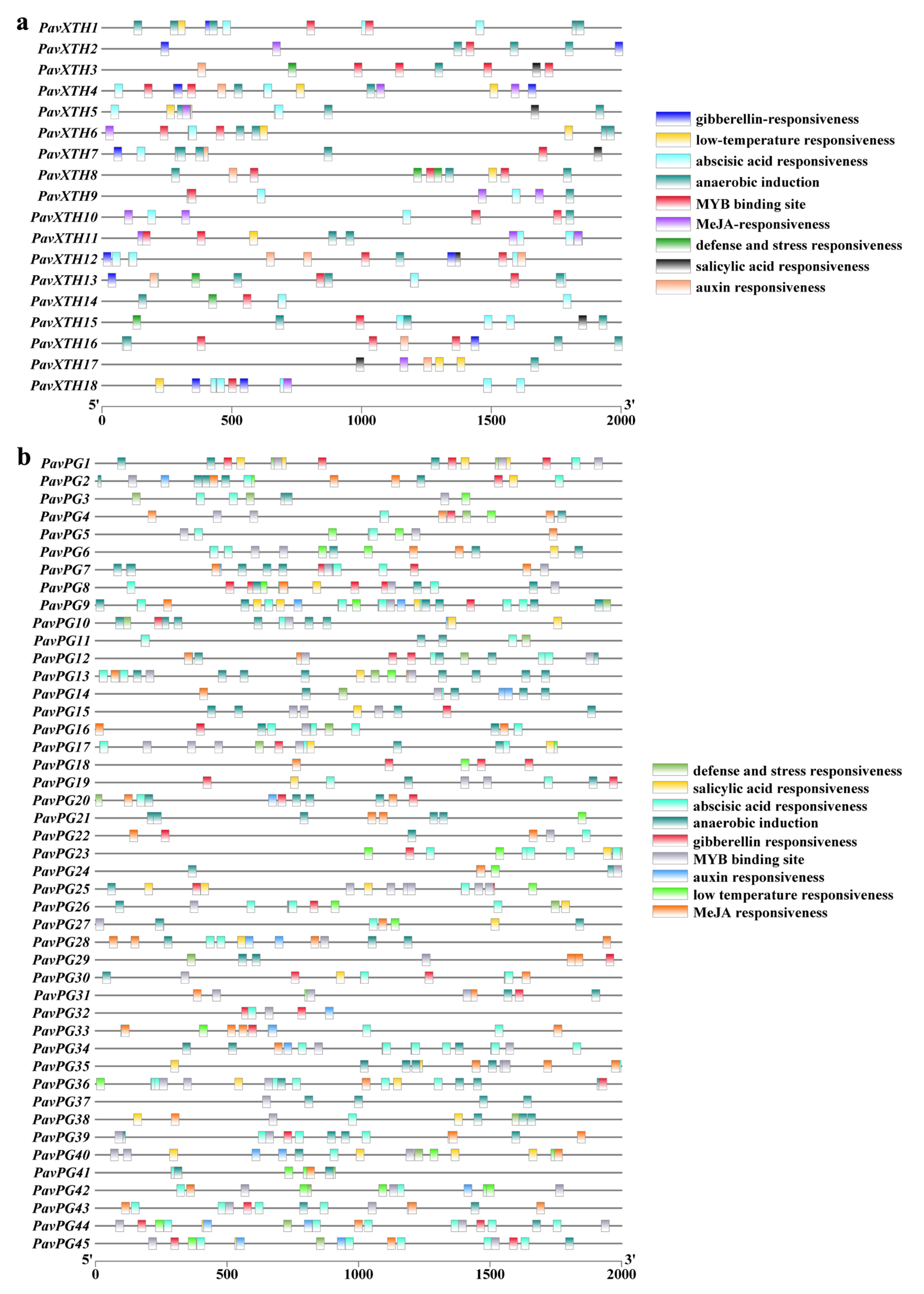
2.3. Gene Structure, Conserved Motif and Cis-Elements Analysis of PavXTHs and PavPGs
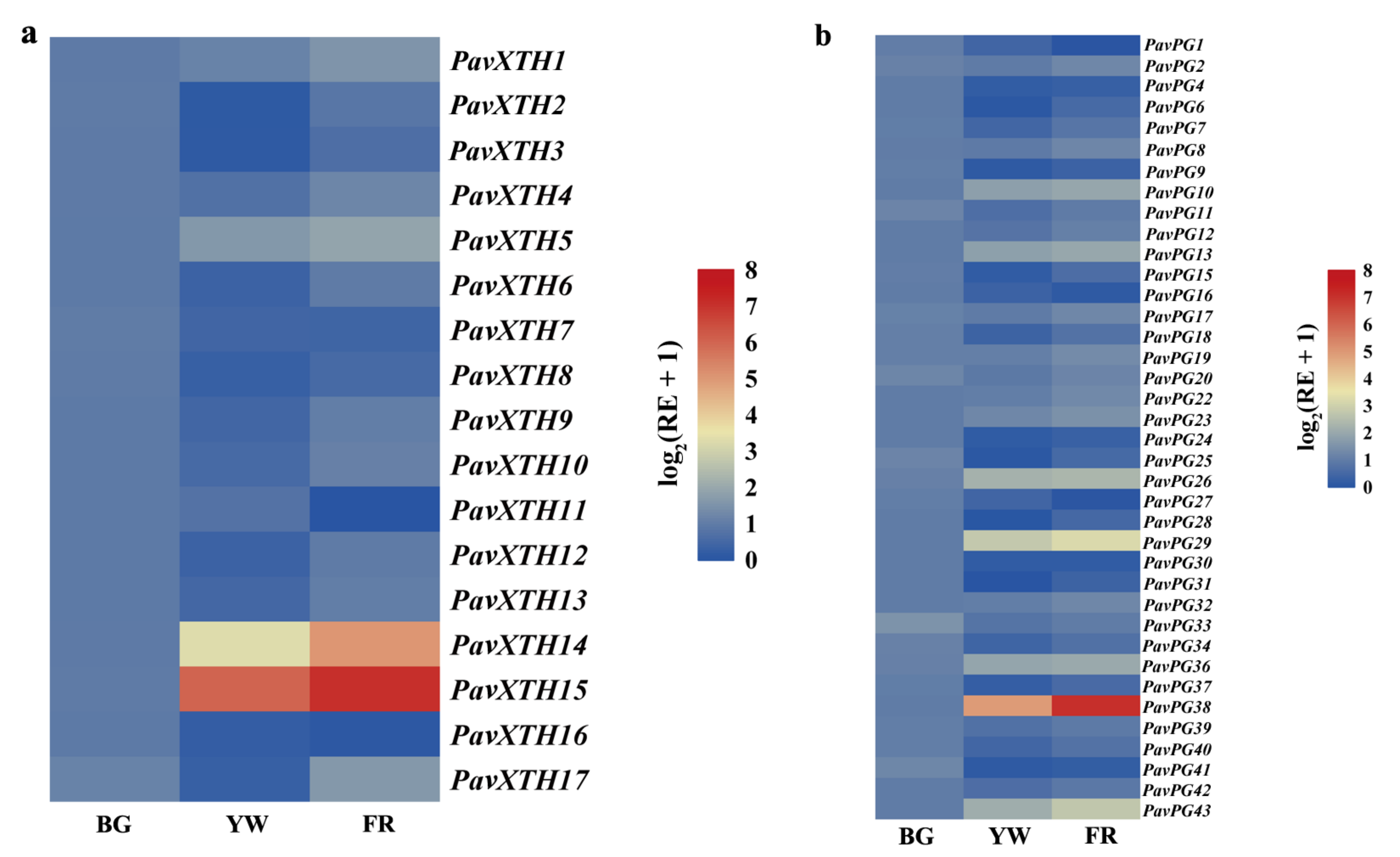
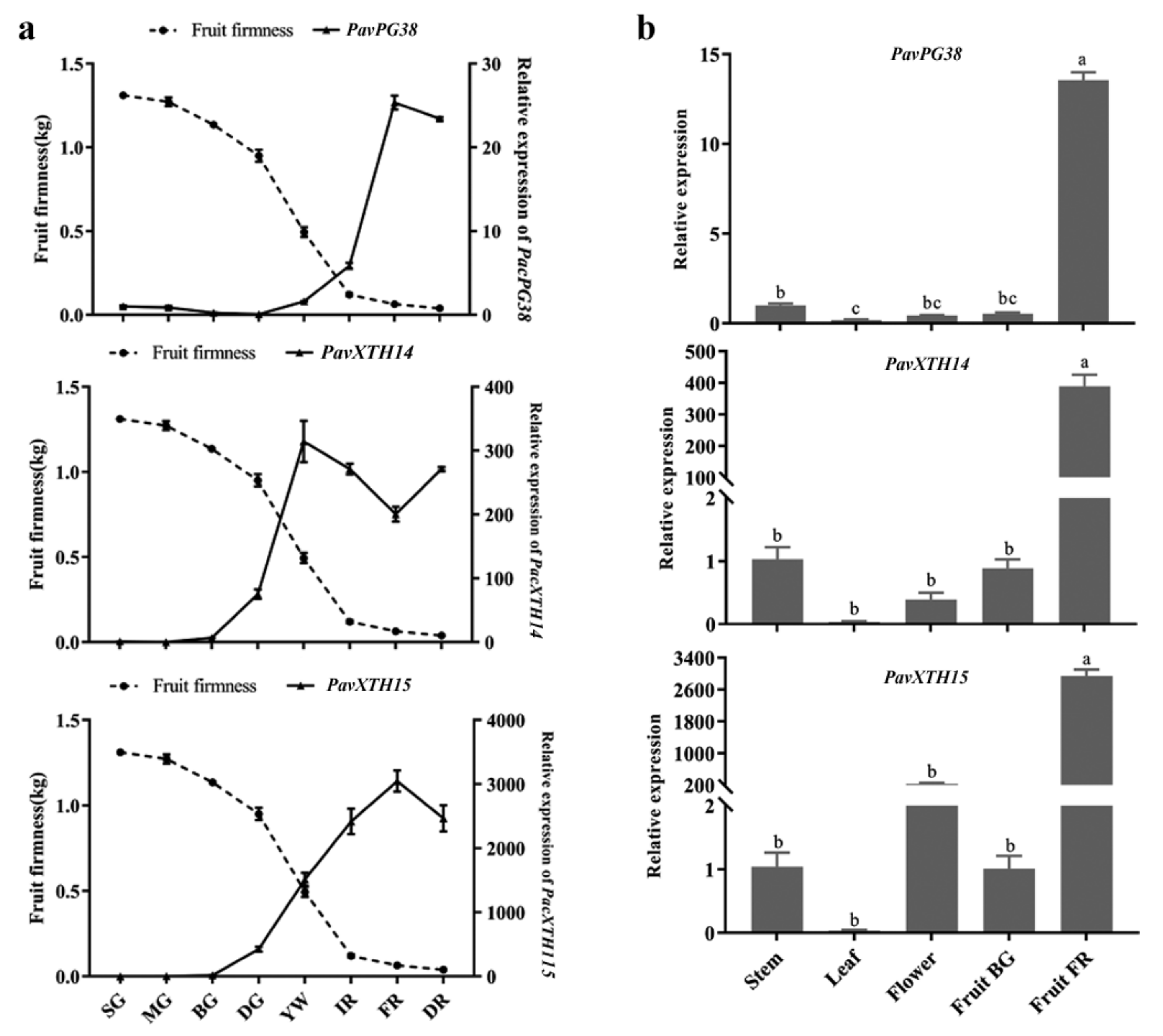
2.4. Identification of PavXTH and PavPG Genes Underlying Involved in Fruit Softening
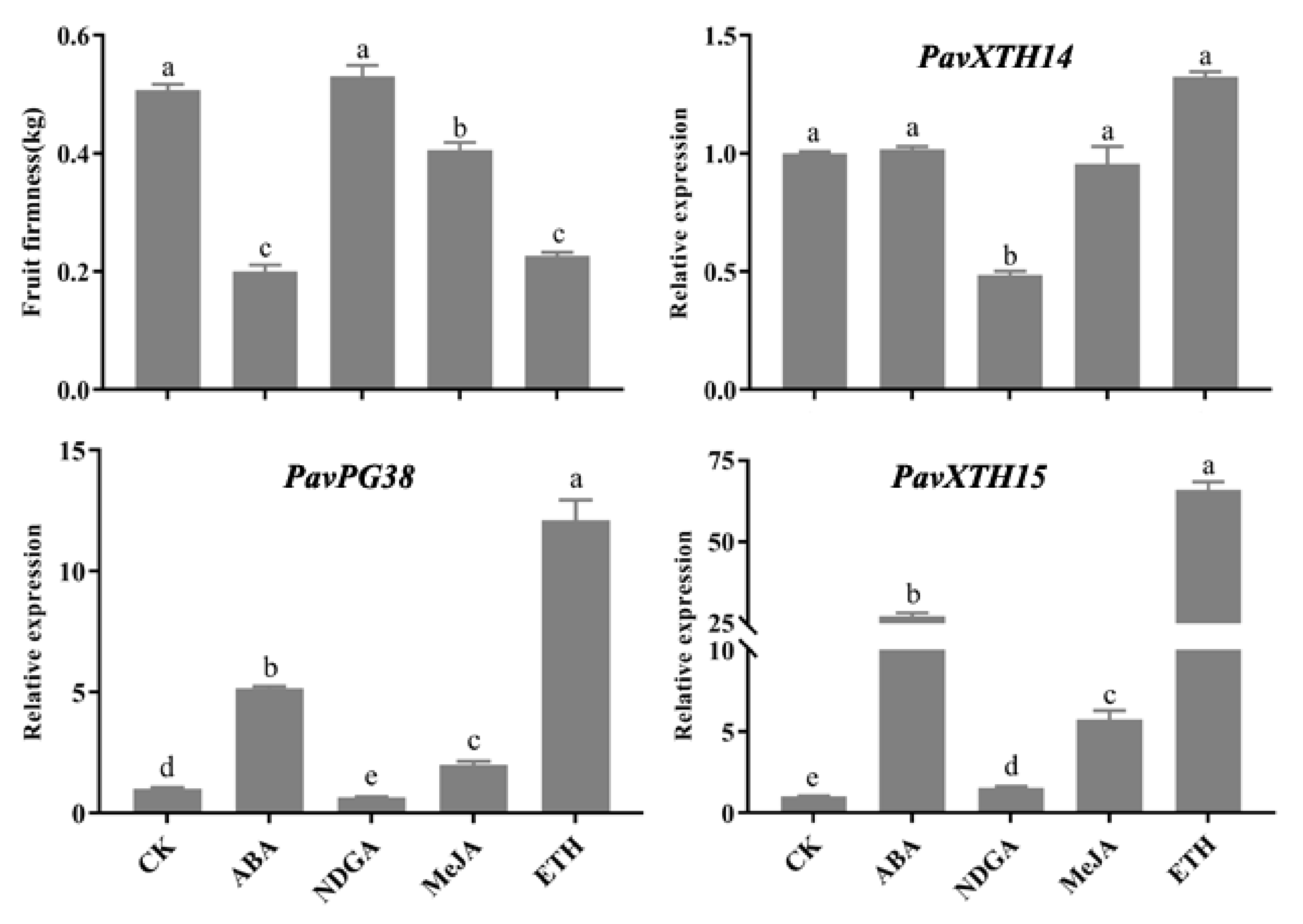
2.5. Expression of PavXTH14, PavXTH15 and PavPG38 Gene Expression in Response to Hormones
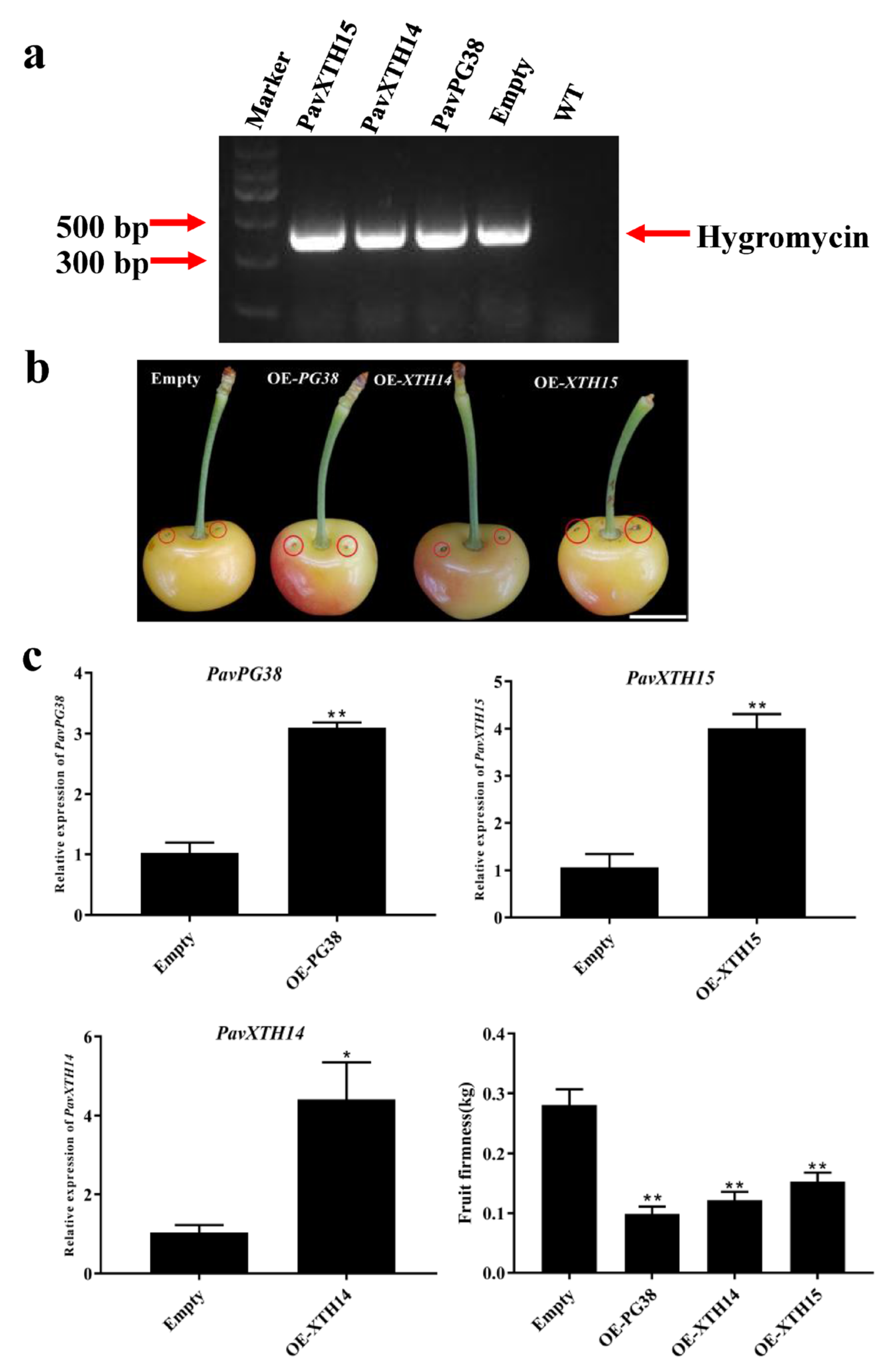
2.6. Transient Overexpression PavXTH14, PavXTH15 and PavPG38 in Cherry Fruits
3. Discussion
3.1. Characteristics of PavXTH and PavPG Gene Families
3.2. Expression Profiles of PavXTH and PavPG Genes
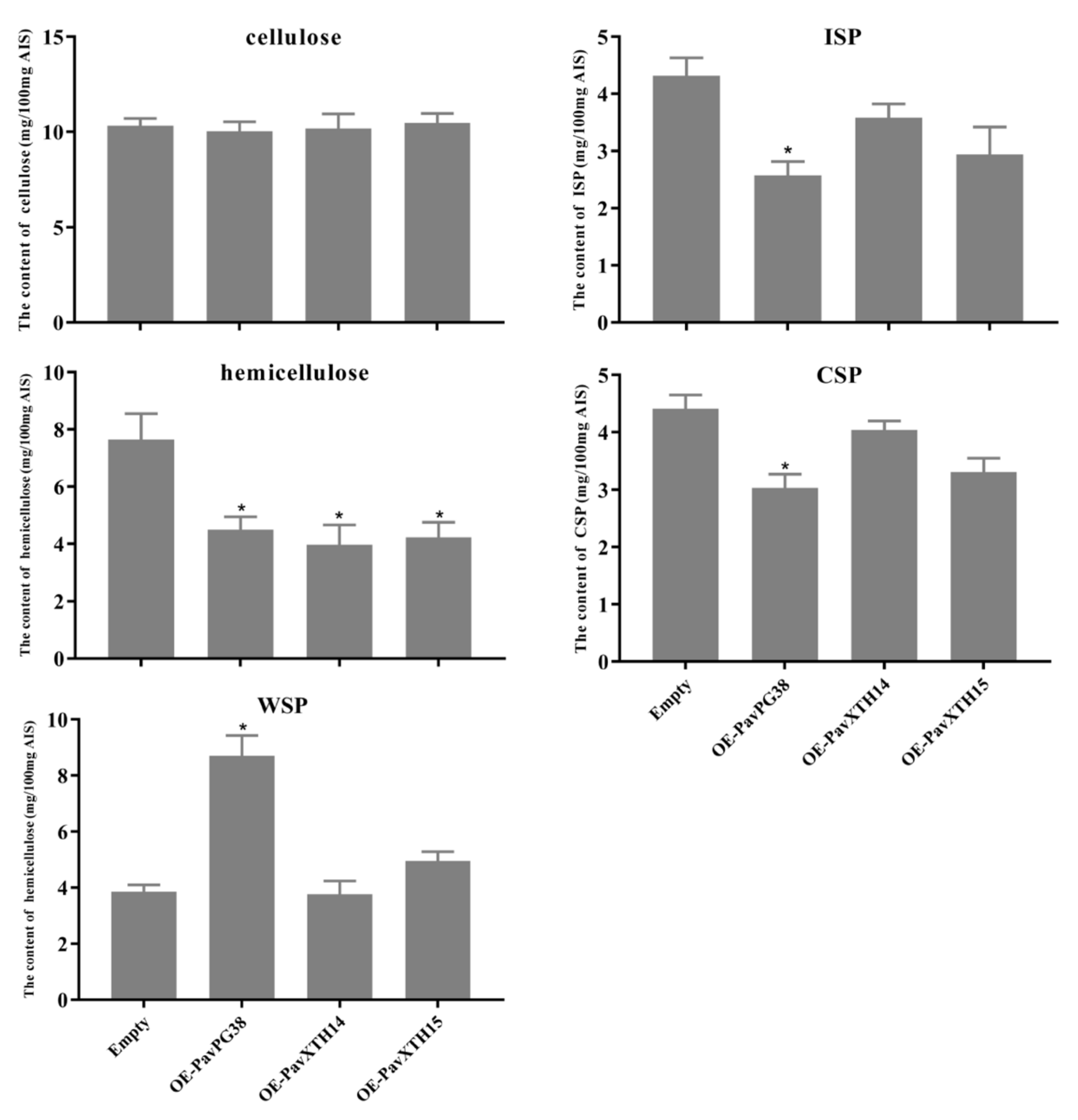
3.3. PavXTH14, PavXTH15 and PavPG38 Participate in Softening by Disrupting the Fruit Cell Wall
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Mining of PavXTH and PavPG Genes in the Prunus Avium Genome
4.3. Phylogenetic Analysis, Multiple Sequence Alignment, Genomic Structure, Chromosomal Location, and Motif Analysis
4.4. Cis-Element Prediction in the PavXTH and PavPG Gene Promoters
4.5. Measurement of Fruit Firmness and Cell Wall Materials
4.6. RT-qPCR Analysis
4.7. Transient Overexpression in Cherry Fruits
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brummell, D.A.; Valeriano, D.C.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [Green Version]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587–599. [Google Scholar] [CrossRef]
- Vicente, A.R.; Saladié, M.; Rose, J.K.C.; Labavitch, J.M. The linkage between cell wall metabolism and fruit softening: Looking to the future. J. Sci. Food Agric. 2007, 87, 1435–1448. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opazo, M.C.; Lizana, R.; Stappung, Y.; Davis, T.M.; Herrera, R.; Moya-Leon, M.A. XTHs from Fragaria vesca: Genomic structure and transcriptomic analysis in ripening fruit and other tissues. BMC Genom. 2017, 18, 852–863. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xu, Z.; Ding, A.; Kong, Y. Genome-wide identification and expression profiling analysis of the Xyloglucan Endotransglucosylase/Hydrolase gene family in Tobacco (Nicotiana tabacum L.). Genes 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Valliyodan, B.; Prince, S.; Wan, J.; Nguyen, H.T. Characterization of the XTH gene family: New insight to the roles in Soybean flooding tolerance. Int. J. Mol. Sci. 2018, 19, 2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, R.; Rose, J.K.; Nishitani, K. A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiol. 2004, 134, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.M.; Liu, C.; Wu, F. Genome-wide identification, characterization and expression analysis of Xyloglucan Endotransglucosylase/Hydrolase genes family in Barley (Hordeum vulgare). Molecules 2019, 24, 1935. [Google Scholar] [CrossRef] [Green Version]
- Miedes, E.; Herbers, K.; Sonnewald, U.; Lorences, E.P. Overexpression of a cell wall enzyme reduces xyloglucan depolymerization and softening of transgenic tomato fruits. J. Agric. Food Chem. 2010, 58, 5708–5713. [Google Scholar] [CrossRef]
- Witasari, L.D.; Huang, F.C.; Hoffmann, T.; Rozhon, W.; Fry, S.C.; Schwab, W. Higher expression of the strawberry xyloglucan endotransglucosylase/hydrolase genes FvXTH9 and FvXTH6 accelerates fruit ripening. Plant J. 2019, 100, 1237–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 39155–39170. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Onelli, E.; Aina, R.; Cocucci, M.; Citterio, S. A comparative study of melting and non-melting flesh peach cultivars reveals that during fruit ripening endo-polygalacturonase (endo-PG) is mainly involved in pericarp textural changes, not in firmness reduction. J. Exp. Bot. 2011, 62, 4043–4054. [Google Scholar] [CrossRef] [Green Version]
- Pose, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Li, F.; Li, F.; Zhang, D.; Zhao, C. Identification and expression analysis of Polygalacturonase family members during peach fruit softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-wide identification and analysis of polygalacturonase genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Carranza, Z.H.; Elliott, K.A.; Roberts, J.A. Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 3719–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Huang, Z.; Song, X.; Liu, T.; Liu, H.; Hou, X.; Li, Y. Comprehensive analysis of the polygalacturonase and pectin methylesterase genes in Brassica rapa shed light on their different evolutionary patterns. Sci. Rep. 2016, 6, 25107. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Zhao, T.; Han, F.; Zhang, Q.; Liu, X.; Jiang, C.; Zhong, C. Genome-wide identification and expression analysis of polygalacturonase gene family in Kiwifruit (Actinidia chinensis) during fruit softening. Plants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dautt-Castro, M.; Lopez-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillon-Sortillon, A.P.; Martinez-Tellez, M.A.; Gonzalez-Aguilar, G.A.; Casas-Flores, J.S.; Sanudo-Barajas, A.; Kuhn, D.N.; et al. Genome-wide identification of Mango (Mangifera indica L.) polygalacturonases: Expression analysis of family members and total enzyme activity during fruit ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-wide identification and expression profiling of the polygalacturonase (PG) and pectin methylesterase (PME) genes in Grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.G.; Schroder, R.; Hallett, I.C.; Cohen, D.; MacRae, E.A. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002, 129, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schroder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus x domestica) fruit. BMC Plant Biol. 2012, 12, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, M.A.; Blanco-Portales, R.; Posé, S.; García-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.S.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Muñoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.Y.; Osborne, E.; Poindexter, P.D.; Somerville, C.R. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 2003, 133, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Karppinen, K.; Tegelberg, P.; Haggman, H.; Jaakola, L. Abscisic Acid regulates anthocyanin biosynthesis and gene expression associated with cell wall modification in ripening Bilberry (Vaccinium myrtillus L.) fruits. Front. Plant Sci. 2018, 9, 1259–1276. [Google Scholar] [CrossRef]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, 11542–11550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serradilla, M.J.; Falagán, N.; Bohmer, B.; Terry, L.A.; Alamar, M.C. The role of ethylene and 1-MCP in early-season sweet cherry ‘Burlat’ storage life. Sci. Hortic. 2019, 258, 108787–108796. [Google Scholar] [CrossRef]
- Saracoglu, O.; Ozturk, B.; Yildiz, K.; Kucuker, E. Pre-harvest methyl jasmonate treatments delayed ripening and improved quality of sweet cherry fruits. Sci. Hortic. 2017, 226, 19–23. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Rose, J.; Janet, B.; Fry, S.C.; Kazuhiko, N. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, H.; Yin, C.; Wang, X.; Jiang, Q.; Zhang, R.; Ge, F.; Chen, Y.; Yang, L. Genome-wide identification and characterization of Xyloglucan Endotransglycosylase/Hydrolase in Ananas comosus during development. Genes 2019, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.J.; Eklof, J.M.; Michel, G.; Kallas, A.M.; Teeri, T.T.; Czjzek, M.; Brumer, H. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: Biological implications for cell wall metabolism. Plant Cell 2007, 19, 1947–1963. [Google Scholar] [CrossRef] [Green Version]
- Mark, P.; Baumann, M.J.; Eklof, J.M.; Gullfot, F.; Michel, G.; Kallas, A.M.; Teeri, T.T.; Brumer, H.; Czjzek, M. Analysis of nasturtium TmNXG1 complexes by crystallography and molecular dynamics provides detailed insight into substrate recognition by family GH16 xyloglucan endo-transglycosylases and endo-hydrolases. Proteins 2009, 75, 820–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Han, M. Identification and phylogenetic analysis of the polygalacturonase gene family in Apple. Hortic. Plant J. 2016, 2, 241–252. [Google Scholar] [CrossRef]
- Khan, N.; Hu, C.M.; Amjad Khan, W.; Hou, X. Genome-wide identification, classification, and expression divergence of Glutathione-Transferase family in Brassica rapa under multiple hormone treatments. Biomed Res. Int. 2018, 2018, 6023457–6023477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, S.; Monteiro, L.; Barreiro, M.G.; Pais, M.S. Expression of genes encoding cell wall modifying enzymes is induced by cold storage and reflects changes in pear fruit texture. J. Exp. Bot. 2005, 56, 2029–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wang, N.; Jiang, S.; Xu, H.; Wang, Y.; Wang, C.; Li, M.; Liu, J.; Qu, C.; Liu, W.; et al. Analysis of the Xyloglucan Endotransglucosylase/Hydrolase gene family during apple fruit ripening and softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Opazo, M.C.; Figueroa, C.R.; Henriquez, J.; Herrera, R.; Bruno, C.; Valenzuela, P.D.; Moya-Leon, M.A. Characterization of two divergent cDNAs encoding xyloglucan endotransglycosylase/hydrolase (XTH) expressed in Fragaria chiloensis fruit. Plant Sci. 2010, 179, 479–488. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; Garcia-Gago, J.A.; Lopez-Casado, G.; Blanco-Portales, R.; Munoz-Blanco, J.; Schuckel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A.; et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef] [PubMed]
- SchroÈder, R.; Atkinson, R.G.; Redgwell, G. Biochemical and molecular characterisation of xyloglucan endotransglycosylase from ripe kiwifruit. Planta 1998, 204, 242–251. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Ren, J.; Zhang, C.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant Growth Regul. 2013, 33, 373–383. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Li, H.; Jin, M.; Wang, X.; Yin, X.; Zhu, Q.; Rao, J. Transcription factors DkBZR1/2 regulate cell wall degradation genes and ethylene biosynthesis genes during persimmon fruit ripening. J. Exp. Bot. 2021, 72, 6437–6446. [Google Scholar] [CrossRef]
- Fu, D.Q.; Zhu, B.Z.; Zhu, H.L.; Jiang, W.B.; Luo, Y.B. Virus-induced gene silencing in tomato fruit. Plant J. 2005, 43, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, K.; Liu, L.; Zhang, K.; Yuan, H.; Liao, X.; Wang, Q.; Guo, X.; Li, F.; Li, T. A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 2014, 55, 862–880. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acid. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dische, Z. Measurement of Cell Wall Neutral Sugars. In Methods in Carbohydrate Chemistry; Whistler, R.L., Wolfrom, M.L., Eds.; Academic Press: New York, NY, USA, 1962; pp. 475–481. [Google Scholar]
| Branch | Gene Locus | Gene Name | Deduced Protein | Signal Peptide | ||
|---|---|---|---|---|---|---|
| Length (aa) | PI | MW (kDa) | ||||
| Ⅰ | Pav_sc0000359.1_g040.1.mk | PavXTH1 | 150 | 6.34 | 17.43 | - |
| Pav_sc0000308.1_g610.1.mk | PavXTH2 | 372 | 5.47 | 42.11 | + | |
| Pav_sc0003915.1_g020.1.mk | PavXTH3 | 176 | 9.68 | 20.5 | - | |
| Pav_sc0000428.1_g530.1.mk | PavXTH8 | 282 | 8.39 | 31.93 | + | |
| Pav_sc0000428.1_g520.1.mk | PavXTH9 | 193 | 9.14 | 22.37 | - | |
| Pav_sc0000428.1_g510.1.mk | PavXTH10 | 316 | 5.2 | 36.53 | - | |
| Pav_sc0001289.1_g270.1.mk | PavXTH13 | 204 | 5.2 | 23.18 | + | |
| Pav_sc0000212.1_g420.1.mk | PavXTH15 | 197 | 6.06 | 22.31 | + | |
| Ⅱ | Pav_sc0001450.1_g080.1.mk | PavXTH4 | 211 | 7.2 | 25.2 | - |
| Ⅲ | Pav_sc0000480.1_g990.1.mk | PavXTH14 | 293 | 6.22 | 32.95 | + |
| Pav_sc0000558.1_g1030.1.mk | PavXTH17 | 639 | 6.44 | 73.43 | - | |
| Ⅳ | Pav_sc0000405.1_g520.1.mk | PavXTH11 | 287 | 6.89 | 32.51 | + |
| Pav_sc0000910.1_g790.1.mk | PavXTH12 | 212 | 4.92 | 24.4 | + | |
| Pav_sc0003766.1_g160.1.mk | PavXTH16 | 277 | 9.41 | 32.62 | + | |
| Pav_sc0006464.1_g040.1.mk | PavXTH18 | 152 | 7.9 | 17.49 | - | |
| Ⅴ | Pav_sc0000354.1_g310.1.mk | PavXTH5 | 314 | 7.95 | 35.1 | + |
| Pav_sc0000893.1_g780.1.mk | PavXTH6 | 168 | 4.98 | 19.01 | + | |
| Pav_sc0001673.1_g050.1.mk | PavXTH7 | 215 | 5.34 | 24.16 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Feng, C.; Wang, Y.; Sun, Y.; Peng, X.; Xiao, Y.; Zhang, X.; Zhou, X.; Jiao, J.; Wang, W.; et al. Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. Int. J. Mol. Sci. 2021, 22, 12331. https://doi.org/10.3390/ijms222212331
Zhai Z, Feng C, Wang Y, Sun Y, Peng X, Xiao Y, Zhang X, Zhou X, Jiao J, Wang W, et al. Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. International Journal of Molecular Sciences. 2021; 22(22):12331. https://doi.org/10.3390/ijms222212331
Chicago/Turabian StyleZhai, Zefeng, Chen Feng, Yanyan Wang, Yueting Sun, Xiang Peng, Yuqin Xiao, Xiang Zhang, Xin Zhou, Jiale Jiao, Weili Wang, and et al. 2021. "Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry" International Journal of Molecular Sciences 22, no. 22: 12331. https://doi.org/10.3390/ijms222212331
APA StyleZhai, Z., Feng, C., Wang, Y., Sun, Y., Peng, X., Xiao, Y., Zhang, X., Zhou, X., Jiao, J., Wang, W., Du, B., Wang, C., Liu, Y., & Li, T. (2021). Genome-Wide Identification of the Xyloglucan endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. International Journal of Molecular Sciences, 22(22), 12331. https://doi.org/10.3390/ijms222212331






